Abstract
Background: Alopecia areata (AA) is a non-scarring auto-immune hair disorder. Recent researches explained the role of growth factors (GFs) in hair follicle cycling. The main reservoir of GFs are alpha-granules of platelets and novel procedures have been implemented aimed at collecting platelet-rich plasma (PRP). PRP has been safely implemented in many medical applications and has also been successfully used as alternative cell-based therapy for the treatment of hair growth disorders, among which also AA.
Objectives: By means of a randomized double-blinded, placebo and active-controlled, parallel group study we have studied the efficacy of a cosmetic product (named TR-M-PRP plus) comprising biomimetic peptides specific for hair growth, mimicking PRP composition for the treatment of AA. Subjects were treated for three months and evaluated, at the end of the study and after one month of follow-up, as regards hair growth using SALT score.
Results: TR-M-PRP plus-like topic produced a statistically significant (p < .001) clinical improvement in SALT score after 3 months of therapy, compared to baseline. Hair growth results further improved after 1 month of follow-up.
Conclusions: This clinical investigation suggests that the biotechnological designed PRP-like cosmetic could represent a valid and safer alternative to autologous PRP for the treatment of AA.
Keywords:
What's already known about this topic?
Platelet-rich plasma (PRP) PRP has been successfully used as alternative cell-based therapies for the treatment of hair growth disorder such as Androgenetic alopecia (AGA) or Alopecia areata (AA).
What does this study add?
We have studied a topical formulation (named TR-M-PRP plus) comprising biomimetic peptides specific for hair growth mimicking PRP composition.
The results obtained with the present clinical investigation suggest that the biotechnological designed PRP-like cosmetic we investigated could represent a valid and safer alternative to autologous PRP for the treatment of AA.
Introduction
Alopecia areata (AA), is a non-scarring auto-immune hair disorder (Citation1,Citation2). Even though AA ethiology is not completely understood, many clinical evidence suggested a role of immunity in the development of such disease (Citation3–5). Therefore, genetic predisposition, environmental factor, psychological stress, hormonal unbalance, concomitance with other skin disorders and gut dysbiosis can contribute to autoimmune mechanism of AA (Citation1,Citation6,Citation7). More recently, a role of scalp microbiome has also been hypothesized (Citation8). AA is the second most common type of alopecia with an incidence higher than 2% and a lifetime risk of 1.7% both in men and women (Citation9).
Currently, available treatment options for AA included: topical (Citation10), intra-lesional (Citation11) or systemic (Citation12) steroids, and immunotherapy or systemic immuno-modulators.
Recent researches (Citation13–16) explained the role of growth factors (GFs), especially polypeptide in the life-long cyclic transformation of the hair follicle, and their activity in control of immune privilege (in particular IGF-1) (Citation17). GFs act by stimulating cell proliferation and differentiation and inhibiting apoptosis on dermal papilla cells and stimulate stem cells of bulge area (Citation18–20). This activity will result in anagen prolongation and catagen delaying (Citation21,Citation22).
The main reservoir of growth factors in the body are alpha-granules of platelets (Citation23) and growth factors involved on hair follicle cycling are mainly vascular endothelial growth factor (VEGF) (Citation24) epidermal growth factor (EGF) (Citation25), fibroblast growth factor (Citation26,Citation27) and insulin-like growth factor (IGF) (Citation28). Adenosine diphosphate (ADP), serotonin and calcium are also released from dense granules and are important in the recruitment of new platelets and coagulation cascade (Citation29).
Novel procedures have been implemented aimed at collecting platelet-rich plasma (PRP) (Citation30–34) releasing growth factors after platelets degranulation (Citation29).
PRP derived from autologous blood has a 1,000,000/UL platelets concentration, which is 3–8 folds higher than normal peripheral blood (range 150,000–350,000 UL) (Citation35,Citation36).
First used in 1987 by Ferrari et al. (Citation37) in transfusion procedure, PRP has then been safely implemented in many application fields, such as orthopedics and sports medicine, dentistry, neurosurgery, ophthalmology, urology, and wound healing (Citation38,Citation39). PRP has also been successfully used as alternative cell-based therapies for the treatment of hair growth disorder such as AGA (Citation36,Citation40) or AA (Citation41,Citation42).
