Abstract
Introduction
In VOYAGE 1 (NCT02207231) and VOYAGE 2 (NCT02207244), guselkumab, an interleukin-23 blocker, was safe and effective in patients with moderate-to-severe plaque psoriasis.
Methods
Patients who self-identified as Hispanic (n = 117) or non-Hispanic (n = 1686) were randomized to guselkumab, placebo, or adalimumab. Efficacy assessments included Psoriasis Area and Severity Index (PASI), Investigator’s Global Assessment (IGA), and Dermatology Life Quality Index (DLQI).
Results
At week 16, treatment differences for guselkumab versus placebo in the Hispanic and non-Hispanic populations were 67.4 (95% confidence interval 50.4, 84.4) and 77.2 (73.5, 80.8) percentage points for IGA 0/1 and 59.2 (41.9, 76.4) and 69.2 (65.7, 72.7) percentage points for PASI 90, respectively. Treatment differences for guselkumab versus adalimumab were 25.9 (6.5, 45.3) and 17.5 (12.8, 22.3) percentage points for IGA 0/1 and 21.4 (–0.1, 42.9) and 23.5 (18.2, 28.9) percentage points for PASI 90, respectively. Week 24 results were similar. Adverse event frequency was greater in adalimumab- versus guselkumab-treated patients in the Hispanic population only through weeks 16 and 28. In both populations, DLQI 0/1 responses were greater in guselkumab-treated versus placebo- and adalimumab-treated patients at week 16 and versus adalimumab-treated patients at week 24.
Conclusions
Guselkumab safety and efficacy were consistent between Hispanic and non-Hispanic populations.
Keywords:
Introduction
Psoriasis is a common, chronic disease that negatively impacts health-related quality of life (HRQoL) (Citation1). Psoriasis affects many ethnic groups worldwide, with prevalence in the USA highest in Caucasians or non-Hispanic whites (3.6% to 3.7%), followed by Black (2.0%) and Hispanic (1.6%) individuals (Citation2,Citation3). However, psoriasis is more frequently undiagnosed in nonwhite individuals, and therefore the prevalence of psoriasis in the Black and Hispanic populations is likely underestimated (Citation4–6). The genetic susceptibility of psoriasis also varies with ethnic groups, although differences are more commonly documented in the Asian population in contrast to the Black and Hispanic populations (Citation6).
Psoriasis has generally been understudied in the Hispanic population, despite the fact that people of Hispanic ethnicity are the largest ethnic minority in the USA, constituting 17.8% of the nation’s total population (Citation7). The limited data available suggest that, compared with patients who are non-Hispanic, psoriasis in patients who are Hispanic is more severe (Citation8,Citation9), has a greater impact on HRQoL (Citation10), and may be undertreated (Citation11). It is important to further investigate the efficacy and safety of psoriasis treatment in this population, since few studies of biologic therapy for the treatment of psoriasis in Hispanic patients have been published (Citation9,Citation10,Citation12,Citation13).
Guselkumab (TREMFYA®; Janssen Research & Development, LLC, Spring House, PA, USA) is an interleukin-23 blocker approved for the treatment of moderate-to-severe plaque psoriasis. In global Phase 3 studies (VOYAGE 1 and VOYAGE 2), guselkumab was well tolerated and demonstrated superior clinical activity compared with adalimumab (HUMIRA®; Abbott Laboratories, North Chicago, IL, USA), a commonly used tumor necrosis factor-α inhibitor (Citation14,Citation15). We conducted an analysis of pooled data from VOYAGE 1 and VOYAGE 2 to compare efficacy and safety between Hispanic and non-Hispanic patients with psoriasis.
