Abstract
Purpose: To evaluate the relative efficacy of brodalumab compared with approved biologic therapies and apremilast for moderate-to-severe psoriasis.
Methods: We searched MEDLINE, Embase, and Cochrane for randomized controlled trials reporting induction phase responses. The primary analysis examined the proportion of patients achieving Psoriasis Area Severity Index (PASI) 50, 75, 90, or 100 responses using a random-effects Bayesian multinomial likelihood model with probit link, with and without adjustment for variation in study-level placebo responses.
Results: A total of 54 studies were included. Based on PASI 100 response, the most efficacious therapies were brodalumab 210 mg every two weeks (Q2W) and ixekizumab. Brodalumab 210 mg Q2W was significantly more efficacious than adalimumab, apremilast, brodalumab 140 mg Q2W, etanercept, infliximab, secukinumab, and ustekinumab. Results were consistent for PASI 50, 75, and 90 outcomes and all sensitivity analyses.
Conclusions: Our findings are consistent with pivotal trials which indicate that high levels of complete clearance can be achieved with brodalumab. Based on existing evidence, induction-phase efficacy of brodalumab is similar to ixekizumab and superior to other approved therapies, including anti-TNFs, apremilast, secukinumab, and ustekinumab.
Introduction
Psoriasis is a chronic, inflammatory, immune-mediated skin disorder which can occur at any age. Psoriasis affects 1.5–3% of the population, with approximately 20% of people with psoriasis having moderate-to-severe disease (Citation1,Citation2). Psoriasis can have a major impact on patients’ health-related quality of life, comparable to other chronic health conditions such as heart disease, diabetes and arthritis (Citation3–6), and is associated with a considerable economic burden (Citation7).
Patients with moderate-to-severe psoriasis who do not respond or are intolerant to systemic therapies are eligible for treatment with biological therapies or with the phosphodiesterase 4 (PDE4) inhibitor apremilast (Citation1,Citation8). The introduction of the anti-tumor necrosis factor (TNF) therapies adalimumab, etanercept, and infliximab and the interleukin (IL)-12/-23 inhibitor ustekinumab transformed the treatment of moderate-to-severe psoriasis (Citation9). However, the majority of patients do not achieve complete skin clearance (Citation10), and many discontinue treatment due to loss of response over time or adverse effects (Citation11–13).
More recently developed biological therapies have focused on the IL-17 pathway, which plays a central role in psoriasis pathogenesis and is a critical therapeutic target (Citation14,Citation15). The three approved IL-17 pathway inhibitors are secukinumab and ixekizumab, both IL-17A inhibitors, and brodalumab, a human monoclonal antibody which targets the IL-17-receptor A (IL-17RA) on keratinocytes and immune cells (Citation16). The first IL-17 A inhibitor to act on the IL-17 receptor, brodalumab blocks the signaling activity of multiple IL-17 ligands, inhibiting keratinocyte activation and inflammatory mediator production (Citation14).
Brodalumab has demonstrated superior efficacy against ustekinumab in the phase 3 randomized controlled trials (RCTs) AMAGINE-2 and AMAGINE-3 (Citation17). To date, however, there has been no thorough assessment of the efficacy of brodalumab relative to other approved biological therapies.
The objective of this study was to compare brodalumab with other biological therapies approved for the treatment of moderate-to-severe psoriasis by conducting a systematic literature review (SLR) and a network meta-analysis (NMA) to estimate relative treatment effects for pairs of therapies not compared directly. The outcome of interest was the improvement in Psoriasis Area and Severity Index (PASI) score (Citation18) at the end of the induction period for each therapy.
Materials and methods
Systematic literature review
An SLR was performed to identify RCT evidence assessing the efficacy of biological therapies or apremilast in the treatment of moderate-to-severe chronic plaque psoriasis. PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) reporting guidelines were followed throughout (Citation19).
Information sources and search strategy
MEDLINE, Embase, and Cochrane Library databases were searched for articles published in English from 2000 to 31 August 2016 using the Ovid interface. Search strings combined terms related to psoriasis, to specific therapies, and to RCTs. Case reports, letters, and editorials were excluded. The bibliography of each relevant article was cross-referenced with the search results to identify additional studies. Abstracts of relevant disease-specific and health economics and outcomes research congresses (see Supplementary Files) from 2013 to 31 August 2016 were searched.
Title/abstract screening and full-text review were conducted by two independent reviewers (EW and NY) against pre-agreed PICOS (Patient population, Intervention, Comparators, Outcomes, and Study design) criteria. Discrepancies were resolved by a third reviewer (IF).
Patient population
This analysis included studies of adult patients with moderate-to-severe chronic plaque-type psoriasis. Studies of patients with both psoriasis and psoriatic arthritis were excluded.
Intervention and comparators
Interventions of interest were adalimumab, apremilast, brodalumab, etanercept, infliximab, ixekizumab, secukinumab, and ustekinumab. Studies involving any comparator, including placebo and unlicensed doses of biological and non-biological systemic therapies, were included. The base-case analysis included only doses of biological therapies licensed by the European Medicines Agency (EMA) and regimens recommended by the National Institute of Health and Care Excellence (NICE), with the exception of brodalumab 140 mg every 2 weeks (Q2W). Other doses of licensed biological therapies, as well as conventional systemic therapies were included in a sensitivity analysis.
