Abstract
Purpose: To evaluate short- and long-term efficacy and safety of ixekizumab in patients according to psoriasis severity.
Materials and methods: Data were integrated from clinical trials (UNCOVER-2, UNCOVER-3). Patients received placebo, 80-mg ixekizumab every 2 weeks (IXEQ2W), every 4 weeks (IXEQ4W), or 50 mg etanercept (ETN) biweekly for 12 weeks, then open-label IXEQ4W (UNCOVER-3). Psoriasis severity was categorized by baseline Psoriasis Area and Severity Index (PASI <20 and ≥20). Efficacy was evaluated by percentage reaching PASI 75, 90, 100, and absolute PASI ≤5, ≤2, and ≤1.
Results: Significantly more patients with PASI ≥20 (vs. PASI <20) were male and had higher body weight. After 12 weeks, both severity groups had significantly more IXEQ2W- than ETN-treated patients reach PASI 75, 90, 100, and absolute PASI ≤5, ≤2, ≤1. Fewer PASI ≥20 vs. PASI <20 patients across treatments reached PASI ≤5, ≤2, and ≤1 at week 12. Efficacy was maintained during 156 weeks of ixekizumab treatment with no differences between groups. The IXEQ2W safety profile was similar between groups except for injection-site reactions (significantly higher in PASI <20).
Conclusions: Ixekizumab demonstrated a high level of efficacy and had a consistent safety profile in patients with different baseline psoriasis severity levels.
Introduction
Ixekizumab is an interleukin (IL)-17A antagonist that has been studied in three randomized, double-blind, placebo-controlled phase 3 trials (UNCOVER-1, UNCOVER-2, and UNCOVER-3). In these studies, ixekizumab was reported to have high efficacy in patients with moderate-to-severe plaque psoriasis (Citation1,Citation2).
The Psoriasis Area and Severity Index (PASI) score, in use since 1978, is the most prevalent measure of psoriasis severity in clinical trials (Citation3,Citation4). A PASI total score 7–12 was introduced to define moderate and ≥12 to define severe psoriasis (Citation3,Citation4). Some studies have demonstrated the need to further differentiate patients with higher levels of severity, proposing an additional threshold of total PASI score (Citation5). For example, National Institute for Health and Clinical Excellence guidelines currently define psoriasis as very severe when patients present with PASI total score ≥20 and Dermatology Life Quality Index (DLQI) total score >18 and European Medicines Agency guidelines define psoriasis as severe when patients present with PASI total score >20 or body surface area involvement >20% (Citation6,Citation7).
There is very limited information on whether the efficacy of biologic therapies is impacted by baseline psoriasis severity as these analyses are rarely reported in clinical trials or registries. In a retrospective analysis of patients evaluated at two clinical centers, Ponnambath et al. (Citation8) observed that within the group of patients with PASI >20, those who were taking etanercept (ETN) discontinued their therapy more often than those treated with other biologics, and hypothesized that this could be related to lower efficacy of ETN in this population. In another report, 6- and 12-month analyses of biologic agents used to treat psoriasis in a real-world setting showed that patients with more severe baseline disease were less likely to achieve clear to minimal disease targets as measured by the Physician Global Assessment Scale (Citation9).
The objective of this post hoc analysis was to compare the short- and long-term efficacy and safety of ixekizumab, used according to the approved labeling, in patients with varying degrees of psoriasis severity (defined by baseline PASI total score <20 [PASI <20] and PASI total score ≥20 [PASI ≥20]) by using integrated data from two active comparator- and placebo-controlled trials, UNCOVER-2 and UNCOVER-3.
