Abstract
Tumor necrosis factor (TNF) inhibitors account for a large proportion of drugs used to treat psoriasis and are indicated first-line options in certain settings. Several biosimilar drugs based on the anti-TNF agents adalimumab, infliximab, and etanercept are now available for use in patients with psoriasis. The favorable cost differential of biosimilars is expected to improve access to biologic therapy for biologic-naive psoriasis patients, who are often undertreated. Also, substantial cost savings can be made if patients are switched to biosimilars. To date, most clinical testing of anti-TNF biosimilars approved for use in psoriasis has been performed in patients with rheumatoid arthritis, and the results extrapolated to psoriasis. Although this may initially raise concerns for clinicians looking to start their psoriasis patients on biologic treatment with a biosimilar or switch from an original biologic to a biosimilar, the process of extrapolation is tightly regulated and scientifically justified. Furthermore, available real-world evidence of the safety and efficacy of anti-TNF agents in patients with psoriasis complements clinical trial data in patients with rheumatoid arthritis. When equipped with the appropriate knowledge, clinicians should have confidence to use biosimilars for the treatment of psoriasis.
Introduction
Biologic therapies, such as monoclonal antibodies and receptor fusion proteins targeting tumour necrosis factor (TNF) or interleukins (ILs), such as IL-12/23 or IL-17, have greatly improved the treatment options and outcomes for patients with moderate-to-severe psoriasis [Citation1,Citation2,Citation3]. Despite being significantly more effective than conventional systemic agents for psoriasis, the high cost of biologics may limit their use and contribute to inequalities of care [Citation1,Citation4]. Data suggest that patients with moderate-to-severe psoriasis who are currently receiving no systemic treatment or treatment with conventional systemic agents, such as methotrexate or acitretin, would benefit from biologic therapy [Citation4]. Under-treatment of psoriasis and accessibility to biologics are expected to improve with the availability of lower cost biosimilar agents [Citation1,Citation3].
Unlike generic copies of small-molecule drugs that are widely used as alternatives to more expensive originator products, the complex molecular structure and cell culture manufacturing process of biologics mean that they cannot be copied exactly [Citation5]. The European Medicines Agency (EMA) states that a “biosimilar is a biological medicinal product that contains a version of the active substance of an already authorised biological medicinal product (reference medicinal product) in the European Economic Area (EAA) [Citation6].” Biosimilar development is tightly regulated, with the EMA stating that “similarity to the reference medicinal product in terms of quality characteristics, biological activity, safety and efficacy based on a comprehensive comparability exercise needs to be established [6].” Likewise, the US Food and Drug Administration (FDA) states that a biosimilar must be “highly similar” to the reference product with “no clinically meaningful differences between the [biosimilar] product and the reference product in terms of the safety, purity, and potency of the product [Citation7].” Overall, the development of a biosimilar product can be as extensive as that of the reference product and is still associated with significant costs, but compared with the development process for reference products, there is more emphasis placed on physicochemical and functional characterisation of biosimilar drugs than on clinical testing [Citation5,Citation8].
The anti-TNF agents adalimumab, etanercept and infliximab were the first biologics to be approved in Europe for use in psoriasis [3]. Biosimilars based on these agents are now marketed for use in psoriasis (Table 1), and many more are in development [Citation1,Citation9]. To date, most clinical testing of anti-TNF biosimilars approved for use in psoriasis has been performed in patients with rheumatoid arthritis [Citation9], who represent a patient population sensitive enough to demonstrate even minor differences in immunogenicity between the biosimilar and the reference product [Citation10]. This is an example of extrapolation, whereby biosimilars are approved for use in indications of the reference product that were not directly studied in clinical trials using the biosimilar [Citation11]. Extrapolation reduces or eliminates the need for duplicative clinical studies, thereby expediting the developmental process [Citation11]. The EMA states that extrapolation must be “scientifically justified” and considered “in the light of the totality of the data, i.e. quality, non-clinical and clinical data [Citation6].” Clinical studies must be conducted in a patient population sensitive enough for the detection of differences in efficacy or safety between the biosimilar and the reference product [Citation6].
In this article, issues relevant to the rational and optimal integration of anti-TNF biosimilars into daily dermatology practice are reviewed, including the role of anti-TNF biologic agents in the treatment of psoriasis, quality attributes of anti-TNF biosimilars in relation to their reference products, and real-world experience with anti-TNF biosimilars in patients with psoriasis.