Outcomes
The outcome of interest was the proportion of patients achieving 50, 75, 90, and 100% improvements in PASI score at the end of the induction period (PASI 50, PASI 75, PASI 90, and PASI 100).
Study design
Only the results of RCTs were included. All other study types, including non-randomized clinical studies, were excluded.
Data extraction and critical appraisal
For each study meeting the PICOS criteria, study design details, patient demographics, therapy details and efficacy, and safety outcomes were extracted. The methodological quality of included studies was assessed and documented using the concise critical appraisal checklists provided by NICE in the Single Technology Appraisal user guide (Citation20) (Supplementary Files). Potential risk of bias was determined by assessing heterogeneity of treatment and outcome characteristics as well as study and patient characteristics.
Network meta-analysis of response rates
The relevant study results were combined by means of a hierarchical Bayesian NMA, a type of meta-analysis that synthesizes direct and indirect evidence simultaneously (Citation21,Citation22). In the primary analysis, outcomes examined were the proportions of patients achieving each level of PASI response.
Analysis of PASI responses was conducted using an ordered probit model to estimate probabilities of achieving different levels of response (Citation21). PASI 50 response outcomes for placebo arms in the included studies were used to inform the baseline event rates (Citation23). PASI response was modeled as a discrete dependent variable that takes ordered multinomial outcomes (e.g. PASI 50, PASI 75, PASI 90, and PASI 100) and no adjustments were made to address between-study differences in potential effect modifiers.
In addition to this unadjusted model, we tested a model that included an adjustment for placebo arm response rates, in accordance with methods recommended by the NICE Decision Support Unit (Citation21,Citation22,Citation24). Placebo arm response rates, which vary considerably among psoriasis trials (Citation25), are an indicator of patient- and study-level factors that can influence response to treatment, and can be a source of significant bias in cross-trial comparisons of treatment outcomes (Citation26–28). Placebo arm adjustment may account for heterogeneity across trials and potentially improve the model fit (Citation21,Citation24,Citation29); this approach has previously been used in an NMA of treatments for psoriasis (Citation30).
For both models, results were generated using random- and fixed-effects assumptions, and compared for goodness of fit to the data. Inconsistency in the direct evidence was assessed using a random-effects unrelated mean effects model (Citation22). No significant inconsistency was identified in the base-case or sensitivity analysis networks.
All analyses were performed using WinBUGS version 1.4 statistical software (Citation31) with non-informative priors. An initial burn-in of at least 20,000 simulations was used, and convergence was confirmed through visual inspection the Brook-Gelman-Rubin diagnostic and history plots. This was followed by 50,000 simulations on three chains to estimate the sampled parameters. Results are calculated as risk ratios (RRs) for each treatment compared with placebo, and for brodalumab 210 mg Q2W compared with each therapy. Point estimates reflecting the median value are presented, along with 95% credible intervals (95% CrI) reflecting the range of true effects with 95% probability.
A sensitivity analysis was performed to test the impact of including additional indirect evidence through the inclusion of data for unlicensed or unapproved doses of biological therapies, and for conventional systemic therapies.
Results
Literature search results
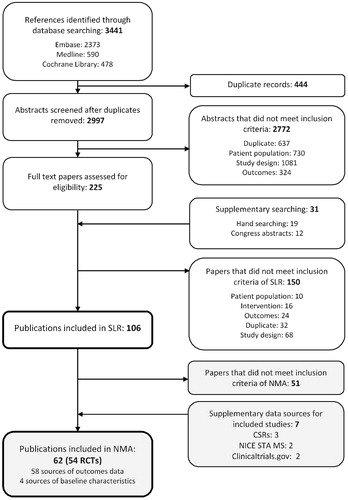
Electronic searches identified 3441 publications, to which supplementary searching added a total of 31 additional references. After deduplication, 2997 titles and abstracts were screened, and full-text versions of 225 publications were assessed. In total, 98 publications, covering 67 RCTs, were included ().
Evidence network
Of the 98 publications identified in the SLR, 62 publications describing 54 RCTs were included in the NMA of PASI responses (); these comprised 58 sources of outcomes data (Citation17,Citation32–88) and four sources of baseline characteristics (Citation89–92).
Table 1. PASI outcomes as reported by all RCTs.
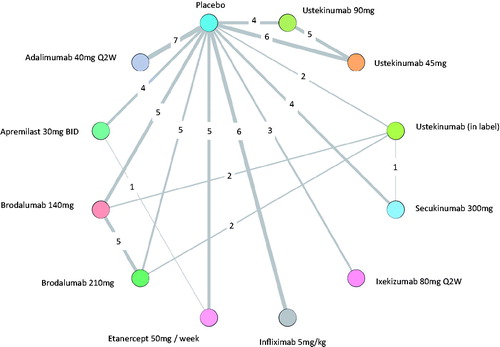
The base-case evidence network for the analysis of PASI responses is shown in , and comprised 41 studies, involving 17,959 patients (Citation17,Citation32–75). Placebo-controlled comparisons were available for all treatments. Direct comparisons between biologic therapies included in the base case were limited to brodalumab or secukinumab versus ustekinumab and etanercept (50 mg per week) versus apremilast. Inclusion criteria relating to body surface area (BSA) involvement and PASI score were generally similar across trials, as was prior exposure to conventional systemic therapies and/or phototherapy. Patient characteristics were broadly similar across studies (Supplementary Files). The mean age of the participants ranged from 39 to 57 years of age, and 40–89% were male. With the exception of eight studies which reported exceptionally low (Citation41,Citation79,Citation82) and high exposure (Citation40,Citation57,Citation59,Citation74,Citation78), previous conventional systemic therapy and phototherapy was reported among 35–77% and 38–84% patients, respectively. The proportion who had received a previous biological therapy ranged from 0% to 53%, with higher levels reported in more recent studies (Citation44,Citation70,Citation71). Overall, the risk of bias for most studies evaluated was low (Supplementary Files).