Materials and methods
Study design
Data presented are based on integrated data from two phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel-group trials that evaluated the efficacy and safety of ixekizumab (UNCOVER-2 [NCT01597245] and UNCOVER-3 [NCT01646177]). The designs of UNCOVER-2 and UNCOVER-3 have been previously described (Citation1,Citation2). Briefly, the UNCOVER-2 and UNCOVER-3 studies had a 12-week placebo-controlled induction dosing period where patients were randomized to receive placebo, 80 mg ixekizumab every 2 weeks (IXEQ2W), 80 mg ixekizumab every 4 weeks (IXEQ4W), or an active comparator, etanercept (ETN; 50 mg twice weekly). UNCOVER-2 had a randomized withdrawal period from Week 12 to Week 60 where patients who had reached static Physician Global Assessment (sPGA) 0 or 1 at Week 12 were re-randomized to receive IXEQ4W, 80 mg ixekizumab every 12 weeks (IXEQ12W), or placebo (UNCOVER-2 data beyond Week 12 are not included in this report). In UNCOVER-3, after Week 12, all patients entered a long-term extension period, during which they received open-label IXEQ4W through Week 264 regardless of their treatment before Week 12. In addition, after Week 60 of the UNCOVER-3 study, at the investigator’s discretion, the dosing frequency could be increased to IXEQ2W for the remainder of the study to maintain disease control. For the purpose of this report, only UNCOVER-3 data are used for analyses beyond Week 12; data from visits where the ixekizumab dose was up-titrated to IXEQ2W were excluded from efficacy analyses, but not safety analyses.
Patients
Detailed inclusion and exclusion criteria have been previously reported (Citation1,Citation2). In both studies, eligible patients were at least 18 years of age with chronic (≥6 months) moderate-to-severe psoriasis (≥10% body surface area involvement, sPGA score ≥3, and PASI total score ≥12 at baseline) and were candidates for phototherapy and/or systemic therapy. Study protocols and informed consent forms were approved by an investigational review board at each site. The studies were conducted in accordance with ethical principles of Good Clinical Practice and the Declaration of Helsinki and its guidelines. Written informed consent was obtained from each patient at study entry before any study procedures as previously described (Citation2).
Assessments
Demographics and disease characteristics, including psoriasis severity measured by PASI, were assessed at baseline.
Short-term (12-week) and long-term (up to 156 weeks [3 years]) efficacy was evaluated by the number (%) of patients with reduction from baseline PASI of at least 75% (PASI 75), at least 90% (PASI 90), and 100% (PASI 100) as well as the number (%) of patients achieving an absolute (total score) PASI ≤5, ≤2, and ≤1.
Safety was evaluated by the incidence of treatment-emergent adverse events (TEAEs) and serious adverse events (SAEs) reported over 12 weeks and 156 weeks (3 years) of ixekizumab treatment. AEs of special interest included, but were not limited to, infections, inflammatory bowel disease, injection-site reactions, and cardiovascular and cerebrovascular events.
Statistical analyses
Short-term analyses used baseline efficacy and safety data from the UNCOVER-2 and UNCOVER-3 studies and long-term analyses used efficacy and safety data from the UNCOVER-3 study only. Baseline, efficacy, and safety data in the overall population have already been reported (Citation1,Citation2,Citation10,Citation11). For the current analyses, patients were grouped by baseline psoriasis severity (PASI <20 and PASI ≥20).
For demographic and baseline variables, p values for the difference between psoriasis severity groups were analyzed using the Cochran–Mantel–Haenszel (CMH) test stratified by study for categorical data and an analysis of variance with treatment, study, and severity group as independent factors for continuous data.
For short-term analyses (up to Week 12) of categorical efficacy variables (i.e. PASI 75, PASI 90, and PASI 100; PASI ≤5, PASI ≤2, and PASI ≤1), a logistic regression analysis was used with treatment, subgroup, and the interaction of treatment-by-subgroup included as factors, and the treatment-by-subgroup interaction. For the within subgroup between treatment comparisons, 95% confidence intervals [CIs] were based on the normal approximation to the binomial distribution. p values from the CMH test stratified by study was used. For the binary PASI outcomes, missing data were imputed using a non-responder imputation (NRI) up to Week 12.