The biologic therapeutic landscape in psoriasis – role of anti-TNF
Available biologics
In addition to three well-established anti-tumor necrosis factor (TNF) agents (adalimumab, etanercept, and infliximab) and the recently approved anti-TNF agent certolizumab pegol, biologic agents approved in Europe for the treatment of psoriasis include agents targeting IL-17 (brodalumab, ixekizumab, and secukinumab), IL-23 (guselkumab), or IL-12/23 (ustekinumab) (Citation1,Citation12). Anti-TNF agents have been available for the treatment of psoriasis for more than 15 years and still account for a large proportion of drugs used to treat psoriasis (51.1% share of 2017 market sales of psoriasis drugs) (Citation3,Citation13). Biologic agents targeting IL-17 and IL-23 were introduced to the market relatively recently (Citation3) and contribute to the substantial cost of treatment, with ustekinumab and secukinumab accounting for 25.6% and 9.3% of 2017 market sales of psoriasis drugs, respectively (Citation13). Although biologic agents targeting ILs have been shown to be very effective in the treatment of psoriasis, long-term efficacy and safety data are relatively limited with these agents (Citation4,Citation14).
Treatment accessibility
Undertreatment is a significant problem in psoriasis, and the high cost of biologic therapy is an important contributing factor (Citation1,Citation15). In a 2011 National Psoriasis Foundation survey in the USA, up to 24% of patients with moderate-to-severe psoriasis were receiving no therapy, and up to 30% of these patients were receiving topical therapies alone (Citation15), which in many cases may not be sufficient. A retrospective study of patients treated with adalimumab, etanercept, ustekinumab, or secukinumab for psoriasis in a Spanish hospital from 2012 to 2016 reported a 30.7% increase in patients receiving biologic treatment, mainly adalimumab and ustekinumab, along with a 42% increase in costs over the time period (Citation16), reflecting the high costs of these drugs (Citation17).
In the last few years, patent expiration and the resultant loss of market exclusivity has facilitated the introduction of biosimilar agents based on the anti-TNF biologic agents etanercept, infliximab and, most recently in October 2018, adalimumab, to clinical care (Citation18–20). Rheumatology data from Sweden, where switching to biosimilars is not mandatory, show that when the etanercept and infliximab reference products went off patent, there was an increase in the overall use of biologic treatment, suggesting that biosimilars were not only replacing the reference products, but were increasing the overall rate of biologic therapy initiation (Citation21). This finding reflects the favorable cost differential of the anti-TNF biosimilars (Citation21). The recent introduction of adalimumab biosimilars to the European market provides further opportunity to improve cost savings and facilitate access to effective biologic therapy (Citation1,Citation18,Citation20). Compared with the current common practice of no or delayed biologic therapy in patients with moderate-to-severe psoriasis, earlier biologic intervention has the potential to improve long-term patient outcomes (Citation22).
Drug survival
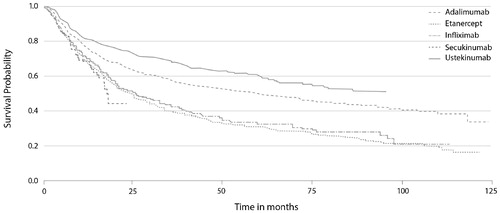
Psoriasis is a chronic disease requiring effective long-term treatment to manage the debilitating consequences, and societal and economic burden of disease (Citation23,Citation24). However, drug survival data from the DERMBIO registry, which contains data on all Danish patients with moderate-to-severe psoriasis treated with biologics, indicate that the investigated biologic drugs (adalimumab, etanercept, infliximab, secukinumab, and ustekinumab) may eventually all lose efficacy () (Citation2). Anti-TNF agents (reference or biosimilar) may therefore be useful in patients experiencing loss of response to biologic agents targeting ILs after long-term use, and vice versa.
Figure 1. The Kaplan–Meier plot of drug survival in patients with moderate-to-severe psoriasis from the DERMBIO registry treated with biologics (Citation2).
Anti-TNF from a comorbidity perspective
Psoriasis affects multiple systems in addition to the skin and is therefore associated with an increased risk of various comorbid diseases (Citation23,Citation25). Treatment should be personalized to address comorbid conditions (Citation25). Anti-TNF biologic agents are the recommended first-line treatment of choice for several patient groups, including those with comorbid psoriatic arthritis (PsA) or IBD (Citation25). In patients with comorbid PsA, which affects approximately 20% of patients (Citation26), ustekinumab may be used as an alternative to anti-TNF agents if psoriasis is severe and arthritis is mild, and IL-17 inhibitors should be considered in patients who do not respond to TNF inhibitors (Citation25). Ustekinumab may also be used in patients with Crohn’s disease, whereas secukinumab and ixekizumab should be used cautiously or avoided, and brodalumab should be avoided (Citation25).