Materials and methods
Patients
VOYAGE 1 and VOYAGE 2 were global studies conducted in the USA, Canada, Poland, Czech Republic, Germany, Hungary, Spain, Russia, Australia, South Korea, and Taiwan (VOYAGE 1 only). Both studies included patients ≥18 years of age who had moderate-to-severe plaque psoriasis (Investigator’s Global Assessment [IGA] score ≥3, Psoriasis Area and Severity Index [PASI] score ≥12, and body surface area involvement ≥10%) for at least 6 months and were candidates for systemic therapy or phototherapy. Full inclusion and exclusion criteria have been previously published (Citation14,Citation15). For this post hoc analysis, patients were divided into self-identified Hispanic and non-Hispanic subpopulations. All patients provided written consent to participate in the studies.
Study design
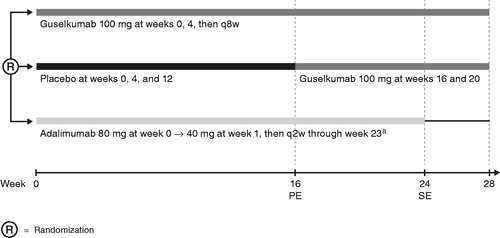
VOYAGE 1 and VOYAGE 2 were Phase 3, randomized, double-blind, placebo- and active-comparator-controlled studies with similar designs through week 28 (Citation14,Citation15). The design of both studies was identical through week 24; patients were randomized to receive guselkumab 100 mg at weeks 0 and 4, then every 8 weeks; placebo at weeks 0, 4, and 12, followed by guselkumab 100 mg at weeks 16 and 20; or adalimumab 80 mg at week 0, 40 mg at week 1, and then 40 mg every 2 weeks through week 23 (). The timing of adalimumab administration between weeks 23 and 28 differed between studies. In VOYAGE 1, patients received adalimumab every 2 weeks through week 28, and in VOYAGE 2, patients randomized to receive adalimumab did not receive any study drug from week 23 until week 28. Data from VOYAGE 1 and VOYAGE 2 were pooled so that outcomes in a relatively large population of Hispanic patients could be compared with outcomes in non-Hispanic patients. Both study protocols were reviewed by an Institutional Review Board or Independent Ethics Committee at participating sites and both VOYAGE 1 (NCT02207231) and VOYAGE 2 (NCT02207244) were registered at ClinicalTrials.gov.
Figure 1. VOYAGE 1 and VOYAGE 2 study designs through week 28. aIn VOYAGE 1, patients randomized to receive adalimumab received adalimumab every 2 weeks through week 28. In VOYAGE 2, patients randomized to receive adalimumab did not receive any study drug from week 23 until week 28. PE: primary endpoint; q2w: every 2 weeks; q8w: every 8 weeks; SE: secondary endpoint.
Study assessments
The co-primary endpoints in VOYAGE 1 and VOYAGE 2 were an IGA score of 0 (cleared) or 1 (minimal) (Citation16) and ≥90% improvement in the PASI score (Citation17) from baseline (PASI 90) at week 16. Secondary endpoints included ≥75% and ≥100% improvement in PASI score from baseline (PASI 75 and PASI 100) and a Dermatology Life Quality Index (DLQI) score of 0 or 1 (DLQI 0/1; no effect at all on patient’s HRQoL) (Citation18). For this analysis, pooled efficacy data for the Hispanic and non-Hispanic patient populations from VOYAGE 1 and VOYAGE 2 were analyzed at week 16 and week 24.
To evaluate safety, pooled data on treatment-emergent adverse events (TEAEs) for the Hispanic and non-Hispanic patient populations from VOYAGE 1 and VOYAGE 2 were analyzed through week 28.
Statistical analyses
Efficacy and baseline characteristics analyses were based on all patients who were randomized at week 0 according to their assigned treatment. Safety analyses were based on patients who received at least 1 dose of study agent according to the actual treatment received during the study. The Cochran–Mantel–Haenszel chi-squared test stratified by study was used to compare the proportion of patients responding to treatment. Treatment differences and confidence intervals (CIs) were adjusted by study (VOYAGE 1 and VOYAGE 2) with Cochran–Mantel–Haenszel. After the treatment failure rules were applied, the remaining missing data were handled using nonresponder imputation.