In a randomized, double-blind, placebo and active-controlled, a half-head study on AA subjects, Trink and collaborators investigated, for the first time, the safety and efficacy of PRP on hair regrowth and dystrophy, burning or itching (Citation43).
During the time, attempts have been made to standardize procedures for PRP preparation in order to reduce variation in the concentration of platelets and differences in manufacturing procedures (Citation44). With a view to the above limitations, the use of biomimetic peptides, mimicking growth factors normally encountered in PRP, could represents a valid alternative. They can be implemented in an topical formulation and used for the treatment many conditions in which modulation by growth factors is involved.
Normally, a biomimetic peptide is an oligopeptide (10–15 aa) that provides similar efficacy of natural or recombinant growth factors but reduce cost and owns more stability. By mean of biotechnological development, a wide range of biomimetic peptides has been developed since the beginning of 2000. Since their birth, these novel discovered molecules represented a very promising application with regard to skin and dermatological applications (Citation45). Several kinds of biomimetic peptides are currently available on the market. They include signal peptides (Citation46,Citation47), carrier peptides (Citation48,Citation49) and also specific peptides targeting hair such as tripeptide-copper complex (Citation50), 5-aminolevulinic acid-GHK (ALAVAX) (Citation51) Octapeptide-2 (Citation52,Citation53), Decapeptide P3 (Citation54) and Sh-polypeptide 9 (Citation55). In a previous randomized trial on 40 women affected by chronic telogen effluvium, we have evaluated the efficacy of a pool of selected mimicking growth factors (IGF 10%, EGF 10%), included in a topical formulation, in preventing dermal papilla apoptosis, prolong anagen phase and delaying catagen and telogen (Citation56).
More recently we have also evaluated in vitro the efficacy of a mix of biomimetic peptides, the same used in the product covered by the present study, for hair growth stimulation (submitted for publishing). Starting from the previously reported evidence we have developed a cosmetic product (named TR-M-PRP plus) for the treatment of AA, comprising biomimetic peptides specific for hair growth and mimicking PRP composition.
Material and methods
Study design and patients
The study was structured in the form of a randomized double-blinded, placebo and active-controlled, parallel group study. 60 subjects with AA of both sex, aged between 18–60 years, were enrolled. For each AA patient, essential background data were collected at baseline according to the guidelines of the National Alopecia Areata Foundation (Citation57,Citation58). AA-grade was assessed according to Severity of Alopecia Tool (SALT) (Citation57) score (S0 = no hair loss; S1 < 25% hair loss; S2 = 25%–49% hair loss; S3 = 50%–74% hair loss; S4 = 75%–99% hair loss; and S5 = 100% hair loss). Efficacy of TR-M-PRP plus treatment was assessed as percentage hair regrowth and the grading of overall improvement, calculated from change in baseline SALT score. Absolute change in SALT score = SALT score at baseline – SALT score at T2. Percentage of hair regrowth was calculated as follows: 100 × (Baseline SALT score − SALT score at T1 or T2)/Baseline SALT score. Assessment of percentage hair regrowth was graded into following 6 grades: A0 = no change or further loss of hairs; A1 = 1–24% regrowth; A2 = 25–49% regrowth; A3 = 50–74% regrowth; A4 = 75–99% regrowth; A5 = 100% regrowth. Subjects had also to accepting to not receive any other drug/cosmetic treatments during the study and had not be involved in a similar study during the previous 6 months. Exclusion criteria included known sensitivity to any compound of the investigational product, pregnancy or breastfeeding, any other medical condition or other scalp or hair disorders.
All patients were evaluated and enrolled in the study by the RS Dermatologic Clinic, Milan, Italy, after signed an informed consent.
The study was under the approval of the Ethical Independent Committee for Clinical, not pharmacological investigation in Genoa (Italy) and in accordance with the ethical standards of the 1964 Declaration of Helsinki.