The evolving landscape of biosimilars – from quality expectations to uninterrupted supply
Biopharmaceutical process modification and variability of biologics
The structural complexity of biologic agents means that even minor alterations in production processes and storage conditions can affect product consistency and cause drifts in quality attributes (Citation27). As a result of intentional changes in production processes, as well as the inevitable endogenous drift inherent to biological systems, quality attributes of the reference products on which biosimilars are based have changed over time and batch-to-batch variability can occur (Citation27–30). Different batches of reference biologic products can be found on the market at the same time and can be used interchangeably (Citation29).
Intentional biologic product manufacturing changes are tightly regulated, and comparability exercises must be performed to show that pre- and post-manufacturing change quality attributes are sufficiently similar to indicate that there will be no impact on clinical efficacy or safety (Citation30,Citation31). Nevertheless, compared with the requirements for demonstrating biosimilarity, less sophisticated methods and less comprehensive characterizations are required to demonstrate batch-to-batch comparability of a biologic product that has undergone a manufacturing change (Citation32,Citation33).
Demonstrating anti-TNF biosimilarity
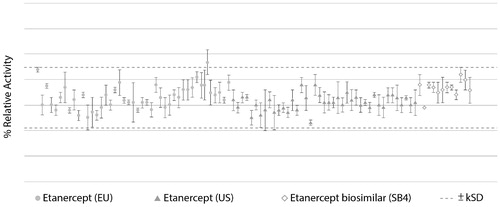
Critical quality attributes (CQAs) of biosimilars are selected based on criticality ranking of quality attributes in relation to their potential impact on immunogenicity, safety, pharmacokinetics, and efficacy (Citation34). For example, the neutralization effect of TNF binding is a CQA of anti-TNF biosimilars (Citation33,Citation35). To ensure that there are no clinically relevant differences between the biosimilar and the reference product, and reduce the need for clinical testing, CQAs must be within similarity ranges that account for batch-to-batch variability of the reference product in recent years (Citation29,Citation32). For example, the results of TNF neutralization assays, which reflect the potency of anti-TNF biosimilar products, should be within the similarity range defined for the anti-TNF reference product, as demonstrated during comparability exercises involving etanercept biosimilar SB4 in relation to multiple batches of EU- and US-sourced product () (Citation35). It is also very important to assess CQAs that can affect the pharmacokinetic profile of anti-TNF biosimilars, such as neonatal Fc receptor binding in the case of monoclonal antibodies, and ensure they are within the similarity range of the reference product (Citation33,Citation36).
Figure 2. Comparison of TNF neutralization activity of SB4 and etanercept reference product (40 lots of EU-sourced product and 40 lots of US-sourced product), with the dotted line indicating the similarity range based on results of etanercept reference product obtained from the EU (Citation35).
Although the characterization of biosimilars in relation to CQAs affecting efficacy and pharmacokinetics is very important, the primary concern with biosimilars is a theoretical risk of immunogenicity, whereby anti-drug antibodies and neutralizing antibodies can adversely affect pharmacokinetics, safety, and efficacy (Citation29,Citation37). However, full characterization of biosimilars using state-of-the-art technology reduces this risk. For example, aggregation of biologic proteins increases the risk of immunogenicity, and levels of aggregate can now be determined by analytical methods, so similar or lower levels of aggregate in the biosimilar compared with the reference product should be demonstrated (Citation29,Citation32). Moreover, in contrast to the last 10–15 years, assays are now available that sensitively characterize antidrug antibodies and neutralizing antibodies in serum samples. Thus, the clinical immunogenicity of biosimilars can be assessed relatively reliably (Citation38). For example, results from a study comparing specific antigenic epitopes between CT-P13 and infliximab reference product have recently been reported, and suggest the two drugs have similar epitope binding profiles and equivalent immunogenicity (Citation39).