Results
Patient disposition and disease characteristics
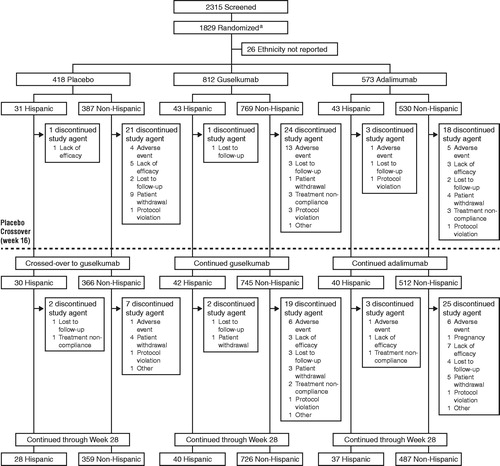
A total of 1803 patients were included in this analysis; 117 randomized patients (6.5%) self-identified as Hispanic (placebo = 31, guselkumab = 43, and adalimumab = 43) and 1686 (93.5%) self-identified as non-Hispanic (placebo = 387, guselkumab = 769, and adalimumab = 530) (). The majority of patients (62.4%) who self-identified as Hispanic were recruited at study sites within the USA, 13.7% were recruited at study sites in Spain, 10.3% were recruited at study sites in Poland, 6.0% were recruited at study sites in Germany, 4.3% were recruited at study sites in Canada, and 1.7% each were recruited at study sites in Australia and Russia.
Baseline demographics were generally comparable between the Hispanic and non-Hispanic populations, although body weight and body mass index (BMI) were slightly higher in the Hispanic population (). Overall, mean body weight was 93 kg for Hispanic patients and 89 kg for non-Hispanic patients, and mean BMI was 31.8 kg/m2 and 29.4 kg/m2, respectively. Severity of disease at baseline was also comparable between the Hispanic and non-Hispanic populations; median body surface area with psoriasis was 20.0% and 23.0%, respectively; mean PASI score was 20.2 and 21.9, respectively; 26.5% and 23.8% of patients, respectively, had an IGA score of 4 (severe); and mean DLQI score was 14.1 and 14.5, respectively.
Table 1. Summary of demographics and psoriasis disease characteristics at baseline in Hispanic and non-Hispanic patients in VOYAGE 1 and VOYAGE 2.
At baseline, a smaller proportion of patients in the Hispanic population had previously been treated with phototherapy (38.5% versus 57.2%, respectively) or nonbiologic systemic therapy (47.0% versus 64.5%, respectively) than patients in the non-Hispanic population. However, the proportion of patients who had prior biologic therapy at baseline was comparable between populations (23.1% in the Hispanic population compared with 20.6% in the non-Hispanic population).
Efficacy
Treatment effects for IGA and PASI responses at week 16 and week 24 were generally consistent between the Hispanic and non-Hispanic populations (). The CIs for the treatment differences across treatments for the Hispanic population were wide and encompassed those of the non-Hispanic population (). At week 16, efficacy responses for guselkumab were greater than those for placebo and adalimumab in both populations. Treatment differences for guselkumab versus placebo were significant for IGA 0/1 and PASI 90 responses (the co-primary end points) in both the Hispanic and non-Hispanic populations (all p < .001); however, they were numerically lower in the Hispanic population (IGA 0/1: 67.4 [95% CI 50.4, 84.4] and 77.2 [95% CI 73.5, 80.8] percentage points in the Hispanic and non-Hispanic populations, respectively; PASI 90: 59.2 [95% CI 41.9, 76.4] and 69.2 [95% CI 65.7, 72.7] percentage points in the Hispanic and non-Hispanic populations, respectively) ( and ). Treatment differences for guselkumab versus adalimumab at week 16 for the IGA 0/1 and PASI 90 responses were generally comparable between populations (IGA 0/1: 25.9 [95% CI 6.5, 45.3] and 17.5 [95% CI 12.8, 22.3] percentage points in the Hispanic and non-Hispanic populations, respectively; PASI 90: 21.4 [95% CI -0.1, 42.9] and 23.5 [95% CI 18.2, 28.9] percentage points in the Hispanic and non-Hispanic populations, respectively) ( and ). Treatment differences for PASI 75 and PASI 100 responses at week 16 were similar to those observed for the PASI 90 response at this time point for both populations ( and ).
