Abstract
Background: It is important to determine the vasoconstrictor potencies of topical corticosteroids used to treat psoriasis to ensure appropriate clinical use.
Objective: To compare the vasoconstrictive potencies of fixed-dose combination calcipotriol (50 μg/g) and betamethasone dipropionate (0.5 mg/g) (Cal/BD) cutaneous foam with other topical corticosteroids.
Methods: In this Phase I, single-center, healthy volunteer study, Cal/BD foam, clobetasol propionate 0.05% cream (CP; very potent), BD 0.05% ointment (potent), mometasone furoate 0.1% cream (MF; potent), hydrocortisone-17-butyrate 0.1% ointment (HB; moderately potent), and foam vehicle were applied, then removed after 16 h. Skin blanching was visually assessed 2 h later (scale of 0–4).
Results: Thirty-six volunteers were randomized. Skin blanching with Cal/BD foam (median [range], 2.00 [0.75–3.00]) was significantly lower than CP cream (3.00 [1.75–4.00]; p < .001), was not significantly different from BD ointment (1.75 [0.75–3.00]; p = .30) and MF cream (2.00 [1.00–3.75]; p = .22), and was significantly greater than HB ointment (1.25 [0.50–3.00]; p < .001) and vehicle (0 [0–0.50]; p < .001). There were no local tolerability reactions or adverse events.
Conclusions: The corticosteroid potency of Cal/BD foam was not significantly different from BD ointment and MF cream, significantly stronger than HB ointment, but weaker than CP cream in healthy volunteers.
Introduction
Most patients with psoriasis vulgaris can effectively manage their disease with topical treatments (Citation1–3). Many topical corticosteroids are available, and are often used in combination with vitamin D analogs. The potencies of corticosteroid preparations differ, and excessive exposure to very potent corticosteroids increases the risk of cutaneous and systemic side effects. Corticosteroids commonly induce skin atrophy and, more rarely, can cause suppression of the hypothalamic–pituitary–adrenal axis (Citation4). Therefore, it is important to determine the potencies of topical corticosteroids to ensure they are used appropriately in clinical practice to minimize the risk of side effects.
Topical corticosteroid potency can be assessed using the McKenzie and Stoughton vasoconstriction assay, which evaluates the blanching (whitening) response of healthy skin induced by a topical corticosteroid (Citation5). Because the blanching response is due to vasoconstriction elicited by the diffusion of corticosteroid across the epidermis, the intensity of blanching is related to the availability of the corticosteroid in the skin and the potency of the applied corticosteroid. This assay has been recommended for ranking the potency of topical corticosteroids based on its correlation with clinical efficacy in patients with psoriasis (Citation6).
In previous clinical trials, the fixed-dose combination ointment of calcipotriol 50 µg/g and betamethasone dipropionate 0.5 mg/g (Cal/BD) formulated as an ointment (Citation7–10) or gel (Citation11–13) displayed increased efficacy and was associated with fewer adverse events (AEs) compared with the individual components. Moreover, Cal/BD formulated as a cutaneous foam displayed greater efficacy and similar tolerability to both gel and ointment formulations in clinical trials (Citation14–19). The greater clinical efficacy of Cal/BD cutaneous foam is related to its greater corticosteroid vasoconstrictive potency compared with the gel or ointment formulations (Citation20).
A prior Phase I study (Citation20) using the McKenzie and Stoughton vasoconstriction assay (Citation5) indicated that Cal/BD cutaneous foam was more potent than Cal/BD ointment and a moderately potent ointment (fluocinolone acetonide 0.25 mg/g [FA]), but less potent than a very potent corticosteroid (clobetasol propionate 0.5 mg/g [CP] cream). In that study, the treatments were applied for 6 h according to McKenzie and Stoughton’s method (Citation5). Briefly, colorimetric and visual vasoconstriction assessments were done at 6 h and 10 min (allowing for product to be removed), and at 8, 10, 12, 24, 28 and 32 h post-application. Potency was assessed in terms of the skin blanching scores at 2 h after the end of exposure or product removal (i.e. at 8 h post-application) and at 32 h post-application. The objective of this study was to reassess the corticosteroid potency of BD in Cal/BD cutaneous foam after exposure for a longer time (in this case, 16 h), with potency assessed at a single time point (2 h after the end of exposure).