Treatment
AA enrolled subjects were randomly divided into 2 groups: group I included 30 AA patients treated with TR-M-PRP plus; group II included 30 AA patients treated with Placebo. Both groups applied the product twice a week (15 ml) for 3 months. Biomimetic peptides used were: Copper Tripeptide-1, Octapeptide-2, Oligopeptide-20, and Acetyl Decapeptide-3. Lactoferrin, lactoglobulin, and melatonin were also included as an anti-inflammatory, ATP stimulator and circadian rhythm regulator agents, respectively.
Assessment of the response
Subjects have been visited three times: at the Randomization Visit (Baseline T0), at the End of Treatment Period Visit at Month 3 (T1, 90 days), and at the Follow Up Visit, one month after treatment end (T2, 120 days). Photography Digital photos were taken for the scalp before therapy and during subsequent visits. Hair regrowth in AA subjects has been evaluated using the SALT score which expresses hair regrowth as a percentage from baseline (Citation59–61). At the end of the study (T1) and at the Follow up Visit (T2), each volunteer has also filled out a questionnaire regarding the perceived efficacy of the treatment and product compliance.
Statistical analysis
A two-sample Student’s t-test was used for comparison at baseline and during the study. p-values less than .05 were considered clinically significant.
Results
A total of 60 subjects (37 men and 23 women) were enrolled and received treatment (). The TR-M-PRP plus-treated group and placebo group had comparable baseline demographics and disease characteristics.
Hair-growth measured after 3 months of treatment and a follow up of one month with TR-M-PRP plus (Group I) were compared with values registered at the baseline and compared to Placebo group (Group II).
Enrolled AA subjects presented a mean of 4.35 symmetrically distributed patches of hair loss and had the last relapse 1–2 years before (mean 1.2). They were no responsive to any other previous treatment including systemic and topical immunosuppressant therapies and phototherapy. Therefore, they received no treatment for at least one year.
Absolute change in the baseline SALT score was calculated. Mean value of the absolute change in SALT score was 18.30 and 8.49 for Group I and II, respectively. Percentage scalp hair regrowth was derived from the absolute change in the baseline SALT score for all the patients. After three months of treatment (T1) the mean values were 57.07% for Group I and 27.96% for Group II (). At T2 a further significative (p < .0001) improvement was found for Group I (68.12% vs 28.89% in Group II).
Table 1. Subjects demographic characteristics.
Table 2. Percentage changes in baseline SALT (Severity of Alopecia Tool) score in Group I and Group II patients (percentage scalp hair regrowth).
In Group I, 53.33% cases showed complete regression (A5 grade) (). A partial regression was also seen in 13.33% of population of Group I, but we did not consider that result as relevant for the present study. 33.34% of subjects from Group I showed no response at all (). Only 3.33% of population of Group II reported a complete regression ().
Table 3. Grading of overall improvement in Group I and II.
No adverse effects were reported after TR-M-PRP plus or Placebo administration. Therefore, all patients under investigation reported a good compliance of the tested product.
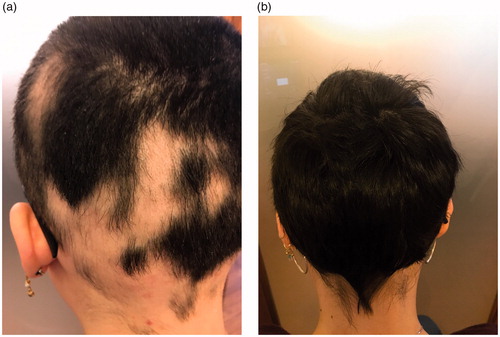
Explicative photographic images showing hair regrowth effect of TR-M-PRP plus were reported in .
Discussion
The prevalence of hair loss in the population and its impact on self-esteem and depression (Citation62,Citation63) poses the need of therapies targeted to reduce the appearance of thinning by delaying, arresting, or reversing hair growth disorder. The synthesis of biotechnological compounds mimicking growth factors opens to novel therapeutic approaches.
The clinical study presented in the current work showed the efficacy of a PRP-like cosmetic product to stimulating hair growth in patients affected by AA.