Although the overall structural, physicochemical and biological quality attributes of the etanercept biosimilar SB4 were shown to be highly similar to the reference product during extensive comparability exercises using state-of-the-art methods, SB4 was found to contain lower levels of potentially immunogenic high molecular weight aggregate (i.e. not within the similarity range of the reference product) (Citation35). It was hypothesized that this could be beneficial in terms of safety and immunogenicity (Citation35). In line with this hypothesis, equivalent efficacy was demonstrated for SB4 and etanercept reference product in relation to the American College of Rheumatology (ACR) 20 response at 24 weeks in a phase III trial of patients with rheumatoid arthritis, but SB4 was associated with significantly fewer injection-site reactions up to week 52, and less immunogenicity than the reference product (Citation40,Citation41). These findings emphasize the importance of publishing biosimilar quality data to better understand biosimilar clinical trial data.
Importance of supply
In the event of a drug shortage, whereby the company cannot supply the market with recently produced drugs, drug batches with different expiry dates and potentially different quality profiles and activities will be on the market. The importance of pharmacovigilance at batch level, and the importance of batch traceability are reflected in the EMA assessment of the infliximab biosimilar CT-P13, in which it was reported that the protein content of the reference product fell below the proposed CT-P13 end-of-shelf-life specification during 12 months of stability testing (Citation42). This was attributed to the fact that the reference product batches were older than the CT-P13 batches when the study was initiated (Citation42). Rigorous post-marketing pharmacovigilance using biosimilar registries is important to monitor the long-term efficacy and safety of anti-TNF biosimilars. In relation to this, prescribers and pharmacists should ensure adequate registration of the biosimilar brand name, batch number, and expiration date.
Biosimilars in daily practice – real-world evidence
With its favorable and well-established safety profile, reliable efficacy and flexibility of dosing, etanercept, which was one of the first biologic drugs approved for use in psoriasis, is still a very important treatment option for patients with psoriasis (Citation43). SB4 was the first biosimilar of etanercept to be authorized for marketing in the EU (Citation35). Real-world data demonstrating the effectiveness and safety of SB4 in patients with psoriasis are now emerging from various national registries to complement clinical trial data in patients with rheumatoid arthritis (Citation2,Citation44,Citation45). Real-world evidence for the safety and effectiveness of SB4 in patients with psoriasis is, however, currently limited to short-term data (6 months) (Citation44,Citation45). Longer-term safety and efficacy monitoring of large populations is necessary and ongoing (Citation44,Citation45). Limited real-world data are also available for the infliximab biosimilar CT-P13 in patients with psoriasis (Citation2,Citation46,Citation47). Adalimumab biosimilars have only very recently entered the European market (Citation20), so there is no substantial real-world data with any of these drugs.
National registry data
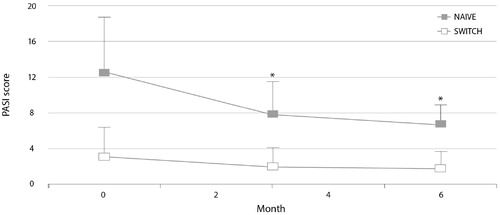
The PsoBiosimilars Registry is an Italian registry of anti-TNF biosimilars in psoriasis and PsA (Citation45,Citation47). As of July 2018, 39 centers had registered a total of 197 patients treated with SB4 (158 patients switched from reference etanercept and 39 etanercept-naive patients). Over 6 months of treatment, the Psoriasis Area and Severity Index (PASI) score was significantly reduced in the etanercept-naive patients, and efficacy was maintained in patients switched from reference etanercept to SB4 () (Citation45). A PASI 75 response occurred in 20 (51%) etanercept-naive patients. There was no significant difference in the number of adverse events between switch patients and etanercept-naive patients. Similar observations from the PsoBiosimilars Registry have been reported for the infliximab biosimilar CT-P13 (Citation47).
Figure 3. Psoriasis Area and Severity Index (PASI) scores during 6 months of treatment with etanercept biosimilar SB4 in patients from the PsoBiosimilars registry switched from reference etanercept and in etanercept-naive patients (Citation45). *p<.05 vs. baseline.
PASI data from the British BADBIR registry also indicate that SB4 is an effective treatment option in patients with psoriasis (Citation44). Of 92 patients who started SB4 in the first half of 2017 for a mean treatment period of 322.3 ± 127.1 days (five patients were switched from reference etanercept), nine patients (9.8%) discontinued treatment (mean treatment time to discontinuation of 137 ± 71.9 days). This was due to lack of effectiveness (n = 6), adverse events (n = 2) or a combination of these problems (n = 1). None of the five patients who switched from reference etanercept to SB4 discontinued treatment during the observation period.