Model fit
Goodness of fit was assessed for the fixed and random effects models of both the model with and without adjustment for cross-trial differences in placebo arm response (). The estimated reference arm adjustment coefficient (β) was estimated to be −0.642 (median, 95% credible interval [CrI] − 0.835 to −0.438) and is statistically significantly different from zero. This indicates that, compared to the unadjusted model, the placebo adjustment reduced unexplained heterogeneity and improved the model. In addition, the 95% CrI of the random effect (τ) was estimated to be from 54.2 to 997.3 in the adjusted model compared with 36.79–4017 in the unadjusted model. The narrowing of the 95% CrI in the adjusted model relative to the unadjusted model demonstrates a reduction in the between-study heterogeneity, which is being captured by the adjustment coefficient (β). The total residual deviance statistic was similar between the two models; however, the DIC for the adjusted model was marginally higher than the DIC for the unadjusted model. Generally, a lower DIC implies a better model fit (Citation21), but relying on DIC alone to assess model fit ignores the statistical advantages of the placebo adjusted model in terms of goodness of fit and the observed heterogeneity of PASI response rates across the included trials. Despite its marginally higher DIC value, the placebo adjusted model is considered more appropriate than the unadjusted model. Results of both models are presented, but more emphasis is given to the adjusted model.
Table 2. Comparison of unadjusted and adjusted models by different diagnostic measures.
Relative efficacy
The treatment effect of all biological therapies and apremilast was significantly higher than that of placebo (), and all therapies were significantly more likely than placebo to achieve PASI 50, PASI 75, PASI 90, and PASI 100 responses ().
Figure 3. Relative treatment effect for all therapies versus placebo. Relative effects are plotted as the median difference in response on the probit scale, where negative values indicate greater efficacy for the intervention and positive values indicate greater efficacy for placebo. Squares indicate the results of the unadjusted analysis. Circles indicate the results of the analysis adjusted to account for cross-trial differences in placebo response. CrI: credible interval.
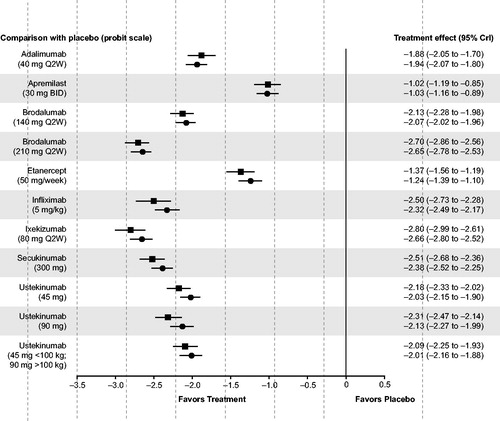
Table 3. Treatment effects at each level of PASI response for interventions versus placebo and brodalumab from adjusted and unadjusted models.
The relative risks for achieving PASI responses with brodalumab 210 mg Q2W, compared to the other therapies of interest, are also shown in . At every level of PASI response, ixekizumab 80 mg Q2W and brodalumab 210 mg Q2W were found to be the most effective therapies in the network, followed by secukinumab 300 mg and infliximab 5 mg/kg. Brodalumab 210 mg Q2W was significantly more efficacious than adalimumab 40 mg Q2W, apremilast 30 mg twice daily (BID), brodalumab 140 mg Q2W, etanercept 50 mg per week (QW), and ustekinumab (45 mg, 90 mg, and weight-based dosing) and was also significantly more efficacious than infliximab 5 mg/kg and secukinumab 300 mg when controlling for cross-trial variation in placebo responses. The size of treatment effects tended to increase with higher levels of PASI response. Based on these results, the probabilities of responding to brodalumab 210 mg Q2W and to ixekizumab 80 mg Q2W are expected to be very similar (median risk ratio at PASI 75: 1.00, 95% CrI 0.96–1.04).
Sensitivity analyses
A total of 54 trials, involving 25,838 patients, were included in the sensitivity analysis (Supplementary Figure S1). Results after including unlicensed doses of biologics as well as conventional systemics like acitretin and methotrexate were generally similar to the base-case analysis. The relative ranking of therapies was preserved, with IL-17 inhibitors outperforming anti-TNFs, ustekinumab and oral therapies. Full sensitivity analysis results are presented in Supplementary Table S6.