In this study, a total significant improvement of SALT score was reported for all 30 subjects enrolled and treated with TR-M-PRP plus. The results show significant changes in the objective parameters tested to evaluate the effectiveness of the treatment. Indeed, the results indicate that the application of biomimetic peptides in patients with AA leads to a decrease in hair loss probably due to prolongation of the anagen phase (probably acting on WNT/β-catenin pathways and via exosomes stimulation) and, consequently, due to the reduction of the telogen phase and possibly, by immunological control.
Following our previous work (Citation43) several published works have been reported as regards efficacy of autologous PRP in AA (Citation64–69). The product under study represents the first biotechnological designed PRP able to reproduce the efficacy of autologous PRP. Many biomimetic peptides are currently available on the market but few reported a well-characterized action on hair growth. We settled up a mix of biomimetic peptides able together to simulate autologous PRP but avoiding its intrinsic limitations (cost, interpersonal variation, invasiveness of the procedure, and reported side effects).
All subjects under investigation well tolerated the treatment and no side effects were identified. Therefore, all used biomimetic peptides are protected by micro-encapsulation to avoid peptidases and proteases degradation and these results in higher stability. Tripeptide-1 (GHK) (Glycyl-l-histidyl-l-lysine) is a biomimetic peptide that is physiologically released during inflammation and wound healing process (Citation48). This peptide shows a high affinity for copper ions, forming a complex (Citation70): Copper Tripeptide-1 (GHK-Cu). It possesses a diverse multiplicity of actions being able to activate many remodeling related processes. Indeed, GHK-Cu is a powerful anti-inflammatory agent in wound-healing (Citation71–74). It also acts on metalloproteinases and on extracellular matrix proteins (Citation70,Citation75–77) and also stimulates angiogenesis (Citation71,Citation78). Most interesting GHK-Cu is able to counteract hair loss through the stimulation of stem cells, increasing hair follicle size (Citation79,Citation80).
We implemented in the formulation also Octapeptide-2 (Thr-Ala-Glu-Glu-His-Glu-Val-Met). It is a mimetic of thymosin-β4 growth factor, a well-known stimulator of hair growth that acts on angiogenesis and promotes the migration of stem cells and their progeny to the base of the follicle. It also stimulates differentiation and extracellular matrix remodeling (Citation53,Citation54).
Acetyl Decapeptide-3 is a Basic Fibroblast Growth Factor (bFGFs) biomimetic whom efficacy has been largely proven in skin regeneration. As biomimetic of bFGFs, it is involved in normal skin growth, healing and wound repair. Most interesting, bFGFs have been shown to be involved in hair development (Citation27,Citation28).
Oligopeptide-20 (8H-Cys-Arg-Lys-Ile-Pro-Asn-Gly-Tyr-Asp-Thr-Leu-OH) is another peptide involved in hair growth mechanisms. It’s supposed to act as an enzyme inhibitor leading to an increase of the synthesis of collagen and glycosaminoglycans. The involvement of collagen, in particular, collagen IV in hair cycling has been recently reported (Citation81). Reduced levels of collagen had also been related to hair follicle aging (Citation82).
We can postulate that the above-reported oligo-peptides may simulate together the efficacy of autologous PRP (Citation43) acting by promoting hair follicle growth probably via stimulation of cell proliferation. Further in vitro experiment could help in confirming mechanism behind their mechanism.
The cosmetic product investigated contained also: (i) lactoferrin that is a potent anti-inflammatory agent (Citation83) for helping in counteract inflammation mechanisms of AA; (ii) lactoglobulin helpful for stimulating mitosis and ATP production (Citation84); (iii) melatonin for clock gene regulation (Citation85), a process strictly involved in hair growth regulation.