Methods
Volunteers
This study enrolled healthy, non-smoking volunteers aged 18–50 years with a skin type of I–IV on the Fitzpatrick scale (Citation21). Volunteers were eligible if they showed an adequate vasoconstriction response, which was defined as a visual skin blanching score of at least 1 unit for 4–6 h after application of non-occlusive BD 0.05% ointment. Volunteers who had received systemic treatments or any medications likely to interfere with skin blanching reaction up to 2 weeks before enrollment, and those who had applied a topical corticosteroid to the forearm up to 4 weeks before enrollment, were excluded from the study. Volunteers were also excluded from the study if they had clear differences in skin color between each arm, skin infections/lesions in the test area, current systemic/cutaneous disease likely to confound interpretation of the results (e.g. atopic dermatitis, contact eczema, or psoriasis), or suspected/known hypersensitivity to the test drugs. All volunteers provided written informed consent.
Study design
This Phase I, single-center, investigator-blinded, vehicle-controlled, intra-individual comparison study (NCT02973776) comprised a screening visit, two test visits, and a follow-up visit.
At the screening visit (up to 15 d before the test visit), volunteers underwent a screening test to select subjects demonstrating adequate skin vasoconstriction (i.e. responders) in which an unoccluded application of 20 μL of BD 0.05% on a 2.2 cm diameter circular area was performed. The skin blanching score (range 0–4) was visually assessed 4–6 h later and only subjects with a score of least 1 unit were eligible. The site used for the screening test was not used during the test phase.
At the first test visit (day 1), six test sites were marked on the anterior surface of the forearms (three per arm) at least 3 cm from the antecubital fossa or the wrist, avoiding the distal part of the wrist, veins, and hair-covered areas. The test sites were circular areas (2.2 cm in diameter) with a gap of at least 0.3 cm between each site.
The treatments used in this study were Cal/BD cutaneous foam, CP 0.05% cream (very potent), BD 0.05% ointment (potent), mometasone furoate 0.1% cream (MF; potent), hydrocortisone-17-butyrate 0.1% ointment (HB; moderately potent) and cutaneous foam vehicle.
Unblinded study personnel used a calibrated Eppendorf pipette (Eppendorf AG, Hamburg, Germany) to transfer 20 μL of CP cream, BD ointment, MF cream, and HB ointment onto dishes, and the cream/ointment was then transferred to the test site. Cal/BD cutaneous foam and cutaneous foam vehicle were sprayed onto a pre-weighed dish and left for 30 s to allow the propellants to evaporate. The dish was then weighed and, if the weight of the foam was within the range of 14.5–19.5 mg (20 μL ±15%), the foam was transferred to the test site. The treatments were massaged into the skin and covered by a non-occlusive system to avoid contamination of other test sites. After the treatments had been applied, the volunteers could leave the study site.
Owing to differences in the formulations of the treatments, a double-blind study design was not possible. Therefore, the treatments were applied by unblinded study personnel; however, visual and safety assessments were made by blinded investigators.
The volunteers returned for the assessments the next day. At 16 h (±0.5 h) after application of the treatments, the study personnel gently wiped off any remaining product using a dry paper towel; washing of the skin was not permitted. Two hours later (±0.5 h), two-blinded trained investigators assessed skin blanching at each of the test sites. One investigator also assessed each site for local tolerability.
A follow-up visit was scheduled up to 14 d (±2 d) later for volunteers with a local tolerability score >0, a non-serious AE considered by the investigator to be possibly or probably related to a test product, or an ongoing serious AE (regardless of tolerability). The follow-up visit could be performed via telephone if deemed appropriate.
This study conformed to the Declaration of Helsinki and Good Clinical Practice guidelines, and was approved by the investigational site’s independent ethics committee.
Study assessments
The primary endpoint was the mean of two visual skin blanching scores, which were assessed by two blinded, trained investigators at 2 h after removing the treatments. Skin blanching was assessed using a scale ranging from 0 to 4 (0, no change in skin color; 1, slight [barely visible] blanching; 2, obvious blanching; 3, intense blanching; and 4, maximal blanching) (Citation5), with half-point scores used for intermediate changes. This scoring method was also used in the screening visit.
Safety assessments included evaluation of local and systemic AEs. The location of cutaneous AEs was classified as being either close to (≤2 cm) or distant from (>2 cm) the application site. Cutaneous reactions were assessed using a scale ranging from 0 to 4 (0, no reaction; 0.5, only slight erythema; 1, only erythema; 2, erythema with papules or edema; 3, erythema, edema with papules, or edema with vesicle; 4, blisters). Irritant reactions, such as miliaria, follicular pustules, burn-like reactions, and dry scales, were recorded as a separate category (termed ‘Irritant reactions’). Cutaneous reactions with a local tolerability score >0 were reported as local AEs.