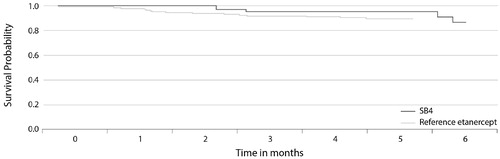
Analysis of data from the Danish DERMBIO registry showed that switching patients with moderate-to-severe psoriasis from reference etanercept to SB4 had no significant impact on drug survival () (Citation2). The hazard ratio (HR) for risk of discontinuation of SB4 versus reference etanercept was 0.46 (p=.297). There was also no significant difference in the risk of discontinuation of CT-P13 versus reference infliximab (HR 1.64; p=.264). In these analyses, a total of 147, 114, 44, and 34 treatment series were included for etanercept, infliximab, SB4, and CT-P13, respectively.
Figure 4. The Kaplan–Meier plot of drug survival in patients with moderate-to-severe psoriasis in the DERMBIO registry treated with reference etanercept or switched from reference etanercept to etanercept biosimilar SB4 (Citation2).
Clinical trial switching data
A recent systematic review of more than 90 studies, including randomized clinical trials and observational studies providing real-world evidence, in 14,225 patients in 14 disease indications, including psoriasis, suggested that patients can be switched from a reference biologic to a biosimilar in any indication without concerns for safety or loss of efficacy (Citation48). The greatest amount of clinical trial switching data for adalimumab, etanercept, and infliximab biosimilars are in patients with rheumatoid arthritis (Citation49–53), but studies have also been conducted with adalimumab and etanercept biosimilars in patients with psoriasis (Citation54–56). Patients with psoriasis were also included in the NOR-SWITCH study, assessing a switch from reference infliximab to CT-P13 versus continued treatment with reference infliximab, but the study was not powered to demonstrate noninferiority of the switch for separate indications (Citation57). Collectively, all of these studies, including those conducted in other indications, supported a switch from reference product to biosimilar in patients with psoriasis ().
Table 1. Biosimilars with EMA approval for the treatment of moderate-to-severe psoriasis (current October 2018) (Citation1, Citation58, Citation59).
Although there may be concerns that the majority of pivotal trials with anti-TNF biosimilars have not been conducted in patients with psoriasis (Citation1,Citation9), the European biosimilar development and approval process, based on the totality of the evidence and appropriate scientific justification (Citation6), means that if an anti-TNF biosimilar drug is shown to be effective in rheumatoid arthritis it is also likely to be effective in psoriasis. National registry data for the etanercept biosimilar SB4 and the infliximab biosimilar CT-P13 support this assertion (Citation44,Citation45,Citation47). Although there are currently no real-world data for adalimumab biosimilars in patients with psoriasis, robust, randomized, double-blind, switching evidence for these drugs comes from phase III studies of ABP 501 and GP2017 in patients with psoriasis (Citation54,Citation56), and BI 695501 and SB5 in patients with rheumatoid arthritis (Citation49,Citation53).
Conclusions
Anti-TNF biosimilars approved by regulatory authorities can be a safe and effective treatment option for patients with psoriasis. In the face of ever-increasing healthcare costs and cost-cutting initiatives, switching patients from reference adalimumab, etanercept, or infliximab to a lower cost biosimilar, and starting biologic-naive patients on the best-value biologic can lower prices for patients and payers without compromising quality of care. Cost savings from use of anti-TNF biosimilars provide an opportunity for physicians to prescribe effective biologic therapy for more patients with moderate-to-severe psoriasis who could benefit from it. However, to realize the cost-saving potential of biosimilars in patients with psoriasis, clinicians must have the confidence to use them. Educating clinicians about the scientific principles underlying biosimilar development and approval, and real-world clinical experience with these drugs in patients with psoriasis may help them to make informed treatment decisions and lead to wider use of biologics in clinical practice (Citation1).
Acknowledgements
The authors acknowledge Weber Shandwick Hong Kong for editorial support in the preparation of this manuscript.
Disclosure statement
Prof. Jonathan Barker has attended advisory boards, received research funding, and/or spoken at sponsored symposia within the past 5 years from: Abbvie, Almirall, Amgen, Boehringer-Ingelheim, Celgene, Creabilis, Janssen, Leo, Lilly, Novartis, Pfizer, Samsung Bioepis, Sienna, and Sun.