Discussion
This systematic review and NMA provides a comprehensive assessment of the comparative efficacy of licensed biological therapies and apremilast for the treatment of moderate-to-severe chronic plaque-type psoriasis in adults. Several novel biologic and non-biologic therapies are now approved for use in this population, with brodalumab being the most recent addition to the list of therapeutic options. With few head-to-head comparisons available, NMA approaches are an important means of integrating the available RCT evidence to determine the relative efficacy of biologic therapies.
Based on the proportion of patients achieving a 75% reduction in PASI from baseline, the base case analysis shows that all therapies are significantly more efficacious than placebo. It also showed brodalumab 210 mg to be significantly more efficacious than apremilast, anti-TNF therapies – adalimumab, etanercept, and infliximab – as well as ustekinumab and secukinumab. In all analyses, the efficacy of brodalumab 210 mg and ixekizumab appeared similar.
In addition to the commonly-used PASI 75 response endpoint (Citation8,Citation18), this NMA investigated the likelihood of achieving PASI 90 and PASI 100, the latter of which represents complete clearing of psoriasis. Notably, there was a trend towards higher treatment effects for brodalumab 210 mg versus the anti-TNF therapies and ustekinumab at these higher PASI response levels. Recent evidence suggests that these differences in higher levels of PASI response will translate to differences in quality of life, as improvements in DLQI have been shown to be significantly higher among PASI 90 responders compared to PASI 75–89 responders (Citation93,Citation94). With PASI 90 and PASI 100 becoming attainable treatment goals, consensus is growing that dermatologists should expect more highly and rapidly effective therapies for their psoriasis patients (Citation95–97).
Fifteen published NMAs have previously compared the efficacy of other biologic therapies for moderate to severe psoriasis during the induction-phase (Citation25,Citation30,Citation98–110), though this is the first to evaluate brodalumab. Our results are similar to those of several other analyses which found ixekizumab and secukinumab to be more effective than infliximab (Citation103,Citation104), and infliximab to be more effective than ustekinumab, adalimumab, and etanercept (Citation30,Citation98,Citation100–102,Citation104,Citation105,Citation107).
Placebo response rates are known to vary considerably between psoriasis trials (Citation25), a reflection of differences in factors such as study design, eligibility criteria, disease duration and/or severity, previous treatments, and concomitant topical therapies. To account for the heterogeneity these differences may introduce, our analysis presented the results of two statistical models: one that adjusted for variation in placebo-arm response and one that did not. Consistent with a previous NMA by Signorovitch et al. (Citation30), the random effects model including the placebo arm adjustment was found to have a closer fit to the data, despite a slightly higher DIC value. This update to their 2015 NMA includes four additional interventions and 27 more studies and utilizes data for PASI 100, a sign of how swiftly the evidence and therapy landscape for psoriasis has evolved.
Strengths and limitations
To the best of our knowledge, this NMA is based on the most recent and comprehensive SLR of the efficacy of biological therapies and apremilast for moderate-to-severe psoriasis. In addition, both the SLR and NMA adhered to best practice methodology throughout. It should be noted that one challenge of comparative effectiveness research in psoriasis is the rapidity with which new data are published. Therefore, although the NMA was performed as soon as possible after the SLR, we are aware that additional clinical evidence has been published and one further therapy, guselkumab, has gained European marketing authorization. A further limitation of the analysis is that only PASI response rates were included. These are the most prominent clinical efficacy data, and agreement between the results obtained with the two measures was consistent. However, assessment of these outcomes does not provide a holistic understanding of patient wellbeing, and further statistical analysis of health-related quality of life (HRQoL) and safety data may be helpful in clinical decision making. Jabbar-Lopez et al. (Citation104) provide the most recent evaluation of HRQoL and tolerability in psoriasis. They found that secukinumab, ixekizumab, infliximab and ustekinumab generate the largest improvements in DLQI and that etanercept and secukinumab had the fewest withdrawals due to adverse events, whilst infliximab and ixekizumab had the most.
This analysis was conducted using responses at the end of the induction phase, which is typically used as the primary endpoint in RCTs. Given the chronic nature of psoriasis and the importance of long-term effectiveness in a real-world clinical setting, a better understanding of the comparative effectiveness of biological therapies beyond the induction phase would be beneficial. Some of the most recent clinical trials have reported 52-week outcomes from active comparator-controlled maintenance phases (Citation17,Citation64,Citation111), a trend that will facilitate future evaluation of these treatments using network meta-analytic methods. Early analysis of these longer-term outcomes suggests that brodalumab is superior to secukinumab, ustekinumab, and etanercept (Citation112).
Conclusions
There is a large body of RCT evidence concerning the efficacy and safety of biological therapies for moderate-to-severe psoriasis, which allows for statistical analysis of commonly-reported efficacy outcomes. The results of this evidence synthesis are consistent with the results from recent pivotal trials, which have shown that high levels of complete skin clearance (PASI 100) can be achieved with the most recent therapies, brodalumab, and ixekizumab (Citation17,Citation33,Citation61). In addition, the NMA results showed that brodalumab was superior, in terms of PASI response, to apremilast, adalimumab, etanercept, infliximab, secukinumab, and ustekinumab. Brodalumab was also associated with a similar likelihood of PASI response to ixekizumab. Future research should focus on comparing the biological therapies that are close to market, such as the IL-23 inhibitors, with all currently licensed treatments as well as on assessing the comparative efficacy and safety of available therapies at longer-term endpoints.