For analyses beyond 12 weeks, only data from visits with treatment at the approved IXEQ4W regimen were considered. Data collected after the first visit with an up-titrated IXEQ2W long-term dosing were excluded before imputations were applied. For binary PASI outcomes, missing data were imputed using a modified NRI (mNRI) for data in the long-term analyses. For the mNRI, the NRI method was used for patients who discontinued due to adverse events or lack of efficacy/relapse; in all other cases of missing data for this analysis, the data were imputed using a partial imputation of non-monotone missing data (i.e. for intermittent missing data) using the Markov chain Monte Carlo method with the simple imputation model, and then for the monotone missing data, a sequential regression multiple imputation with the baseline score (Citation12,Citation13).
For adverse event data, p values for the difference between psoriasis severity groups were calculated using the CMH test stratified by study for data up to Week 12. For all analyses, p values ≤.05 indicated statistical significance and no correction for multiplicity was applied due to the exploratory nature of the analyses.
For the absolute PASI response animation, a cubic Bezier interpolation was used to interpolate (for non-scheduled time points) through observations over time for each patient. Observations at each visit were obtained, and if any data were missing at a scheduled visit, it was imputed using the last observation carried forward method. In the animation, each patient is represented by a dot; the dot color is associated with PASI change from baseline. For each arm, a stacked bar chart (upper left of figures in animation) presents the distribution of patients by baseline PASI.
Results
A total of 2570 patients were enrolled and randomized in the UNCOVER-2 and UNCOVER-3 studies (Citation2). Of these patients, 1590 (61.9%) had baseline PASI <20 and 977 (38.0%) had baseline PASI ≥20. The remaining three patients had missing baseline PASI values and were not included in this analysis. The distributions of baseline PASI scores are shown in Supplemental Figure 1. A total of 362 patients who received IXEQ2W during the 12-week induction entered the long-term extension of UNCOVER-3 and received open-label IXEQ4W. Between Week 60 and Week 156, 55 of the 362 patients (15.2%) were up-titrated to IXEQ2W (data from visits where the ixekizumab dosing was up-titrated were excluded from efficacy analyses).
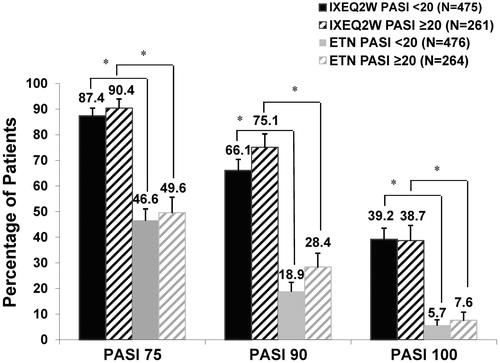
Figure 1. PASI 75, PASI 90, and PASI 100 response at week 12 for patients administered IXEQ2W or ETN by baseline psoriasis severity (NRI). ETN: etanercept; IXEQ2W: ixekizumab 80 mg every 2 weeks; N: total number of patients; NRI: non-responder imputation; PASI: Psoriasis Area and Severity Index. *p < .001, IXEQ2W vs. ETN.
Baseline patient demographics and clinical characteristics by baseline psoriasis severity are reported in . Several baseline variables were statistically significantly different between the psoriasis severity groups. Patients with more severe psoriasis (PASI ≥20) were more frequently male (71.3% vs. 65.3%), from Europe (53.5% vs. 36.5%), and had a higher mean body weight (93.7 kg vs. 89.9 kg) compared to patients with less severe psoriasis (PASI <20). More patients with baseline PASI ≥20 than those with PASI <20 reported previous treatments with non-biologic systemic drugs (58.2% vs. 49.3%), but not with biologics (17.3% vs. 20.6%). Patients with baseline PASI ≥20 also had a higher prevalence of nail (67.0% vs. 58.1%), face (51.1% vs. 39.9%), and palm/sole (30.4% vs. 24.6%) involvement, a higher frequency of psoriatic arthritis (27.0% vs. 18.7%), and a worse mean baseline DLQI score (13.5 vs. 11.3) than those with PASI <20.
