Psoriasis patients want drugs that are highly effective and that work fast. Understanding the relative differences between the many available agents is important. In head-to-head studies to analyze these differences, response curves are generated. These curves provide information on overall efficacy and the speed of response. The interpretation of these curves may be analogous to the interpretation of pharmacological dose-response curves.
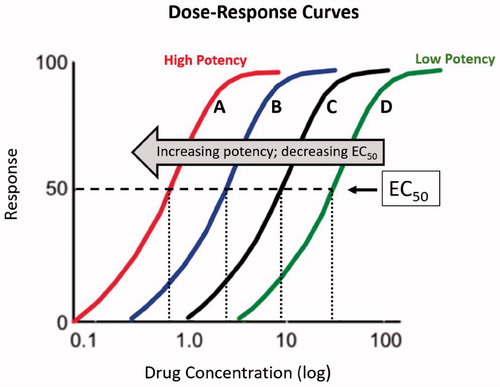
In dose-response curves, the independent variable (x-axis) is typically drug concentration, and the dependent variable (y-axis) is response rate. The results form sigmoidal, or “S” shaped, curves that reflect the strength of drugs in pharmacological studies (). The curve parameters are used to measure efficacy (a measure of maximal response) and potency (the concentration of drug that achieves a given efficacy level). The maximal height along the y-axis defines efficacy. The concentration of a drug, measured on the x-axis, which induces 50% response is the half maximal effective concentration (EC50) and is a measure of potency. The curve of more potent drugs is leftward of a less potent agent ().
Figure 1. Dose-response curves for drugs A-D. Increasing drug potency decreases half maximal effective concentration (EC50).
The ECLIPSE study recently compared the efficacy of guselkumab and secukinumab for the treatment of adults with plaque psoriasis. Psoriasis Area of Severity Index (PASI) 90 response over time in this study produces sigmoidal curves. Guselkumab had greater efficacy in PASI 90 response (84.5%) compared to secukinumab (70.0%) after 48 weeks of treatment (Citation1). The efficacy of both drugs is similar at weeks 16 through 20, and then favors guselkumab to week 48. At earlier time points, the secukinumab curve is shifted to the left corresponding to faster PASI 90 response for secukinumab at weeks 4 and 8. Using 50% response in PASI90 (which we can abbreviate PASI9050 analogous to EC50) as the measure of speed of response, secukinumab acts between one and two weeks faster than guselkumab ().
Figure 2. PASI 90 response over time in ECLIPSE study (Citation2). The efficacy of both drugs is similar at weeks 16 through 20, and then favors guselkumab to week 48. Speed of response illustrated using PASI9050, favors secukinumab by more than one week.
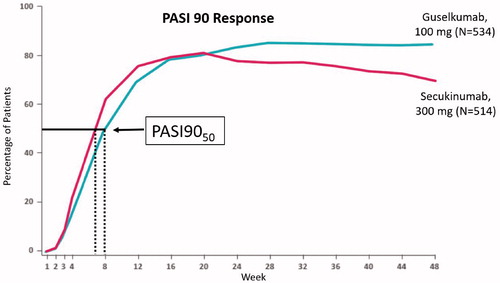
Figure 3. Concentration-response curves illustrating the concept of potency. For a response of 25%, Drug B is more potent. For a response of 75%, Drug A is more potent.
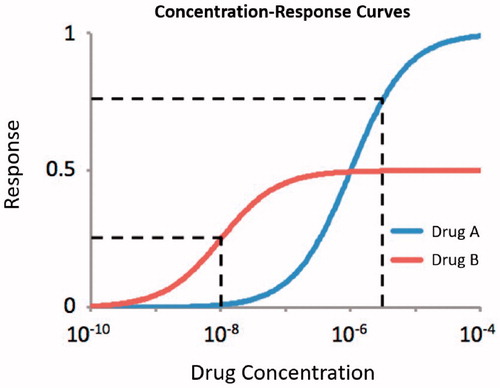
Figure 4. PASI 75 response over time in FIXTURE study.3 Green and orange arrows indicate weeks to PASI7550 for secukinumab 300 mg and etanercept respectively. Secukinumab 300 mg achieved PASI7550 in less than 6 weeks compared to more than 13 weeks for etanercept.