IJDT_1427205_Supplementary_Material.pdf
Download PDF (471 KB)Acknowledgements
The authors thank Sarah Bermingham for providing data management support and Paul Overton of Beacon Medical Communications for editorial assistance.
Disclosure statement
Laura Sawyer, Iain Fotheringham, Najeeda Yasmeen and Emily Wright are Symmetron employees and consultants to LEO Pharma for this study; Carl Gibbons and Anders Holmen Møller are LEO Pharma employees.
Additional information
Funding
References
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826–850.
- Goff KL, Karimkhani C, Boyers LN, et al. The global burden of psoriatic skin disease. Br J Dermatol. 2015;172:1665–1668.
- Weiss SC, Kimball AB, Liewehr DJ, et al. Quantifying the harmful effect of psoriasis on health-related quality of life. J Am Acad Dermatol. 2002;47:512–518.
- Obradors M, Blanch C, Comellas M, et al. Health-related quality of life in patients with psoriasis: a systematic review of the European literature. Qual Life Res. 2016;25:2739–2754.
- Rapp SR, Feldman SR, Exum ML, et al. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41(3 Pt 1):401–407.
- de Korte J, Sprangers MA, Mombers FM, et al. Quality of life in patients with psoriasis: a systematic literature review. J Invest Dermatol Symp Proc. 2004;9:140–147.
- Feldman SR, Burudpakdee C, Gala S, et al. The economic burden of psoriasis: a systematic literature review. Expert Rev Pharmacoecon Outcomes Res. 2014;14:685–705.
- Smith CH, Anstey AV, Barker JN, et al. British Association of Dermatologists' guidelines for biologic interventions for psoriasis 2009. Br J Dermatol. 2009;161:987–1019.
- Gaspari AA, Tyring S. New and emerging biologic therapies for moderate-to-severe plaque psoriasis: mechanistic rationales and recent clinical data for IL-17 and IL-23 inhibitors. Dermatol Ther. 2015;28:179–193.
- Malakouti M, Jacob SE, Anderson NJ. Treatment challenges in the management of moderate-to-severe plaque psoriasis – role of secukinumab. Clin Cosmet Invest Dermatol. 2016;9:347–355.
- Carrascosa JM, van Doorn MB, Lahfa M, et al. Clinical relevance of immunogenicity of biologics in psoriasis: implications for treatment strategies. J Eur Acad Dermatol Venereol. 2014;28:1424–1430.
- Warren RB, Smith CH, Yiu ZZ, et al. Differential drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Invest Dermatol. 2015;135:2632–2640.
- Gniadecki R, Bang B, Bryld LE, et al. Comparison of long-term drug survival and safety of biologic agents in patients with psoriasis vulgaris. Br J Dermatol. 2015;172:244–252.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141–150.
- Wasilewska A, Winiarska M, Olszewska M, et al. Interleukin-17 inhibitors. A new era in treatment of psoriasis and other skin diseases. Postepy Dermatol Alergol. 2016;33:247–252.
- Papp KA, Reid C, Foley P, et al. Anti-IL-17 receptor antibody AMG 827 leads to rapid clinical response in subjects with moderate to severe psoriasis: results from a phase I, randomized, placebo-controlled trial. J Invest Dermatol. 2012;132:2466–2469.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318–1328.
- Feldman S, Krueger G. Psoriasis assessment tools in clinical trials. Ann Rheum Dis. 2005;64(Suppl 2):ii65–iii8.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100.
- National Institute for Health and Care Excellence [Internet]. Single technology appraisal: user guide for company evidence submission template. 2015 [cited 2017 Mar 8]. Available from: https://www.nice.org.uk/process/pmg24/resources/single-technology-appraisal-user-guide-for-company-evidence-submission-template-pdf-72286715419333
- Dias S, Sutton AJ, Ades AE, et al. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making. 2013;33:607–617.
- Dias S, Welton NJ, Sutton AJ, et al. Evidence synthesis for decision making 4: inconsistency in networks of evidence based. Med Decis Making. 2013;33:641–656.
- Dias S, Welton NJ, Sutton AJ, et al. Evidence synthesis for decision making 5: the baseline natural history model. Med Decis Making. 2013;33:657–670.
- Achana FA, Cooper NJ, Dias S, et al. Extending methods for investigating the relationship between treatment effect and baseline risk from pairwise meta-analysis to network meta-analysis. Stat Med. 2013;32:752–771.
- Lamel SA, Myer KA, Younes N, et al. Placebo response in relation to clinical trial design: a systematic review and meta-analysis of randomized controlled trials for determining biologic efficacy in psoriasis treatment. Arch Dermatol Res. 2012;304:707–717.
- Sharp SJ, Thompson SG. Analysing the relationship between treatment effect and underlying risk in meta-analysis: comparison and development of approaches. Statist Med. 2000;19:3251–3274.
- Sharp SJ, Thompson SG, Altman DG. The relation between treatment benefit and underlying risk in meta-analysis. BMJ. 1996;313:735–738.