Table 1. Baseline patient demographics and clinical characteristics by baseline psoriasis severity.
Short-term (12-week) efficacy
The animation (Supplemental Figure 2) presents the evolution of PASI scores (LOCF) through the induction phase of UNCOVER-2 and -3 for all randomized patients treated with IXEQ2W and ETN, as well as the distribution of the percentages of patients reaching PASI 50, PASI 75, PASI 90, and PASI 100 among the PASI <20 and the PASI ≥20 groups.
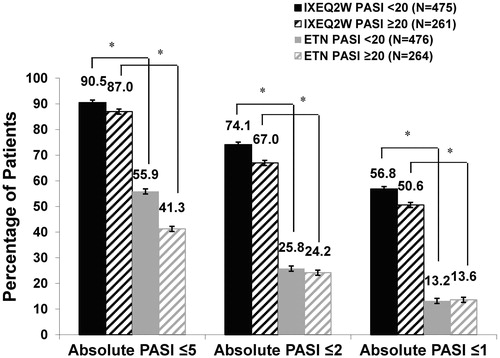
Figure 2. Absolute PASI ≤5, PASI ≤2, and PASI ≤1 by baseline psoriasis severity at Week 12 (NRI). ETN: etanercept; IXEQ2W: ixekizumab 80 mg every 2 weeks; N: total number of patients; NRI: non-responder imputation; PASI: Psoriasis Area and Severity Index. *p < .001, IXEQ2W vs. ETN.
At 12 weeks, there was no meaningful impact of baseline psoriasis severity on the efficacy of IXEQ2W as the percentage of patients reaching PASI 75, PASI 90, and PASI 100 at Week 12 were similar in patients with baseline PASI >20 and patients with baseline PASI <20, respectively (, NRI). A significantly higher percentage of IXEQ2W-treated patients than ETN-treated patients reached PASI 75 (236 [90.4%] vs. 131 [49.6%] patients, p < .001), PASI 90 (196 [75.1%] vs. 75 [28.4%] patients, p < .001), and PASI 100 (101 [38.7%] vs. 20 [7.6%] patients, p < .001) in the PASI ≥20 group (, NRI). Similarly, a significantly higher percentage of IXEQ2W- than ETN-treated patients reached PASI 75 (415 [87.4%] vs. 222 [46.6%] patients, p < .001), PASI 90 (314 [66.1%] vs. 90 [18.9%] patients, p < .001), and PASI 100 (186 [39.2%] vs. 27 [5.7%] patients, p < .001) in the PASI <20 group (, NRI). There were no statistically significant interactions between baseline PASI score and treatment.
In the case of high baseline PASI values, patients with PASI 75 may still present with significant disease. For this reason, we also evaluated efficacy data in terms of absolute PASI values and observed, again, limited impact of baseline severity on the efficacy of IXEQ2W. By Week 12, a significantly higher percentage of IXEQ2W-treated patients than ETN-treated patients reached PASI ≤5 (227 [87.0%] vs. 109 [41.3%] patients, p < .001), PASI ≤2 (175 [67.0%] vs. 64 [24.2%] patients, p < .001) and PASI ≤1 (132 [50.6%] vs. 36 [13.6%] patients, p < .001) in the PASI ≥20 group (, NRI). Similarly, a significantly higher percentage of IXEQ2W- than ETN-treated patients reached PASI ≤5 (430 [90.5%] vs. 266 [55.9%] patients, p < .001), PASI ≤2 (352 [74.1%] vs. 123 [25.8%] patients, p < .001), and PASI ≤1 (270 [56.8%] vs. 63 [13.2%] patients, p < .001) in the PASI <20 group (, NRI). Of note, a slightly lower percentage of patients with PASI ≥20 than with PASI <20 reached PASI <5, <2, and <1 at Week 12. There were no statistically significant interactions between baseline PASI score and treatment. The animation shows the comparative dynamic of PASI improvement between treatment groups and across different baseline disease severity (Supplemental Figure 2).