Conclusion
The results obtained in the present clinical investigation suggest that the biotechnological designed PRP-like cosmetic we investigated could represent a valid and safer alternative to autologous PRP for the treatment of AA. Further in vitro and in vivo studies may help into better underline the mechanism behind its efficacy.
| Abbreviations | ||
| PRP | = | Platelet-rich plasma; |
| AGA | = | Androgenetic alopecia; |
| AA | = | Alopecia areata |
| GFs | = | growth factors |
| VEGF | = | vascular endothelial growth factor |
| EGF | = | epidermal growth factor |
| FGF | = | fibroblast growth factor |
| IGF | = | insulin-like growth factor |
| ADP | = | Adenosine diphosphate |
| SALT | = | Severity of Alopecia Tool |
| bFGFs | = | Basic Fibroblast Growth Factor |
Disclosure statement
R.F. and S.E. serve as a consultant for Giuliani S.p.A. P.D. and M.B. are employed by Giuliani S.p.A.
Additional information
Funding
References
- Syed SA, Sandeep S. Alopecia areata: a review. J Saudi Soc Dermatol Dermatol Surg. 2013;17:37–45.
- D'Ovidio R. Alopecia Areata: news on diagnosis, pathogenesis and treatment. G Ital Dermatol Venereol. 2014;149:25–45.
- McDonagh AJ, Tazi-Ahnini R. Epidemiology and genetics of alopecia areata. Clin Exp Dermatol. 2002;27:405–409.
- Hordinsky M, Ericson M. Autoimmunity: alopecia areata. J Investig Dermatol Symp Proc. 2004;9:73–78.
- Brenner W, Diem E, Gschnait F. Coincidence of vitiligo, alopecia areata, onychodystrophy, localized scleroderma and lichen planus. Dermatologica. 1979;159:356–360.
- Borde A, Åstrand A. Alopecia areata and the gut-the link opens up for novel therapeutic interventions. Expert Opin Ther Targets. 2018;22:503–511.
- Rebello D, Wang E, Yen E. Hair growth in two alopecia patients after fecal microbiota transplant. ACG Case Rep J. 2017;13:e107.
- Rinaldi F, Pinto D, Marzani B, et al. Human microbiome: what's new in scalp diseases. J Transl Sci. 2018;4:1–4.
- Dawber R. Alopecia areata. Monogr Dermatol. 1989;2:89–102.
- Tosti A, Iorizzo M, Botta GL, et al. Efficacy and safety of a new clobetasol propionate 0.05% foam in alopecia areata: a randomized, double-blind placebo-controlled trial. J Eur Acad Dermatol Venerol. 2006;20:1243–1247.
- Sardesai VR, Prasad S, Agarwal TD. A study to evaluate the efficacy of various topical treatment modalities for alopecia areata. Int J Trichol. 2012;4:265–270.
- Kar BR, Handa S, Dogra S, et al. Placebo-controlled oral pulse prednisolone therapy in alopecia areata. J Am Acad Dermatol. 2005;52:287–290.
- Takakura N, Yoshida H, Kunisada T, et al. Involvement of platelet-derived growth factor receptor-alpha in hair canal formation. J Invest Dermatol. 1996;107:770–777.
- Yano K, Brown LF, Detmar M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J Clin Invest. 2001;107:409–417.
- Rishikaysh P, Dev K, Diaz D, et al. Signaling Involved in hair follicle morphogenesis and development. Int J Mol Sci. 2014;15:1647–1670.
- Jain R, De-Eknamkul W. Potential targets in the discovery of new hair growth promoters for androgenic alopecia. Expert Opin Ther Targets. 2014;18:787–806.
- Pai VV, Bhandari P, Shukla P. Topical peptides as cosmeceuticals. Indian J Dermatol Venereol Leprol. 2017;83:9–18.
- Böhlen P, Esch F, Baird A, et al. Acidic fibroblast growth factor (FGF) from bovine brain: amino-terminal sequence and comparison with basic FGF. Embo J. 1985;4:1951–1956.
- Kawano M, Komi-Kuramochi A, Asada M, et al. Comprehensive analysis of FGF and FGFR expression in skin: FGF18 is highly expressed in hair follicles and capable of inducing anagen from telogen stage hair follicles. J Invest Dermatol. 2005;124:877–885.
- Kimura-Ueki M, Oda Y, Oki J, et al. Hair cycle resting phase is regulated by cyclic epithelial FGF18 signaling. J Invest Dermatol. 2012;132:1338–1345.