Figure 3. Proportions of Hispanic and non-Hispanic patients in VOYAGE 1 and VOYAGE 2 who achieved an IGA score of 0 or 1 and PASI 75, PASI 90, and PASI 100 responses at week 16. *p < .001; **p < .05. p-values are versus guselkumab and based on Cochran–Mantel–Haenszel chi-squared test stratified by study. IGA: Investigator’s Global Assessment; PASI: Psoriasis Areas Severity Index.
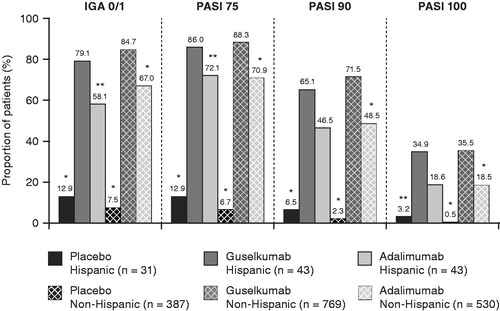
Figure 4. Proportions of Hispanic and non-Hispanic patients in VOYAGE 1 and VOYAGE 2 who achieved an IGA score of 0 or 1 and PASI 75, PASI 90, and PASI 100 responses at week 24. *p < .001; **p < .05. p-values are versus guselkumab and based on Cochran–Mantel–Haenszel chi-squared test stratified by study. IGA: Investigator’s Global Assessment; PASI: Psoriasis Areas Severity Index.
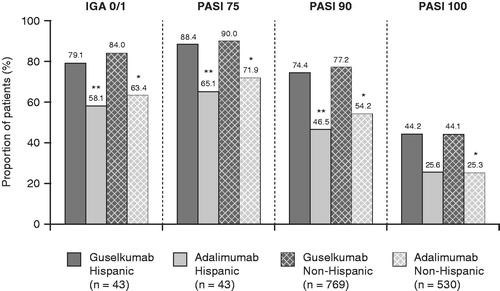
Figure 5. Proportion differences and 95% confidence intervals in IGA and PASI responses at week 16 and week 24 for guselkumab-treated versus placebo- or adalimumab-treated Hispanic and non-Hispanic patients in VOYAGE 1 and VOYAGE 2. Treatment differences and confidence intervals were adjusted by study (VOYAGE 1 and VOYAGE 2) with Cochran–Mantel–Haenszel. p-values are based on Cochran–Mantel–Haenszel chi-squared test stratified by study. CI: confidence interval; IGA: Investigator’s Global Assessment; PASI: Psoriasis Areas Severity Index.
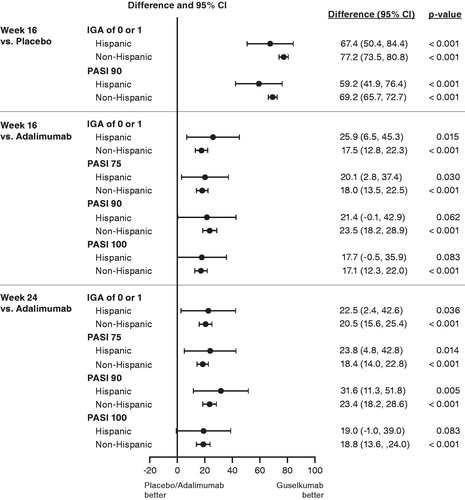
The superior efficacy response with guselkumab versus adalimumab was maintained through week 24. Treatment differences for guselkumab versus adalimumab treatment remained significant for IGA 0/1 and PASI 90 responses (all p ≤ .036) and were comparable between the Hispanic and non-Hispanic populations (IGA 0/1: 22.5 [95% CI 2.4, 42.6] and 20.5 [95% CI 15.6, 25.4] percentage points in the Hispanic and non-Hispanic populations, respectively; PASI 90: 31.6 [95% CI 11.3, 51.8] and 23.4 [95% CI 18.2, 28.6] percentage points in the Hispanic and non-Hispanic populations, respectively) ( and ). Similar treatment differences were observed for PASI 75 and PASI 100 responses at this time point ( and ).