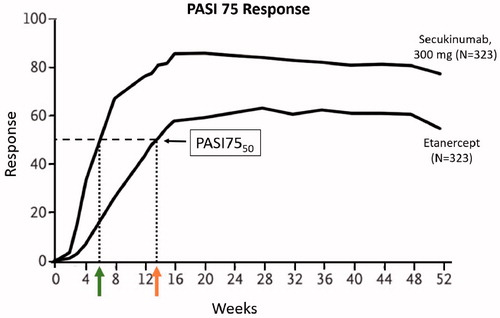
Clinical trials may compare drugs with different maximal response (efficacy). The speed of response can be defined using a response intensity independent of maximal response (). Potency (i.e. speed of response) is an imprecise term and should always be further defined. In , comparison of the speed of response for Drug A and B is possible if a 25% response is used to define the speed of response. Defining the speed of response using a 75% response does not allow comparison, as Drug B does not have the efficacy to reach that response. If each drug in a study achieves at least 50% response, time to 50% response can be used to compare the speed of response for curves with different maximal efficacy. The FIXTURE study compared secukinumab and etanercept, which have different efficacy (Citation3). In this study secukinumab 300 mg achieved PASI7550 in less than 6 weeks compared to more than 13 weeks for etanercept ().
Efficacy and the speed of improvement are important to patients. These two parameters can be assessed from graphs of clinical trial data. The height of the curve tells us overall efficacy; the time to 50% improvement can give us a measure of the speed of response that can be used to compare how fast drugs work.
Center for Dermatology Research, Department of Dermatology, Wake Forest School of Medicine, Winston-Salem, NC, USA
[email protected]
Steven R. Feldman
Center for Dermatology Research, Department of Dermatology, Wake Forest School of Medicine, Winston-Salem, NC, USA
Department of Pathology, Wake Forest School of Medicine, Winston-Salem, NC, USA
Department of Social Sciences & Health Policy, Wake Forest School of Medicine, Winston-Salem, NC, USA
Department of Dermatology, University of Southern Denmark, Odense, Denmark
Disclosure statement
Feldman has received research, speaking and/or consulting support from a variety of companies including Galderma, GSK/Stiefel, Almirall, Leo Pharma, Boehringer Ingelheim, Mylan, Celgene, Pfizer, Valeant, Abbvie, Samsung, Janssen, Lilly, Menlo, Merck, Novartis, Regeneron, Sanofi, Novan, Qurient, National Biological Corporation, Caremark, Advance Medical, Sun Pharma, Suncare Research, Informa, UpToDate and National Psoriasis Foundation. He is founder and majority owner of http://www.DrScore.com and founder and part owner of Causa Research, a company dedicated to enhancing patients’ adherence to treatment. Jeremy G. Light and Wasim Haidari have no conflicts to disclose.
References
- Langley R, Blauvelt A, Armstrong A, et al. Guselkumab demonstrates superior long-term responses to secukinumab at week 48 in the treatment of moderate to severe psoriasis: results from the ECLIPSE trial. Poster presented at: 3rd Inflammatory Skin Disease Summit; 2018 December 12–15; Vienna, Austria.
- Langley R, Blauvelt A, Armstrong A, et al. Proportion of patients achieving a PASI 90 response (with 95% confidence interval) through week 48 by visit. Guselkumab comparison to secukinumab in the treatment of adults with moderate to severe plaque psoriasis. Janssen Biotech, Inc. [cited 2019 Jun 27].
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis – results of two phase 3 trials. N Engl J Med. 2014;371:326–338.