- Mak KK, Chan SY. Epidermal growth factor as a biologic switch in hair growth cycle. J Biol Chem. 2003;278:26120–26126.
- Rinaldi F, Sorbellini E, Bezzola P. The role of up-stimulation of growth factors in hair transplantation: improve the revascularization of transplanted hair growth mediated by angiogenesis. Forum. 2007;2.
- Jungbluth P, Grassmann J-P, Thelen S, et al. Concentration of platelets and growth factors in platelet-rich plasma from Goettingen minipigs. GMS Interdisciplinar Plastic Reconstruct Surger DGPW. 2014;3:Doc11.
- Kozlowska U, Blume-Peytavi U, Kodelja V, et al. Expression of vascular endothelial growth factor (VEGF) in various compartments of the human hair follicle. Arch Dermatol Res. 1998;290:661–668.
- Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–494.
- du Cros DL. Fibroblast growth factor and epidermal growth factor in hair development. J Invest Dermatol. 1993;101:106S–113S.
- Lin WH, Xiang LJ, Shi HX, et al. Fibroblast growth factors stimulate hair growth through β-catenin and Shh expression in C57BL/6 mice. Biomed Res Int. 2015;2015:730139.
- Yoon SY, Kim K-T, Jo SJ, et al. Induction of hair growth by insulin-like growth factor-1 in 1,763 mhz radiofrequency-irradiated hair follicle cells. PLoS ONE. 2011;6:e28474.
- Rossano F, Di Martino S, Iodice L, et al. Correlation between individual inflammation genetic profile and platelet rich plasma efficacy in hair follicle regeneration: a pilot study reveals prognostic value of IL-1a polymorphism. Eur Rev Med Pharmacol Sci. 2017;21:5247–5257.
- Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489–496.
- Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P–PRP) to leucocyte- and platelet-rich fibrin (LPRF). Trends Biotechnol. 2009;27:158–167.
- Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999;14:529–14535.
- Dohan Ehrenfest DM, de Peppo GM, Doglioli P, et al. Slow release of growth factors and thrombospondin-1 in Choukroun’s platelet-rich fibrin (PRF): A gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors. 2009;27:63–69.
- Marx RE, Carlson ER, Eichstaedt RM, et al. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–646.
- Li ZJ, Choi HI, Choi DK, et al. Autologous platelet-rich plasma: a potential therapeutic tool for promoting hair growth. Dermatol Surg. 2012;38:1040–1046.
- Alves R, Grimalt R. Randomized placebo-controlled, double-blind, half-head study to assess the efficacy of platelet-rich plasma on the treatment of androgenetic alopecia. Dermatol Surg. 2016;42:491–497.
- Ferrari M, Zia S, Valbonesi M, et al. A new technique for hemodilution, preparation of autologous platelet-rich plasma and intraoperative blood salvage in cardiac surgery. Int J Artif Organs. 1987;10:47–50.
- Anitua E, Sánchez M, Nurden AT, et al. New insights into and novel applications for platelet-rich fibrin therapies. Trends Biotechnol. 2006;24:227–234.
- Foster TE, Puskas BL, Mandelbaum BR, et al. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37:2259–2272.
- Mapar MA, Shahriari S, Haghighizadeh MH. Efficacy of platelet-rich plasma in the treatment of androgenetic (male-patterned) alopecia: a pilot randomized controlled trial. J Cosmet Laser Ther. 2016;18:452–455.
- Cole JP, Cole MA, Insalaco C, et al. Alopecia and platelet-derived therapies. Stem Cell Investig. 2017;4:88.
- Sorbellini E, Trink A, Rinaldi F. Experimental clinical assessment of the use of platelet-rich plasma in dermatology and rationale for its use in the treatment of non-scarring alopecia. Presented at the 35th La Medicina Estetica 4 October 2011.
- Trink A, Sorbellini E, Bezzola P, et al. A randomized, double-blind, placebo- and active-controlled, half-head study to evaluate the effects of platelet-rich plasma on alopecia areata. Br J Dermatol. 2013;169:690–694.