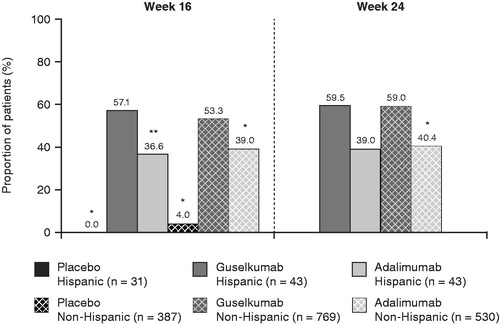
The HRQoL improvement with treatment was comparable between the Hispanic and non-Hispanic populations (). At week 16, DLQI 0/1 was achieved by 0% of placebo-treated patients, 57.1% of guselkumab-treated patients, and 36.6% of adalimumab-treated patients in the Hispanic population and by 4.0% of placebo-treated patients, 53.3% of guselkumab-treated patients, and 39.0% of adalimumab-treated patients in the non-Hispanic population. Similar results were observed at week 24.
Figure 6. Proportions of Hispanic and non-Hispanic patients in VOYAGE 1 and VOYAGE 2 with a DLQI score of 0 or 1 at week 16 or week 24. *p < .001; **p < .05. p-values are versus guselkumab and based on Cochran–Mantel–Haenszel chi-squared test stratified by study. DLQI: Dermatology Life Quality Index.
Safety
Through week 16, the frequency of adverse events was comparable between guselkumab- and placebo-treated patients in both the Hispanic and non-Hispanic populations (). The frequency of adverse events was numerically greater in adalimumab-treated patients compared with guselkumab-treated patients only in the Hispanic population through week 16 and week 28. Although no pattern accounted for the difference in the frequency of events between guselkumab- and adalimumab-treated patients in the Hispanic population at week 16 or week 28, the highest rates of adverse events in adalimumab-treated patients in the Hispanic population were in the system organ class of infections and infestations. A greater proportion of adalimumab-treated Hispanic patients reported at least 1 infection and at least 1 infection requiring treatment compared with guselkumab-treated Hispanic patients. Serious adverse events, major adverse cardiovascular events, malignancies other than nonmelanoma skin cancer, and nonmelanoma skin cancer occurred infrequently across all treatment groups in both the Hispanic and non-Hispanic populations through week 16 and week 28 ().
Table 2. Adverse events through week 16 and week 28 in Hispanic and non-Hispanic patients in VOYAGE 1 and VOYAGE 2.
Discussion
Guselkumab, an interleukin-23 blocker, is approved for the treatment of moderate-to-severe plaque psoriasis. Taken together, the pivotal guselkumab studies (VOYAGE 1 and VOYAGE 2) included 117 Hispanic patients and 1686 self-identified non-Hispanic patients. This pooled analysis of the Hispanic and non-Hispanic populations in these studies adds to the limited literature on the characteristics of psoriasis in Hispanic patients. Results from previous studies that included Hispanic patients suggested that psoriasis is more severe in Hispanic (Citation8,Citation9) and Asian (Citation10) patients than in patients of other ethnicities; however, in our analysis, baseline disease severity was generally comparable between the Hispanic and non-Hispanic populations. It has also been reported that compared with non-Hispanic patients with the same severity of psoriasis, Hispanic patients are more likely to have a lower HRQoL (Citation10). In this analysis, however, mean baseline DLQI scores were comparable between the Hispanic and non-Hispanic populations. Finally, review of data suggests that psoriasis may be undertreated in nonwhite individuals (Citation5,Citation11). In our analysis, fewer patients in the Hispanic population than in the non-Hispanic population had received phototherapy and nonbiological systemic therapy at baseline; however, rates of prior biologic treatment were comparable between populations. It should be noted that the majority of Hispanic patients in this study (62.4%) were from the USA and that disease characteristics may not be representative of the general Hispanic population in the rest of the world.