Long-term (156-week) efficacy
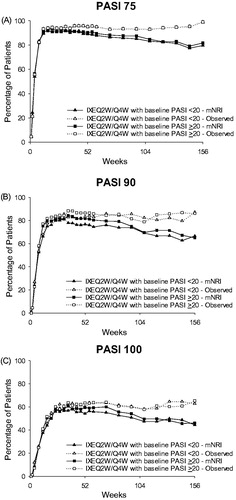
As shown in , a high level of efficacy was maintained with IXEQ2W/IXEQ4W over 156 weeks of treatment in both baseline psoriasis severity groups. PASI 75 (mNRI) was reached by 90.2% and 89.1% of ixekizumab-treated patients in the PASI ≥20 and <20 groups, respectively, at Week 52, by 84.7% and 83.1% of patients at Week 108, and by 81.7% and 79.6% at Week 156. PASI 90 (mNRI) was reached by 81.7% and 76.7% of ixekizumab-treated patients in the PASI ≥20 and <20 groups, respectively, at Week 52, by 69.7% and 69.2% at Week 108, and by 65.1% and 66.6% at Week 156. PASI 100 (mNRI) was reached by 59.4% and 54.1% of ixekizumab-treated patients in the PASI ≥20 and <20 groups, respectively, at Week 52, by 49.5% and 47.4% of patients at Week 108, and by 46.0% and 44.4% of patients at Week 156. PASI improvement results based on observed data were better than the corresponding mNRI results. For instance, PASI 75 (observed) was reached by 95.1% and 94.9% of ixekizumab-treated patients in the PASI ≥20 and <20 groups, respectively, at Week 52, by 93.0% and 94.9% of patients at Week 108, and by 99.0% and 95.9% at Week 156 (). Consistent with the PASI 75 data, the pattern of results based on observed data for PASI 90 and PASI 100 was also better than the corresponding mNRI data ().
Figure 3. PASI 75, PASI 90, and PASI 100 response by baseline psoriasis severity at Week 156 (mNRI and observed). (A) PASI 75 over 156 weeks of treatment. Some values are obscured where data overlap for the two observed groups. (B) PASI 90 over 156 weeks of treatment. (C) PASI 100 over 156 weeks of treatment. Abbreviations: IXEQ2W: ixekizumab 80 mg every 2 weeks; mNRI: modified non-responder imputation; N: total number of patients; PASI: Psoriasis Area and Severity Index; Q4W: every 4 weeks.
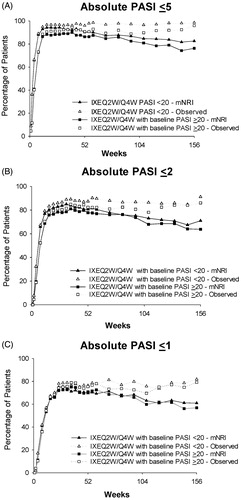
For analyses of absolute PASI, a consistent pattern of efficacy was also observed between the two groups of patients. Among IXEQ2W/IXEQ4W-treated patients, PASI ≤5 (mNRI) was reached by 86.3% in the PASI ≥20 group and 90.3% in the PASI <20 group at Week 52, by 80.9% and 84.7% at Week 108, and by 76.3% and 82.6% at Week 156 (). A PASI ≤2 (mNRI) was reached by 77.6% in the PASI ≥20 group and 80.7% in the PASI <20 group at Week 52, by 68.0% and 73.1% at Week 108, and by 63.8% and 71.0% at Week 156 (). Among IXEQ2W/IXEQ4W-treated patients, 70.8% in the PASI ≥20 group and 70.4% in the PASI <20 group achieved PASI ≤1 (mNRI) at Week 52, while 59.8% and 63.1% reached PASI ≤1 at Week 108, and 56.8% and 61.0% at Week 156 (). Absolute PASI results based on observed data were better than the corresponding mNRI results. For instance, PASI ≤5 (observed) was reached by 90.8% in the PASI ≥20 group and 96.0% in the PASI <20 group at Week 52, by 89.8% and 96.6% at Week 108, and by 96.0% and 98.6% at Week 156 (). Consistent with the absolute PASI ≤5 data, the pattern of results based on observed data for absolute PASI ≤2 and PASI ≤1 was also better than the corresponding mNRI data ().