- Giordano S, Romeo M, di Summa P, et al. A meta-analysis on evidence of platelet-rich plasma for androgenetic alopecia. Int J Trichology. 2018;10:1–10.
- Reddy B, Jow T, Hantash BM. Bioactive oligopeptides in dermatology: Part I. Exp Dermatol. 2012;21:563–568.
- Schagen SK. Topical peptide treatments with effective anti-aging results. Cosmetics. 2017;4:16.
- Lintner K, Peschard O. Biologically active peptides: from a laboratory bench curiosity to a functional skin care product. Int J Cosmet Sci. 2000;22:207–218.
- Gorouhi F, Maibach HI. Role of topical peptides in preventing or treating aged skin. Int J Cosmet Sci. 2009;31:327–345.
- Snyder EL, Dowdy SF. Recent advances in the use of protein transduction domains for the delivery of peptides, proteins and nucleic acids in vivo. Expert Opin Drug Deliv. 2005;2:43–51.
- Pyo HK, Yoo HG, Won CH, et al. The effect of tripeptide-copper complex on human hair growth in vitro. Arch Pharm Res. 2007;30:834–839.
- Lee WJ, Sim HB, Jang YH, et al. Efficacy of a complex of 5-aminolevulinic acid and glycyl-histidyl-lysine peptide on hair growth. Ann Dermatol. 2016;28:438–443.
- Philp D, Nguyen M, Scheremeta B, et al. Thymosin beta4 increases hair growth by activation of hair follicle stem cells. Faseb J. 2004;18:385–387.
- Philp D, St-Surin S, Cha HJ, et al. Thymosin beta 4 induces hair growth via stem cell migration and differentiation. Ann N Y Acad Sci. 2007;1112:95–103.
- Ito C, Saitoh Y, Fujita Y, et al. Decapeptide with fibroblast growth factor (FGF)-5 partial sequence inhibits hair growth suppressing activity of FGF-5. J Cell Physiol. 2003;197:272–283.
- Bassino E, Zanardi A, Gasparri F, et al. Effects of the biomimetic peptide Sh-Polypeptide 9 (CG-VEGF) on cocultures of human hair follicle dermal papilla cells and microvascular endothelial cells. Exp Dermatol. 2016;25:237–239.
- Rinaldi F, Sorbellini E, Castiglioni M, et al. The role of mimicking growth factors to control anagen phase: evaluation in vitro and in vivo. JADD. 2010;62:AB74.
- Olsen EA, Hordinsky MK, Price VH, et al. Alopecia areata investigational assessment guidelines–Part II. National Alopecia Areata Foundation. J Am Acad Dermatol. 2004;51:440–447.
- Olsen E, Hordinsky M, McDonald-Hull S, et al. Alopecia areata investigational assessment guidelines. National Alopecia Areata Foundation. J Am Acad Dermatol. 1999;40:242–246.
- Olsen EA. Investigative guidelines for alopecia areata. Dermatol Ther. 2011;24:311–319.
- Price VH, Hordinsky MK, Olsen EA, et al. Subcutaneous efalizumab is not effective in the treatment of alopecia areata. J Am Acad Dermatol. 2008;58:395–402.
- Williamson D, Gonzalez M, Finlay AY. The effect of hair loss on quality of life. J Eur Acad Dermatol Venereol. 2001;15:137–139.
- Phillips TG, Slomiany WP, Allison R. Hair loss: common causes and treatment. Am Fam Physician. 2017;96:371–378.
- Singh S. Role of platelet-rich plasma in chronic alopecia areata: our centre experience. Indian J Plast Surg. 2015;48:57–59.
- Munki T. Platelet-rich plasma combined with intralesional triamcinolone acetonide for the treatment of alopecia areata: a case report. J Dermatol Dermatol Surg. 2016;20:87–90.
- Garg S, Manchanda S. Platelet-rich plasma-an 'Elixir' for treatment of alopecia: personal experience on 117 patients with review of literature. Stem Cell Investig. 2017;4:64.