Heart rate and blood pressure were also measured at each visit.
Statistical analyses
The null hypothesis for each pairwise comparison was no difference in skin blanching between Cal/BD and each comparator. Using a paired t-test, a sample size of 30 volunteers would provide a probability of 98% for detecting a mean difference in skin blanching score of 0.5 units between treatments at a two-sided significance level of 5%, and a standard deviation (SD) for difference of 0.65 (based on results of an earlier similarly designed study) (Citation20).
Because four active treatments were to be tested, a sample size of 30 volunteers would provide power of at least 92% (0.98), assuming the true difference in visual score between Cal/BD cutaneous foam and the active comparators was at least 0.5 units. For the comparison of Cal/BD cutaneous foam and the cutaneous foam vehicle, a score difference of at least 1 unit was assumed and this comparison was expected to have a negligible impact on the overall power.
Allowing for a dropout rate of about 15%, a total of 36 volunteers were to be enrolled and randomized.
The Shapiro–Wilk test of normality was used to test whether the data followed a normal distribution. The protocol specified that skin blanching scores were to be analyzed using analysis of variance (ANOVA), with subject and treatment as factors. The Kruskal–Wallis test (for the overall product effect) and Wilcoxon signed rank test (for pairwise comparisons) were to be used if the scores deviated from a normal distribution. Baseline characteristics, AEs, and vital signs were summarized descriptively in terms of the mean ± SD and n (%).
Results
Volunteers
Thirty-six volunteers (14 males and 22 females) with a median age of 34.5 years (range 19−50) were randomized and received all six treatments (). The Fitzpatrick skin type was II in five volunteers, III in 30 volunteers, and IV in one volunteer. The median skin blanching score was 1.0 (range 1.0–1.5) at the screening test.
Table 1. Baseline characteristics of the volunteers.
Assessment of skin blanching
Because the skin blanching data were not normally distributed for three of the treatments (MF [p = .0248], HB [p = .0045] and cutaneous foam vehicle [p < .0001]), the Kruskal–Wallis and Wilcoxon signed rank tests were used. All five active treatments were associated with greater skin blanching compared with cutaneous foam vehicle (; ). The Kruskal–Wallis test revealed a statistically significant overall effect for differences among the six treatments (p < .001). As indicated in , the skin blanching score with Cal/BD cutaneous foam was significantly lower than that of CP cream (p < .001), was not significantly different to those of BD ointment (p = .30) and MF cream (p = .22), and was significantly greater than those of HB ointment and cutaneous foam vehicle (both p < .001) (Wilcoxon signed rank test). Using a Bonferroni-corrected significance level of 0.01 (0.05/5) to account for multiplicity, the results were not affected.
Figure 1. Box plot of visually assessed skin blanching scores at 2 h after treatment application for 16 h.The box plot shows the median (horizontal lines), range (boxes), 1.5× interquartile range (whiskers) and mean (circles). BD: betamethasone dipropionate 0.05%; Cal/BD: calcipotriol 50 μg/g and betamethasone dipropionate 0.5 mg/g; CP: clobetasol propionate 0.05%; HB: hydrocortisone-17-butyrate 0.1%; MF: mometasone furoate 0.1%; vehicle, foam vehicle.
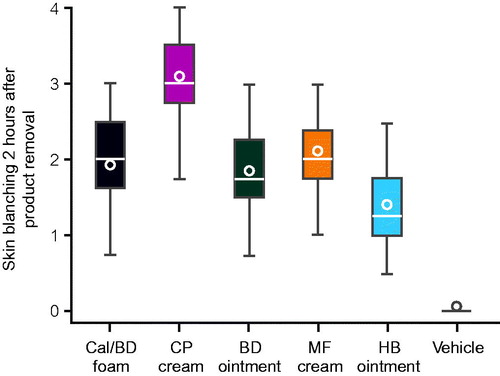
Table 2. Visually assessed skin blanching scores at 2 h after non-occlusive application of the treatments for 16 hTable Footnotea (N = 36).
ANOVA without correction for multiplicity was also performed because the data for three treatments (Cal/BD cutaneous foam, CP, and BD) were normally distributed. ANOVA revealed a statistically significant overall effect for differences among the six treatments (p < .001). The skin blanching score for Cal/BD cutaneous foam was significantly different to the scores for CP cream, HB ointment, and cutaneous foam vehicle (all p < .0001), but not for BD ointment (p = .44) or MF cream (p = .09) (). These results support those obtained using the Kruskal–Wallis and Wilcoxon signed rank tests.