Figure 4. Absolute PASI ≤5, PASI ≤2, and PASI ≤1 by baseline psoriasis severity at Week 156 (mNRI and observed). (A) PASI ≤5 over 156 weeks of treatment. (B) PASI ≤2 over 156 weeks of treatment. (C) PASI ≤1 over 156 weeks of treatment. Abbreviations: IXEQ2W: ixekizumab 80 mg every 2 weeks; mNRI: modified non-responder imputation; N: total number of patients; PASI: Psoriasis Area and Severity Index; Q4W: every 4 weeks.
A similar pattern of results was observed for PASI improvement and absolute PASI data among patients in the PASI ≥20 and PASI <20 groups who switched to IXEQ4W after receiving ETN in the first 12 weeks of the study (data not shown).
Short-term (12-week) safety
The frequency of TEAEs and SAEs through Week 12 by group of psoriasis severity are presented in . At Week 12, in general, the safety profile of IXEQ2W was comparable between patients with baseline PASI ≥20 and PASI <20. The frequencies of SAEs were similar in the IXEQ2W and ETN treatment arms, while SAEs were significantly higher in the placebo arm for patients with PASI ≥20 compared to those with PASI <20 (4.7% vs. 0.5%; p = .009). In addition, in all treatment arms, the frequency of injection-site reactions was significantly higher in the patients with baseline PASI <20 than in those with PASI ≥20 (placebo: 5.7% vs. 0.7%, p = .012; IXEQ2W: 19.7% vs. 13.4%, p = .050; ETN: 19.8% vs. 10.6%, p = .002).
Table 2. Week 12 rates of treatment-emergent adverse events and serious adverse events by baseline psoriasis severity.
Long-term (3-year) safety
Rates of TEAEs and SAEs over 156 weeks by psoriasis severity are presented in . Similar to the 12-week induction period, the frequencies of TEAEs and SAEs remained generally consistent for patients in the PASI ≥20 and PASI <20 groups. Two deaths were reported in the IXEQ2W/IXEQ4W group. A lower percentage of patients with baseline PASI ≥20 than in those with PASI <20 discontinued due to TEAEs in the IXEQ2W/IXEQ4W treatment group. The frequency of injection-site reactions remained lower in the PASI ≥20 than the PASI <20 group, although in general, these rates decreased from the Week 12 rates in the IXEQ2W/IXEQ4W group. The frequency of cerebrovascular/cardiovascular events was numerically lower in the PASI <20 group than the PASI ≥20 group.
Table 3. Week 12–156 rates of treatment-emergent adverse events and serious adverse events by baseline psoriasis severity.
Discussion
There is very limited data on the potential impact of baseline psoriasis severity on the efficacy and safety of biologic therapies. In this report, we compared for the first time the long-term efficacy and safety of ixekizumab in patients with baseline PASI <20 and ≥20.
Patients with more severe psoriasis (PASI ≥20) were characterized by higher mean body weight, were more frequently male, had higher prevalence of nail, face, palm, and sole involvement, were more frequently from Europe, had more frequent prior treatment with non-biologic systemic drugs, had higher frequency of psoriatic arthritis, and had a higher baseline DLQI score. Consistent with the findings of this study, differences in psoriasis severity have been reported between men and women (Citation14,Citation15) as well as patients with facial involvement versus those without (Citation16,Citation17). The higher prevalence of European patients in the group of baseline PASI ≥20 may reflect differences of practice between Europe and North America, with later use of biologics in Europe (also reflected by the more frequent exposure to conventional systemic drugs in this population) (Citation18).