- Greco J, Brandt R. The effects of autologous platelet rich plasma and various growth factors on non-transplanted miniaturized hair. Hair Transplant Forum Int. 2009;19:49–50.
- Donovan J. Successful treatment of corticosteroid-resistant ophiasis-type alopecia areata (AA) with platelet-rich plasma (PRP). J JAAD Case Rep. 2015;1:305–307.
- El Taieb MA, Ibrahim H, Nada EA, et al. Platelets rich plasma versus minoxidil 5% in treatment of alopecia areata: a trichoscopic evaluation. Dermatol Ther. 2017;30:1–6.
- Maquart FX, Pickart L, Laurent M, et al. Stimulation of collagen synthesis in fibroblast cultures by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+. FEBS Lett. 1988;238:343–346.
- Pickart L, Downey D, Lovejoy S, et al. Gly-l-his-l-lys:copper(II) – A human plasma factor with superoxide dismutase-like and wound-healing properties, In: Superoxide and Superoxide Dismutase. Edit Rotilio, Amsterdam: Elsevier, 1986. p. 555–558.
- Miller DM, DeSilva D, Pickart L, et al. Effects of glycyl-histidyl-lysyl chelated Cu(II) on ferritin dependent lipid peroxidation. Adv Exp Med Biol. 1990;264:79–84.
- Vinci C, Caltabiano V, Santoro AM, et al. Copper addition prevents the inhibitory effects of interleukin 1-beta on rat pancreatic islets. Diabetologia. 1995;38:39–45.
- McCormack MC, Nowak KC, Koch RJ. The effect of copper tripeptide and tretinoin on growth factor production in a serum-free fibroblast model. Arch Facial Plast Surg. 2001;3:28–32.
- Simeon A, Monier F, Emonard H, et al. Expression and activation of matrix metalloproteinases in wounds: modulation by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+. J Invest Dermatol. 1999;12:957–964.
- Simeon A, Wegrowski Y, Bontemps Y, et al. Expression of glycosaminoglycans and small proteoglycans in wounds: modulation by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu(2+). J Invest Dermatol. 2000;115:962–968.
- Simeon A, Emonard H, Hornebeck W, et al. The tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+ stimulates matrix metalloproteinase-2 expression by fibroblast cultures. Life Sci. 2000;18:2257–2665.
- Raju KS, Alessandri G, Gullino PM. Characterization of a chemoattractant for endothelium induced by angiogenesis effectors. Cancer Res. 1984;44:1579–1584.
- Pickart L, New metal peptide complexes and derivatives used for stimulating growth of hair in warm-blooded animals, especially humans, US Patent 5,120,831; Compositions for stimulating hair growth containing cupric complexes of peptide derivatives including. glycyl-l-histidyl-l-lysine n-octyl ester. US Patent 5,177,061; New glycyl-histidyl-lysyl copper compounds used in stimulating hair growth; US Patent 5,214,032; Metal-peptide compositions and methods for stimulating hair growth, US Patent 5,550,183.
- Uno H, Kurata S. Chemical agents and peptides affect hair growth. J Invest Dermatol. 1993;101:143S–147S.
- Chen P, Cescon M, Bonaldo P. Lack of collagen VI promotes wound-induced hair growth. J Invest Dermatol. 2015;135:2358–2367.
- Matsumura H, Mohri Y, Binh NT, et al. Hair follicle aging is driven by transepidermal elimination of stem cells via COL17A1 proteolysis. Science. 2016;351:aad395.
- Kruzel ML, Zimecki M, Actor JK, et al. Lactoferrin in a context of inflammation-induced pathology. Frontiers Immunol. 2017;8:1438.
- Zhang K, Letham DS, John PC. Cytokinin controls the cell cycle at mitosis by stimulating the tyrosine dephosphorylation and activation of p34cdc2-like H1 histone kinase. Planta. 1996;200:2–12.
- Tai CS, Chen YY, Chen WL. β-lactoglobulin influences human immunity and promotes cell proliferation. BioMed Res Int. 2016;2016:1.
- Geyfman M, Andersen B. Clock genes, hair growth and aging. Aging (Albany NY). 2010;2:122–128.