The efficacy of guselkumab treatment in patients with moderate-to-severe psoriasis was consistent between the Hispanic and non-Hispanic populations in this analysis. Treatment differences were generally consistent between the populations for all efficacy endpoints at weeks 16 and 24, although the treatment differences for guselkumab versus placebo for IGA 0/1 and PASI responses were numerically lower in the Hispanic population. However, the CIs for the treatment effects in the Hispanic population were wide, as expected due to smaller sample size, and encompassed those of the non-Hispanic population. Improvements in DLQI scores with guselkumab treatment compared with placebo or adalimumab treatment at week 16 and adalimumab treatment at week 24 were also comparable between the populations. Furthermore, guselkumab was generally well tolerated in the Hispanic and non-Hispanic populations, and no new safety concerns emerged within the Hispanic population. However, it is notable that through week 16 and week 28, the frequency of adverse events was greater in adalimumab-treated patients compared with guselkumab-treated patients in the Hispanic population only. The increase in adverse event rates for adalimumab was mostly attributable to infection-related adverse events, including infections and infections requiring treatment.
Limitations of this analysis include its duration of only 28 weeks and the relatively small number of patients, which limits the precision of the results. In addition, the term ‘Hispanic’ is primarily used within the USA to denote a person of Cuban, Mexican, Puerto Rican, South or Central American, or other Spanish culture or origin, regardless of race (Citation19), and may be confusing to patients in other countries. Therefore, because Hispanic status was patient-reported, there is the possibility that patients could be mis-identified. However, because of the considerable size of the non-Hispanic population, this is not likely to affect results. It is interesting to note that of the 68 patients recruited in Spain, 23.5% self-identified as Hispanic and 76.5% self-identified as non-Hispanic, which was similar to what was observed in patients recruited in the USA (20.5% and 79.5%, respectively). Despite these limitations, considering the small number of published studies evaluating psoriasis in Hispanic patients (Citation9,Citation10,Citation12,Citation13), this analysis adds significantly to the literature covering biologics for the treatment of psoriasis in this population.
In summary, in this analysis of a significant number of Hispanic patients with moderate-to-severe psoriasis from the VOYAGE 1 and VOYAGE 2 studies, the efficacy and safety of guselkumab were generally comparable between the Hispanic and non-Hispanic populations. These results suggest that the efficacy, safety, and resulting benefit/risk analyses of guselkumab are applicable to Hispanic patients with moderate-to-severe psoriasis.
Acknowledgements
The authors thank Holly Capasso-Harris, Synchrogenix, a Certara Company, Wilmington, DE; Cynthia Guzzo, Kelly Global Services, LLC, Troy, MI; and Cynthia Arnold, Janssen Scientific Affairs, LLC, Spring House, PA for their support.
Disclosure statement
Dr. Puig has received consultancy and/or speaker’s honoraria from and/or participated in clinical trials sponsored by AbbVie, Almirall, Amgen, Baxalta, Biogen, Boehringer Ingelheim, Celgene, Gebro, Janssen, LEO Pharma, Eli Lilly, Merck-Serono, MSD, Mylan, Novartis, Pfizer, Regeneron, Roche, Sandoz, Samsung-Bioepis, Sanofi, and UCB.
Dr. Wu has been an investigator for AbbVie, Amgen, Eli Lilly, Janssen, and Novartis; a consultant for AbbVie, Almirall, Amgen, Bristol-Myers Squibb, Celgene, Dermira, Dr. Reddy’s Laboratories, Eli Lilly, Janssen, LEO Pharma, Novartis, Promius Pharma, Regeneron, Sun Pharmaceutical, UCB, and Valeant Pharmaceuticals North America LLC; and a speaker for AbbVie, Celgene, Novartis, Regeneron, Sun Pharmaceutical, UCB, and Valeant Pharmaceuticals North America LLC.