Superior efficacy of ixekizumab over placebo and ETN has been reported previously (Citation1,Citation2). The additional analyses presented here show the consistent response with ixekizumab treatment across differing disease severities and over time (with up to 156 weeks of treatment). Consistency across severity groups was observed not only for PASI improvement, but more importantly, for absolute PASI data as well, with comparable percentage of patients reaching a high level of clearance (PASI ≤2 and PASI ≤1). The efficacy of ETN was also quite consistent across psoriasis severity groups in this study, although data were only collected out to 12 weeks for the ETN treatment arm. Of note, more patients with PASI ≥20 than PASI <20 achieved relative improvements in PASI (i.e. PASI 75 and 90), whereas the reverse was observed for absolute PASI (i.e. PASI ≤5, ≤2, and ≤1). For patients with higher baseline PASI scores, the changes in the individual components of the PASI may be more dramatic, making it easier for them to achieve relative improvements in PASI. Thus, absolute PASI data should also be taken into consideration when determining the improvements patients may achieve with treatment.
Overall, with long-term ixekizumab treatment (up to 156 weeks), patients demonstrated a high level of efficacy that was maintained across patients with different baseline psoriasis severity.
The safety profile of ixekizumab in patients with moderate-to-severe plaque psoriasis was comparable to that previously reported (Citation1,Citation2,Citation11,Citation19,Citation20). Discontinuation rates were ≤2% and were not significantly different between severity groups. The incidence of cerebro-cardiovascular events was higher among patients with more severe disease, which is consistent with severe psoriasis being a known risk factor for cardiovascular events (Citation21,Citation22). Of note, the incidence of injection-site reactions was consistently lower in patients with more severe disease across treatment arms. All other adverse events were comparable between the baseline psoriasis severity groups.
Limitations
Some limitations to this analysis should be considered. First, all analyses within this report are post hoc analyses. In addition, the active comparator, ETN, was only evaluated out to 12 weeks and the long-term treatment period was open-label.
Conclusions
Overall, ixekizumab provided a high level of response with a favorable safety profile to patients with moderate-to-severe plaque psoriasis independent of baseline PASI severity level. Notably, patients with more severe psoriasis (PASI ≥20) were more frequently characterized by certain traits, such as higher mean body weight and higher prevalence of face, nail, palm, and sole involvement. In both baseline psoriasis severity groups, IXEQ2W demonstrated superior efficacy to placebo and ETN at Week 12, and a high level of efficacy was maintained with up to 156 weeks of IXEQ2W/IXEQ4W treatment. The safety profiles of the two psoriasis severity groups were maintained over 3 years.
Supplemental Material
Download Zip (3.7 MB)Acknowledgements
The authors would like to thank all the investigators, their clinical staff, and the patients who participated in these studies. The authors would also like to thank Beatrice Augendre-Ferrante, Laboratoires Lilly France, for her contributions to data analysis and interpretation, and Syneos Health for writing and editorial assistance with the preparation of this manuscript.
Disclosure statement
Dr. Kemény is an advisory board member for Eli Lilly and Company, Novartis, Janssen-Cilag, and AbbVie. Ms. Berggren is a contractor working part-time for Eli Lilly and Company. Drs. Dossenbach and Dutronc are full-time employees of, and shareholders in, Eli Lilly and Company. Dr. Paul is a consultant and investigator for Amgen, AbbVie, Almirall, Boerhinger Ingelheim, Celgene, Dermira, Janssen-Cilag, Leo Pharma, Eli Lilly and Company, GlaxoSmithKline, Novartis, Pfizer, Pierre Fabre, Regeneron, Sanofi Genzyme, and UCB Pharma.
Additional information
Funding
References
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345–356.
- Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541–551.
- Schmitt J, Wozel G. The psoriasis area and severity index is the adequate criterion to define severity in chronic plaque-type psoriasis. Dermatology (Basel). 2005;210:194–199.