- Thompson SG, Smith TC, Sharp SJ. Investigating underlying risk as a source of heterogeneity in meta-analysis. Stat Med. 1997;16:2741–2758.
- Dias S, Sutton AJ, Welton NJ, et al. Evidence synthesis for decision making 3: heterogeneity–subgroups, meta-regression, bias, and bias-adjustment. Med Decis Making. 2013;33:618–640.
- Signorovitch JE, Betts KA, Yan YS, et al. Comparative efficacy of biological treatments for moderate-to-severe psoriasis: a network meta-analysis adjusting for cross-trial differences in reference arm response. Br J Dermatol. 2015;172:504–512.
- Lunn DJT, A, Best N, Spiegelhalter D. WinBUGS – a Baysian modelling framework: concepts, structure, and extensibility. Stat Comput. 2000;10:325–337.
- National Institute for Health and Care Excellence. Single technology appraisal: ixekizumab for treating moderate to severe plaque psoriasis [ID904] Committee Papers [Internet]. London, UK: National Institute for Health and Care Excellence. 2016 [cited 2017 Jan 31]. Available from: https://www.nice.org.uk/guidance/GID-TA10063/documents/committee-papers-3
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273–286.
- Amgen Inc. A phase 3 study to evaluate the efficacy, safety, and effect of withdrawal and retreatment with brodalumab in subjects with moderate to severe plaque psoriasis: AMAGINE-1 (Clinical Study Report). 15 May 2015. Data on file. Thousand Oaks (CA): Amgen Inc.
- Amgen Inc. A phase 3 study to evaluate the efficacy and safety of induction and maintenance regimens of brodalumab compared with placebo and ustekinumab in subjects with moderate to severe plaque psoriasis: AMAGINE-2 (Clinical Study Report). 30 March 2015. Data on file. Thousand Oaks (CA): Amgen Inc.
- Amgen Inc. Phase 3 Study to Evaluate the Efficacy and Safety of Induction and Maintenance Regimens of Brodalumab Compared With Placebo and Ustekinumab in Subjects With Moderate to Severe Plaque Psoriasis: AMAGINE-3 (Clinical Study Report). 27 March 2015. Data on file. Thousand Oaks (CA): Amgen Inc.
- Nakagawa H, Niiro H, Ootaki K. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci. 2016;81:44–52.
- Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181–1189.
- Saurat JH, Stingl G, Dubertret L, et al. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol. 2008;158:558–566.
- Cai L, Gu J, Zheng J, et al. Efficacy and safety of adalimumab in Chinese patients with moderate-to-severe plaque psoriasis: results from a phase 3, randomized, placebo-controlled, double-blind study. J Eur Acad Dermatol Venereol. 2016;31:89–95.
- Menter A, Tyring SK, Gordon K, et al. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol. 2008;58:106–115.
- Asahina A, Nakagawa H, Etoh T, et al. Adalimumab in Japanese patients with moderate to severe chronic plaque psoriasis: efficacy and safety results from a Phase II/III randomized controlled study. J Dermatol. 2010;37:299–310.
- Gordon KB, Langley RG, Leonardi C, et al. Clinical response to adalimumab treatment in patients with moderate to severe psoriasis: double-blind, randomized controlled trial and open-label extension study. J Am Acad Dermatol. 2006;55:598–606.
- Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373:136–144.
- Bissonnette R, Tardif JC, Harel F, et al. Effects of the tumor necrosis factor-alpha antagonist adalimumab on arterial inflammation assessed by positron emission tomography in patients with psoriasis: results of a randomized controlled trial. Circ Cardiovasc Imaging. 2013;6:83–90.
- Papp K, Cather JC, Rosoph L, et al. Efficacy of apremilast in the treatment of moderate to severe psoriasis: a randomised controlled trial. Lancet. 2012;380:738–746.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37–49.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387–1399.
- Reich K, Soung J, Gooderham M, et al. Sustained efficacy of apremilast in patients with moderate to severe psoriasis who continued on apremilast or switched from etanercept treatment: 52-week results from the LIBERATE study. J Am Acad Dermatol. 2016;74:AB276.
- Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349:2014–2022.
- Gottlieb AB, Matheson RT, Lowe N, et al. A randomized trial of etanercept as monotherapy for psoriasis. Arch Dermatol. 2003;139:1627–1632.
- Papp KA, Tyring S, Lahfa M, et al. A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. Br J Dermatol. 2005;152:1304–1312.
- van de Kerkhof PC, Segaert S, Lahfa M, et al. Once weekly administration of etanercept 50 mg is efficacious and well tolerated in patients with moderate-to-severe plaque psoriasis: a randomized controlled trial with open-label extension. Br J Dermatol. 2008;159:1177–1185.
- Yang HZ, Wang K, Jin HZ, et al. Infliximab monotherapy for Chinese patients with moderate to severe plaque psoriasis: a randomized, double-blind, placebo-controlled multicenter trial. Chin Med J (Engl). 2012;125:1845–1851.
- Reich K, Nestle FO, Papp K, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005;366:1367–1374.
- Chaudhari U, Romano P, Mulcahy LD, et al. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trial. Lancet. 2001;357:1842–1847.
- Gottlieb AB, Evans R, Li SM, et al. Infliximab induction therapy for patients with severe plaque-type psoriasis: a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2004;51:534–542.