Table 3. Results of analysis of variance of visually assessed skin blanching scores (N = 36).
Safety/tolerability
There were no local tolerability reactions, irritant reactions or AEs. In addition, there were no clinically relevant changes in heart rate or blood pressure from baseline.
Discussion
The degree of skin blanching after applying a topical corticosteroid is representative of the corticosteroid’s potency and ability to diffuse into the skin. This study, using a vasoconstriction assay, revealed that Cal/BD cutaneous foam has a potency not significantly different to BD ointment and MF cream, both of which are classified as potent corticosteroids. Cal/BD cutaneous foam was less potent than CP cream and more potent than HB ointment.
These results support those of an earlier study that compared the potencies of Cal/BD cutaneous foam, CP 0.5 mg/g cream, Cal/BD ointment, FA 0.25 mg/g ointment, and BD cutaneous foam after application for 6 h. In that study, Cal/BD cutaneous foam had the same potency as BD foam, was more potent than Cal/BD ointment and FA ointment, and was less potent than CP cream in terms of the area under the curve for skin blanching scores over 32 h after application and at 2 h after removing the treatments (Citation20). The results of both that study and a study (Citation22) that used an in vitro skin penetration model suggest that Cal/BD formulated as a cutaneous foam shows increased skin penetration compared with the ointment formulation and the individual active components.
Consistent with the vasoconstrictor results, clinical trials have demonstrated greater clinical efficacy of Cal/BD cutaneous foam on psoriasis than Cal/BD ointment or the individual components (Citation14–19). The greater efficacy of Cal/BD cutaneous foam might be related to its greater vasoconstrictive potency and increased bioavailability compared with other formulations and the individual components (Citation23). The increased bioavailability of Cal/BD in the cutaneous foam has been attributed to the formation of a supersaturated environment after evaporation of dimethyl ether present in the cutaneous foam (Citation23).
Cal/BD cutaneous foam was well tolerated with no local tolerability reactions, AEs or clinically relevant changes in heart rate or blood pressure from baseline, consistent with the prior study. Data from this and the previous study can only demonstrate that no AEs or local tolerability reactions were provoked by a single exposure (16 h in this study and 6 h in the previous study) of healthy skin to the test formulation. Nevertheless, Phase II and Phase III studies have demonstrated the favorable safety and tolerability profile of Cal/BD cutaneous foam in patients with psoriasis (Citation14–19).
Conclusions
This study showed that, consistent with corticosteroid potency classifications, the steroid potency of Cal/BD cutaneous foam was not significantly different to that of BD ointment and MF cream, significantly stronger than that of HB ointment, but weaker than that of very potent CP cream in healthy volunteers.
Previous presentation
Poster presented at the 26th EADV Congress, Geneva, Switzerland, September 13–17 2017.
Disclosure statement
C. Queille-Roussel reports subject fees paid by LEO Pharma; J. Nielsen is an employee of LEO Pharma; J.-P. Lacour reports reimbursement for conference attendance and honoraria for consultancy activity from LEO Pharma.
Additional information
Funding
References
- Schon MP, Boehncke WH. Psoriasis. N Engl J Med. 2005;352:1899–1912.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643–659.
- Samarasekera E, Sawyer L, Parnham J, et al. Assessment and management of psoriasis: summary of NICE guidance. BMJ. 2012;345:e6712.
- Stein L. Clinical studies of a new vehicle formulation for topical corticosteroids in the treatment of psoriasis. J Am Acad Dermatol. 2005;53:S39–S49.
- McKenzie AW, Stoughton RB. Method for comparing percutaneous absorption of steroids. Arch Dermatol. 1962;86:608–610.
- Cornell RC, Stoughton RB. Correlation of the vasoconstriction assay and clinical activity in psoriasis. Arch Dermatol. 1985;121:63–67.
- Guenther L, van de Kerkhof PC, Snellman E, et al. Efficacy and safety of a new combination of calcipotriol and betamethasone dipropionate (once or twice daily) compared to calcipotriol (twice daily) in the treatment of psoriasis vulgaris: a randomized, double-blind, vehicle-controlled clinical trial. Br J Dermatol. 2002;147:316–323.