Prof. Giampiero Girolomoni has been principal investigator in clinical trials sponsored by and/or has received personal fees from AbbVie, Abiogen, Almirall, Amgen, Bayer, Biogen, Celgene, Eli-Lilly, Genzyme, Leo Pharma, Menlo therapeutics, Merck, MSD, Novartis, Pfizer, Pierre Fabre, Regeneron, Samsung, Sandoz, Sanofi, and Sun Pharma.
Prof. Alexander Egeberg has received research funding from Pfizer, Eli Lilly, the Danish National Psoriasis Foundation and the Kgl Hofbundtmager Aage Bang Foundation, and honoraria as a consultant and/or speaker from Almirall, Leo Pharma, Samsung Bioepis Co., Ltd., Pfizer, Eli Lilly, Novartis, Galderma, and Janssen Pharmaceuticals.
Prof. Joao Goncalves has provided consultancy services to Sandoz, Celltrion, Samsung, Novartis, Pfizer, Amgen, and Sanofi.
Dr. Burkhard Pieper and Taegyun Kang are employees of Biogen and Samsung Bioepis, respectively.
Additional information
Funding
References
- Carrascosa J-M, Jacobs I, Petersel D, et al. Biosimilar drugs for psoriasis: principles, present, and near future. Dermatol Ther (Heidelb). 2018;8:173–194.
- Egeberg A, Ottosen MB, Gniadecki R, et al. Safety, efficacy and drug survival of biologics and biosimilars for moderate-to-severe plaque psoriasis. Br J Dermatol. 2018;178:509–519.
- Rønholt K, Iversen L. Old and new biological therapies for psoriasis. Int J Mol Sci. 2017;18. DOI: 10.3390/ijms18112297
- Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2017;12:CD011535.
- Declerck P, Farouk Rezk M. The road from development to approval: evaluating the body of evidence to confirm biosimilarity. Rheumatology (Oxford, England). 2017;56:iv4–iv13.
- European Medicines Agency. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues. London: Committee for Medicinal Products for Human Use; 2014.
- US Food Drug Administration Center for Drug Evaluation Research Center for Biologics Evaluation Research. Scientific considerations in demonstrating biosimilarity to a reference product: guidance for industry. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER); 2015.
- GBI Research. Biosimilar development: the incentives and challenges. GBI Research; 2017.
- Blauvelt A, Puig L, Chimenti S, et al. Biosimilars for psoriasis: clinical studies to determine similarity. Br J Dermatol. 2017;177:23–33.
- Castañeda-Hernández G, González-Ramírez R, Kay J, et al. Biosimilars in rheumatology: what the clinician should know. RMD Open. 2015;1:e000010.
- Tesser JR, Furst DE, Jacobs I. Biosimilars and the extrapolation of indications for inflammatory conditions. BTT. 2017;11:5–11.
- European Medicines Agency. Assessment report: Cimzia. EMA/502221/2018; 2018.
- Mihel I, Huntley C, Kurniawan A. Psoriatic arthritis market and forecast analysis 2035. London: Datamonitor Healthcare; 2018.
- Bilal J, Berlinberg A, Bhattacharjee S, et al. A systematic review and meta-analysis of the efficacy and safety of the interleukin (IL)-12/23 and IL-17 inhibitors ustekinumab, secukinumab, ixekizumab, brodalumab, guselkumab and tildrakizumab for the treatment of moderate to severe plaque psoriasis. J Dermatol Treatment. 2018;29:569–578.
- Armstrong AW, Robertson AD, Wu J, et al. Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: findings from the National Psoriasis Foundation Surveys, 2003–2011. JAMA Dermatol. 2013;149:1180–1185.
- Selvi P, Pérez OM, Madroñal IC, et al. 4CPS-134 Evolution and analysis of spending on biological medication in psoriasis. Section 4: clinical pharmacy services. BMJ. 25:A104.1–A104.
- Burgos-Pol R, Martínez-Sesmero JM, Ventura-Cerdá JM, et al. Coste de la psoriasis y artritis psoriásica en cinco países de Europa: una revisión sistemática [The cost of psoriasis and psoriatic arthritis in 5 European countries: a systematic review]. Actas Dermo-Sifiliograficas. 2016;107:577–590.
- Aideed H. The European biosimilars landscape: what to expect in the year ahead; 2018; [cited 2018 Dec]. Available from: https://www.biosimilardevelopment.com/doc/the-european-biosimilars-landscape-what-to-expect-in-the-year-ahead-0001
- Calo-Fernández B, Martínez-Hurtado JL. Biosimilars: company strategies to capture value from the biologics market. Pharmaceuticals (Basel, Switzerland). 2012;5:1393–1408.