- Menter A, Feldman SR, Weinstein GD, et al. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2007;56:31 e1–15.
- Torii H, Nakagawa H. Japanese Infliximab Study i. Infliximab monotherapy in Japanese patients with moderate-to-severe plaque psoriasis and psoriatic arthritis. A randomized, double-blind, placebo-controlled multicenter trial. J Dermatol Sci. 2010;59:40–49.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345–356.
- Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541–551.
- Blauvelt A, Prinz JC, Gottlieb AB, et al. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015;172:484–493.
- National Institute for Health and Care Excellence. Single technology appraisal: secukinumab for the treatment of moderate to severe plaque psoriasis [TA350] Committee Papers [Internet]. London, UK: National Institute for Health and Care Excellence. 2015 [cited 2017 Jan 31]. Available from: https://www.nice.org.uk/guidance/TA350/documents/secukinumab-for-treating-moderate-to-severe-plaque-psoriasis-committee-papers2
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis-results of two phase 3 trials. N Engl J Med. 2014;371:326–338.
- Novartis Pharmaceuticals. Efficacy and safety of subcutaneous secukinumab for moderate to severe chronic plaque-type psoriasis for up to 1 year (ERASURE). In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. 2000 [cited 2017 Jan 31]. Available from: https://clinicaltrials.gov/ct2/show/results/NCT01365455?sect=Xd0156
- Paul C, Lacour JP, Tedremets L, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. 2015;29:1082–1090.
- Novartis Pharmaceuticals. Efficacy and safety of subcutaneous secukinumab (AIN457) for moderate to severe chronic plaque-type psoriasis assessing different doses and dose regimens (SCULPTURE). In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine; 2000 [cited 2017 Jan 31]. Available from: https://clinicaltrials.gov/ct2/show/results/NCT01406938?term=SCULPTURE&rank =1
- Thaci D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400–409.
- Tsai TF, Ho JC, Song M, et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL). J Dermatol Sci. 2011;63:154–163.
- Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371:1665–1674.
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675–1684.
- Zhu X, Zheng M, Song M, et al. Efficacy and safety of ustekinumab in Chinese patients with moderate to severe plaque-type psoriasis: results from a phase 3 clinical trial (LOTUS). J Drugs Dermatol. 2013;12:166–174.
- Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118–128.
- Igarashi A, Kato T, Kato M, et al. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. 2012;39:242–252.
- Krueger GG, Langley RG, Leonardi C, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580–592.
- Bachelez H, van de Kerkhof PC, Strohal R, et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet. 2015;386:552–561.
- Bagel J, Lynde C, Tyring S, et al. Moderate to severe plaque psoriasis with scalp involvement: a randomized, double-blind, placebo-controlled study of etanercept. J Am Acad Dermatol. 2012;67:86–92.
- de Vries AC, Thio HB, de Kort WJ, et al. A prospective randomised controlled trial comparing infliximab and etanercept in patients with moderate to severe chronic plaque type psoriasis: the psoriasis infliximab versus etanercept Comparison Evaluation, the PIECE study. Br J Dermatol. 2017;176:624–633.
- Gottlieb AB, Leonardi C, Kerdel F, et al. Efficacy and safety of briakinumab vs. etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol. 2011;165:652–660.
- Mrowietz U, Leonardi CL, Girolomoni G, et al. Secukinumab retreatment-as-needed versus fixed-interval maintenance regimen for moderate to severe plaque psoriasis: a randomized, double-blind, noninferiority trial (SCULPTURE). J Am Acad Dermatol. 2015;73:27–36.e1.
- Papp KA, Kaufmann R, Thaci D, et al. Efficacy and safety of apremilast in subjects with moderate to severe plaque psoriasis: results from a phase II, multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison study. J Eur Acad Dermatol Venereol. 2013;27:e376–e383.
- Strober BE, Crowley JJ, Yamauchi PS, et al. Efficacy and safety results from a phase III, randomized controlled trial comparing the safety and efficacy of briakinumab with etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol. 2011;165:661–668.
- Strohal R, Puig L, Chouela E, et al. The efficacy and safety of etanercept when used with as-needed adjunctive topical therapy in a randomised, double-blind study in subjects with moderate-to-severe psoriasis (the PRISTINE trial). J Dermatolog Treat. 2013;24:169–178.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35.
- Barker J, Hoffmann M, Wozel G, et al. Efficacy and safety of infliximab vs. methotrexate in patients with moderate-to-severe plaque psoriasis: results of an open-label, active-controlled, randomized trial (RESTORE1). Br J Dermatol. 2011;165:1109–1117.
- Caproni M, Antiga E, Melani L, et al. Serum levels of IL-17 and IL-22 are reduced by etanercept, but not by acitretin, in patients with psoriasis: a randomized-controlled trial. J Clin Immunol. 2009;29:210–214.
- Gisondi P, Del Giglio M, Cotena C, et al. Combining etanercept and acitretin in the therapy of chronic plaque psoriasis: a 24-week, randomized, controlled, investigator-blinded pilot trial. Br J Dermatol. 2008;158:1345–1349.