- Kragballe K, Noerrelund KL, Lui H, et al. Efficacy of once-daily treatment regimens with calcipotriol/betamethasone dipropionate ointment and calcipotriol ointment in psoriasis vulgaris. Br J Dermatol. 2004;150:1167–1173.
- Kragballe K, van de Kerkhof PC. Consistency of data in six phase III clinical studies of a two-compound product containing calcipotriol and betamethasone dipropionate ointment for the treatment of psoriasis. J Eur Acad Dermatol Venerol. 2006;20:39–44.
- Saraceno R, Andreassi L, Ayala F, et al. Efficacy, safety and quality of life of calcipotriol/betamethasone dipropionate (Dovobet®) versus calcipotriol (Daivonex®) in the treatment of psoriasis vulgaris: a randomized, multicentre, clinical trial. J Dermatolog Treat. 2007;18:361–365.
- Bottomley JM, Taylor RS, Ryttov J. The effectiveness of two-compound formulation calcipotriol and betamethasone dipropionate gel in the treatment of moderately severe scalp psoriasis: a systematic review of direct and indirect evidence. Curr Med Res Opin. 2011;27:251–268.
- Fleming C, Ganslandt C, Guenther L, et al. Calcipotriol plus betamethasone dipropionate gel compared with its active components in the same vehicle and the vehicle alone in the treatment of psoriasis vulgaris: a randomised, parallel group, double-blind, exploratory study. Eur J Dermatol. 2010;20:465–471.
- Langley RG, Gupta A, Papp K, et al. Calcipotriol plus betamethasone dipropionate gel compared with tacalcitol ointment and the gel vehicle alone in patients with psoriasis vulgaris: a randomized, controlled clinical trial. Dermatology. 2011;222:148–156.
- Koo J, Tyring S, Werschler WP, et al. Superior efficacy of calcipotriene and betamethasone dipropionate aerosol foam versus ointment in patients with psoriasis vulgaris - A randomized phase II study. J Dermatolog Treat. 2016;27:120–127.
- Lebwohl M, Tyring S, Bukhalo M, et al. Fixed combination aerosol foam calcipotriene 0.005% (Cal) plus betamethasone dipropionate 0.064% (BD) is more efficacious than Cal or BD aerosol foam alone for psoriasis vulgaris: a randomized, double-blind, multicenter, three-arm, Phase II study. J Clin Aesthet Dermatol. 2016;9:34–41.
- Leonardi C, Bagel J, Yamauchi P, et al. Efficacy and safety of calcipotriene plus betamethasone dipropionate aerosol foam in patients with psoriasis vulgaris - a randomized Phase III study (PSO-FAST). J Drugs Dermatol. 2015;14:1468–1477.
- Paul C, Stein Gold L, Cambazard F, et al. Calcipotriol plus betamethasone dipropionate aerosol foam provides superior efficacy versus gel in patients with psoriasis vulgaris: randomized, controlled PSO-ABLE study. J Eur Acad Dermatol Venereol. 2017;31:119–126.
- Queille-Roussel C, Olesen M, Villumsen J, et al. Significant reduction in psoriatic clinical signs with an innovative aerosol foam formulation of fixed combination calcipotriol plus betamethasone dipropionate in patients with psoriasis vulgaris. Clin Drug Investig. 2015;35:239–245.
- Stein GL, Lebwohl M, Menter A, et al. Aerosol foam formulation of fixed combination calcipotriene plus betamethasone dipropionate is highly efficacious in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. J Drugs Dermatol. 2016;15:951–957.
- Queille-Roussel C, Bang B, Clonier F, et al. Enhanced vasoconstrictor potency of the fixed combination calcipotriol plus betamethasone dipropionate in an innovative aerosol foam formulation versus other corticosteroid psoriasis treatments. J Eur Acad Dermatol Venereol. 2016;30:1951–1956.
- Fitzpatrick TB. Soleil et peau. J Méd Esthétique. 1975;2:33–34.
- Hollesen Basse L, Olesen M, Lacour JP, et al. Enhanced in vitro skin penetration and antipsoriatic effect of fixed combination calcipotriol plus betamethasone dipropionate in an innovative foam vehicle. J Invest Dermatol. 2014;134:abst 192.
- Lind M, Nielsen KT, Schefe LH, et al. Supersaturation of calcipotriene and betamethasone dipropionate in a novel aerosol foam formulation for topical treatment of psoriasis provides enhanced bioavailability of the active ingredients. Dermatol Ther (Heidelb). 2016;6:413–425.