- Morriss E. Adalimumab biosimilars launched as Humira patent expires; 2018; [cited 2018 Dec]. Available from: https://pharmafield.co.uk/pharma_news/adalimumab-biosimilars-launched-humira/
- Di Giuseppe D, Frisell T, Ernestam S, et al. Uptake of rheumatology biosimilars in the absence of forced switching. Expert Opin Biol Ther. 2018;18:499–504.
- Girolomoni G, Griffiths CEM, Krueger J, et al. Early intervention in psoriasis and immune-mediated inflammatory diseases: a hypothesis paper. J Dermatol Treatment. 2015;26:103–112.
- Feldman SR, Zhao Y, Shi L, et al. Economic and comorbidity burden among patients with moderate-to-severe psoriasis. JMCP. 2015;21:874–888.
- Löfvendahl S, Petersson IF, Theander E, et al. Incremental costs for psoriasis and psoriatic arthritis in a population-based cohort in southern Sweden: is it all psoriasis-attributable morbidity? J Rheumatol. 2016;43:640–647.
- Amin M, No DJ, Egeberg A, et al. Choosing first-line biologic treatment for moderate-to-severe psoriasis: what does the evidence say? Am J Clin Dermatol. 2018;19:1–13.
- Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2018;80(1):251–265.e19.
- Kim S, Song J, Park S, et al. Drifts in ADCC-related quality attributes of Herceptin®: impact on development of a trastuzumab biosimilar. mAbs. 2017;9:704–714.
- Cline A, Feldman SR. Biologics are too complicated to duplicate: should we be worried about biosimilars? Br J Dermatol. 2018;179:557–558.
- Gonçalves J, Araújo F, Cutolo M, et al. Biosimilar monoclonal antibodies: preclinical and clinical development aspects. Clin Exp Rheumatol. 2016;34:698–705.
- Vezér B, Buzás Z, Sebeszta M, et al. Authorized manufacturing changes for therapeutic monoclonal antibodies (mAbs) in European Public Assessment Report (EPAR) documents. Curr Med Res Opin. 2016;32:829–834.
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH harmonised tripartite guideline comparability of biotechnological/biological products subject to changes in their manufacturing process Q5E; 2004.
- European Medicines Agency. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substances: quality issues (revision 1). London: Committee for Medicinal Products for Human Use (CHMP); 2014.
- Hong J, Lee Y, Lee C, et al. Physicochemical and biological characterization of SB2, a biosimilar of Remicade® (infliximab). mAbs. 2017;9:364–382.
- Vandekerckhove K, Seidl A, Gutka H, et al. Rational selection, criticality assessment, and tiering of quality attributes and test methods for analytical similarity evaluation of biosimilars. AAPS J. 2018;20:68.
- Cho IH, Lee N, Song D, et al. Evaluation of the structural, physicochemical, and biological characteristics of SB4, a biosimilar of etanercept. mAbs. 2016;8:1136–1155.
- Abdiche YN, Yeung YA, Chaparro-Riggers J, et al. The neonatal Fc receptor (FcRn) binds independently to both sites of the IgG homodimer with identical affinity. mAbs. 2015;7:331–343.
- Araújo FC, Fonseca JE, Goncalves J. Switching to biosimilars in inflammatory rheumatic conditions: current knowledge. EMJ Rheumatol. 2018;5:66–74.
- Emery P, Vencovský J, Kang JW, et al. Confirmation on the immunogenicity assay used in the SB4 phase III study: response to the comments by Meacci et al. Ann Rheum Dis. 2016;75:e40.
- Goncalves J, Santos M, Acurcio R, et al. Antigenic response to CT-P13 and infliximab originator in inflammatory bowel disease patients shows similar epitope recognition. Aliment Pharmacol Ther. 2018;48:507–522.
- Girolomoni G, Feldman SR, Emery P, et al. Comparison of injection-site reactions between the etanercept biosimilar SB4 and the reference etanercept in patients with rheumatoid arthritis from a phase III study. Br J Dermatol. 2018;178:e215–e216.
- Emery P, Vencovský J, Sylwestrzak A, et al. 52-week results of the phase 3 randomized study comparing SB4 with reference etanercept in patients with active rheumatoid arthritis. Rheumatology (Oxford, England). 2017;56:2093–2101.
- European Medicines Agency, Committee for Medicinal Products for Human Use. Assessment report: Remsima. EMA/CHMP/589317/2013; 2013.