- Goldminz AM, Suarez-Farinas M, Wang AC, et al. CCL20 and IL22 messenger RNA expression after adalimumab vs methotrexate treatment of psoriasis: a randomized clinical trial. JAMA Dermatol. 2015;151:837–846.
- Reich K, Nestle FO, Papp K, et al. Improvement in quality of life with infliximab induction and maintenance therapy in patients with moderate-to-severe psoriasis: a randomized controlled trial. Br J Dermatol. 2006;154:1161–1168.
- Reich K, Segaert S, Van de Kerkhof P, et al. Once-weekly administration of etanercept 50 mg improves patient-reported outcomes in patients with moderate-to-severe plaque psoriasis. Dermatology (Basel). 2009;219:239–249.
- Shikiar R, Heffernan M, Langley RG, et al. Adalimumab treatment is associated with improvement in health‐related quality of life in psoriasis: Patient‐reported outcomes from a Phase II randomized controlled trial. J Dermatolog Treat. 2007;18:25–31.
- Tyring S, Gordon KB, Poulin Y, et al. Long-term safety and efficacy of 50 mg of etanercept twice weekly in patients with psoriasis. Arch Dermatol. 2007;143:719–726.
- Elewski BE, Puig L, Mordin M, et al. Psoriasis patients with psoriasis area and severity index (PASI) 90 response achieve greater health-related quality-of-life improvements than those with PASI 75–89 response: results from two phase 3 studies of secukinumab. J Dermatolog Treat. 2017;28:492–499.
- Puig L, Thom H, Mollon P, et al. Clear or almost clear skin improves the quality of life in patients with moderate-to-severe psoriasis: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2017;31:213–220.
- Manalo IF, Gilbert KE, Wu JJ. Time to raise the bar to psoriasis area severity index 90 and 100. J Drugs Dermatol. 2015;14:1086–1088.
- Puig L. PASI90 response: the new standard in therapeutic efficacy for psoriasis. J Eur Acad Dermatol Venereol. 2015;29:645–648.
- Torres T, Puig L. Treatment goals for psoriasis: Should PASI 90 become the standard of care?. Acta Dermosifiliogr. 2015;106:155–157.
- Bansback N, Sizto S, Sun H, et al. Efficacy of systemic treatments for moderate to severe plaque psoriasis: systematic review and meta-analysis. Dermatology (Basel). 2009;219:209–218.
- Collins A, Hawe E, Vickers A, et al. Secukinumab 300 mg demonstrates higher probability of efficacy than other biologics in psoriasis: indirect comparison. Poster presented at the 48th Australasian College of Dermatologists Annual Scientific Meeting, Adelaide, South Australia; 2015.
- Fan T, Bennett H, Smith N, et al. Mixed treatment comparison of infliximab with ustekinumab in patients with moderate to severe psoriasis. Br J Dermatol. 2011;165:e38–e39.
- Galvan-Banqueri M, Gil RM, Ramos BS, et al. Biological treatments for moderate-to-severe psoriasis: indirect comparison. J Clin Pharm Ther. 2013;38:121–130.
- Gupta AK, Daigle D, Lyons DCA. Network meta-analysis of treatments for chronic plaque psoriasis in Canada. J Cutan Med Surg. 2014;18:371–378.
- Hartz S, Walzer S, Dutronc Y, et al. Network meta-analysis to evaluate the efficacy of ixekizumab in the treatment of moderate to severe psoriasis. Value Health. 2016;19:A576.
- Jabbar-Lopez ZK, Yiu ZZN, Ward V, et al. Quantitative evaluation of biologic therapy options for psoriasis: a systematic review and network meta-analysis. J Invest Dermatol. 2017;137:1646–1654.
- Lin VW, Ringold S, Devine EB. Comparison of ustekinumab with other biological agents for the treatment of moderate to severe plaque psoriasis: a Bayesian network meta-analysis. Arch Dermatol. 2012;148:1403–1410.
- Odom D, Brogan A, Talbird S, et al. Meta-analysis of randomized, controlled trials of ustekinumab and adalimumab for moderate-to-severe psoriasis. Value Health. 2013;16:A112.
- Reich K, Burden AD, Eaton JN, et al. Efficacy of biologics in the treatment of moderate to severe psoriasis: a network meta-analysis of randomized controlled trials. Br J Dermatol. 2012;166:179–188.
- Strober B, Checchio T, Gupta P, et al. A dose-response model-based meta-analysis to compare tofacitinib to other psoriasis treatments. J Eur Acad Dermatol Venereol. 2016;30 (Suppl. 6):94.
- Wilson JL, Standfield L, Paech D, Mulani P. Comparative effectiveness of adalimumab and etanercept in patients with chronic plaque psoriasis. Aust J Dermatol. 2012;53:54.
- Woolacott N, Hawkins N, Mason A, et al. Etanercept and efalizumab for the treatment of psoriasis: a systematic review. Health Technol Assess. 2006;10:1–258.
- Blauvelt A, Reich K, Tsai TF, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol. 2017;76:60–69.e9.
- Sawyer L, Fotheringham I, Cornic L, , et al. Assessing the longer-term efficacy of biologic therapies and apremilast for patients with moderate-to-severe psoriasis: a systematic review and network meta-analysis. Value Health. 2017;20:A801.