- Feldman SR. A quantitative definition of severe psoriasis for use in clinical trials. J Dermatolog Treat. 2004;15:27–29.
- Weisman S, Pollack CR, Gottschalk RW. Psoriasis disease severity measures: comparing efficacy of treatments for severe psoriasis. J Dermatolog Treat. 2003;14:158–165.
- National Institute for Health and Clinical Excellence. Psoriasis: the management of psoriasis [Internet]. London: National Institute for Health and Clinical Excellence; 2012 [cited 2018 May 14]. Available from: https://www.nice.org.uk/guidance/cg153/documents/psoriasis-nice-guideline2
- European Medicines Agency. Guideline on Clinical Investigation of Medicinal Products Indicated for the Treatment of Psoriasis [Internet]. London: European Medicines Agency; 2004 [cited 2018 May 14]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003329.pdf
- Ponnambath N, Kalavala M, Anstey AV, et al. Practical experience of biologics for treatment of very severe psoriasis: a retrospective case cohort study of patients with a baseline Psoriasis Area and Severity Index greater than 20. Clin Exp Dermatol. 2016;41:95–96.
- Strober BE, Bissonnette R, Fiorentino D, et al. Comparative effectiveness of biologic agents for the treatment of psoriasis in a real-world setting: results from a large, prospective, observational study (Psoriasis Longitudi-nal Assessment and Registry [PSOLAR]). J Am Acad Dermatol. 2016;74:851–861.
- Gottlieb AB, Lacour JP, Korman N, et al. Treatment outcomes with ixekizumab in patients with moderate-to-severe psoriasis who have or have not received prior biological therapies: an integrated analysis of two Phase III randomized studies. J Eur Acad Dermatol Venereol. 2017;31:679–685.
- Blauvelt A, Gooderham M, Iversen L, et al. Efficacy and safety of ixekizumab for the treatment of moderate-to-severe plaque psoriasis: results through 108 weeks of a randomized, controlled phase 3 clinical trial (UNCOVER-3)). J Am Acad Dermatol. 2017;77:855–862.
- Schafer JL. Analysis of incomplete multivariate data. New York (NY): Chapman and Hall; 1997.
- Rubin DB. Multiple imputation for nonresponse in surveys. New York (NY): John Wiley & Sons, Inc; 1987.
- Hägg D, Eriksson M, Sundström A, et al. The higher proportion of men with psoriasis treated with biologics may be explained by more severe disease in men. PLoS One. 2013;8:e63619.
- Hägg D, Sundström A, Eriksson M, et al. Severity of psoriasis differs between men and women: a study of the clinical outcome measure Psoriasis Area and Severity Index (PASI) in 5438 Swedish register patients. Am J Clin Dermatol. 2017;18:583–590.
- van de Kerkhof P, Murphy GM, Austad J, et al. Psoriasis of the face and flexures. J Dermatolog Treat. 2007;18:351–360.
- Young Park J, Hyun Rim J, Beom Choe Y, et al. Facial psoriasis: comparison of patients with and without facial involvement. J Am Acad Dermatol. 2004;50:582–584.
- Tabolli S, Paradisi A, Giannantoni P, et al. Factors associated with the prescription of “traditional” or “biological” systemic treatment in psoriasis. J Dermatolog Treat. 2015;26:37–40.
- Gordon KB, Leonardi CL, Lebwohl M, et al. A 52-week, open-label study of the efficacy and safety of ixekizumab, an anti-interleukin-17A monoclonal antibody, in patients with chronic plaque psoriasis. J Am Acad Dermatol. 2014;71:1176–1182.
- Strober B, Leonardi C, Papp K, et al. Short- and long-term safety outcomes with ixekizumab from 7 clinical trials in psoriasis: etanercept comparisons and integrated data. J Am Acad Dermatol. 2017;76:432–440.
- Gefland JM, Neimann AL, Wang X, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735–1741.
- Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149:1173–1179.