- Prinz JC, Puig L, Girolomoni G. Treatment of psoriasis with etanercept: the typical patient profile. J Eur Acad Dermatol Venereol. 2016;30:1092–1099.
- Egeberg A. Real world SB4 (etanercept biosimilar) use in patients with psoriasis: data from British Association of Dermatologist Biologic Interventions Registry (BADBIR): Abstract #P1839. 27th EADV Congress; Paris, France; 2018.
- Gisondi P, Bianchi L, Calzavara-Pinton P, et al. Etanercept biosimilar SB4 in the treatment of chronic plaque psoriasis: data from the Psobiosimilars registry. Br J Dermatol. 2019;180(2):409–410.
- Dapavo P, Vujic I, Fierro MT, et al. The infliximab biosimilar in the treatment of moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:736–739.
- Gisondi P, Bianchi L, Conti A, et al. Infliximab biosimilar CT-P13 in the treatment of chronic plaque psoriasis: data from the Psobiosimilars registry. Br J Dermatol. 2017;177:e325–e326.
- Cohen HP, Blauvelt A, Rifkin RM, et al. Switching reference medicines to biosimilars: a systematic literature review of clinical outcomes. Drugs. 2018;78:463–478.
- Cohen SB, Alonso-Ruiz A, Klimiuk PA, et al. Similar efficacy, safety and immunogenicity of adalimumab biosimilar BI 695501 and Humira reference product in patients with moderately to severely active rheumatoid arthritis: results from the phase III randomised VOLTAIRE-RA equivalence study. Ann Rheum Dis. 2018;77:914–921.
- Emery P, Vencovský J, Sylwestrzak A, et al. Long-term efficacy and safety in patients with rheumatoid arthritis continuing on SB4 or switching from reference etanercept to SB4. Ann Rheum Dis. 2017. DOI:10.1136/annrheumdis-2017-211591
- Smolen JS, Choe J-Y, Prodanovic N, et al. Safety, immunogenicity and efficacy after switching from reference infliximab to biosimilar SB2 compared with continuing reference infliximab and SB2 in patients with rheumatoid arthritis: results of a randomised, double-blind, phase III transition study. Ann Rheum Dis. 2018;77:234–240.
- Yoo DH, Prodanovic N, Jaworski J, et al. Efficacy and safety of CT-P13 (biosimilar infliximab) in patients with rheumatoid arthritis: comparison between switching from reference infliximab to CT-P13 and continuing CT-P13 in the PLANETRA extension study. Ann Rheum Dis. 2017;76:355–363.
- Weinblatt ME, Baranauskaite A, Dokoupilova E, et al. Switching from reference adalimumab to SB5 (adalimumab biosimilar) in patients with rheumatoid arthritis: fifty-two-week phase III randomized study results. Arthritis Rheumatol. 2018;70:832–840.
- Blauvelt A, Lacour J-P, Fowler JF, et al. Phase III randomized study of the proposed adalimumab biosimilar GP2017 in psoriasis: impact of multiple switches. Br J Dermatol. 2018;179:623–631.
- Griffiths CEM, Thaçi D, Gerdes S, et al. The EGALITY study: a confirmatory, randomized, double-blind study comparing the efficacy, safety and immunogenicity of GP2015, a proposed etanercept biosimilar, vs. the originator product in patients with moderate-to-severe chronic plaque-type psoriasis. Br J Dermatol. 2017;176:928–938.
- Papp K, Bachelez H, Costanzo A, et al. Clinical similarity of the biosimilar ABP 501 compared with adalimumab after single transition: long-term results from a randomized controlled, double-blind, 52-week, phase III trial in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2017;177:1562–1574.
- Jørgensen KK, Olsen IC, Goll GL, et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet (London, England). 2017;389:2304–2316.
- Generics Biosimilars Initiative. Adalimumab and trastuzumab biosimilars gain EC approval; 2018 [cited 2018 Dec]. Available from: http://www.gabionline.net/Biosimilars/News/Adalimumab-and-trastuzumab-biosimilars-gain-EC-approval
- Generics Biosimilars Initiative. EC approval for adalimumab and pegfilgrastim biosimilars Hulio and Udenyca; 2018 [cited 2018 Dec]. Available from: http://www.gabionline.net/Biosimilars/News/EC-approval-for-adalimumab-and-pegfilgrastim-biosimilars-Hulio-and-Udenyca