Dr. Gooderham has been an investigator, speaker, advisory board member, or consultant for AbbVie, Actelion Pharma, Amgen, Akros, Arcutis, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Dermira, Eli Lilly, Galderma, GlaxoSmithKline, Janssen, Kyowa Kirin, LEO Pharma, Medimmune, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi Genzyme, Sun Pharmaceuticals, UCB, and Valeant Pharmaceuticals North America LLC.
Drs. You, Shen, and Randazzo are employed by Janssen Research & Development, LLC and own stock in Johnson & Johnson.
Dr. Kerdel has been an investigator (receiving grants) for Actelion, Amgen, AbbVie, AstraZeneca, Janssen, Celgene, Dermira, Dr. Reddy’s, Eli Lilly, LEO Pharma, Menlo, Novartis, Ortho, Pfizer, Regeneron, Sanofi, UCB, and X-Biotech; a speaker (receiving honoraria) for Actelion, Amgen, AbbVie, Janssen, Celgene, Eli Lilly, Novartis, Pfizer, Ortho, Regeneron, Sanofi, and Valeant Pharmaceuticals North America LLC; and served on advisory boards for Eli Lilly and Celgene.
Data availability statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Additional information
Funding
References
- Global report on psoriasis. Geneva, Switzerland: World Health Organization; 2016. Available at: http://apps.who.int/iris/bitstream/10665/204417/1/9789241565189_eng.pdf.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70(3):512–516.
- Helmick CG, Lee-Han H, Hirsch SC, et al. Prevalence of psoriasis among adults in the U.S.: 2003–2006 and 2009–2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47(1):37–45.
- McMichael AJ, Jackson S. Issues in dermatologic health care delivery in minority populations. Dermatol Clin. 2000;18(2):229–233.
- Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003–2004. J Am Acad Dermatol. 2009;60(2):218–224.
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19(3):405–423.
- Profile America Facts for Features: Hispanic Heritage Month 2017. United States Census Bureau; 2017. Available at: https://www.census.gov/content/dam/Census/newsroom/facts-for-features/2017/cb17-ff17.pdf.
- Abrouk M, Lee K, Brodsky M, et al. Ethnicity affects the presenting severity of psoriasis. J Am Acad Dermatol. 2017;77(1):180–182.
- Adsit S, Zaldivar ER, Sofen H, et al. Secukinumab is efficacious and safe in Hispanic patients with moderate-to-severe plaque psoriasis: pooled analysis of four Phase 3 trials. Adv Ther. 2017;34(6):1327–1339.
- Shah SK, Arthur A, Yang YC, et al. A retrospective study to investigate racial and ethnic variations in the treatment of psoriasis with etanercept. J Drugs Dermatol. 2011;10(8):866–872.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11(4):466–473.
- McMichael A, Desai SR, Qureshi A, et al. Efficacy and safety of brodalumab in patients with moderate-to-severe plaque psoriasis and skin of color: Results from the pooled AMAGINE-2/-3 randomized trials. Am J Clin Dermatol. 2019;20(2):267–276.
- Stengel FM, Petri V, Campbell GA, et al. Control of moderate-to-severe plaque psoriasis with efalizumab: 24-week, open-label, phase IIIb/IV Latin American study results. Arch Drug Info. 2009;2(4):71–78.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–417.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76(3):418–431.
- Langley RG, Feldman SR, Nyirady J, et al. The 5-point Investigator’s Global Assessment (IGA) Scale: a modified tool for evaluating plaque psoriasis severity in clinical trials. J Dermatolog Treat. 2015;26(1):23–31.
- Fredriksson T, Pettersson U. Severe psoriasis—oral therapy with a new retinoid. Dermatologica. 1978;157(4):238–244.
- Basra MKA, Fenech R, Gatt RM, et al. The Dermatology Life Quality Index 1994-2007: a comprehensive review of validation data and clinical results. Br J Dermatol. 2008;159(5):997–1035.
- United States Census Bureau [Internet]. United States Census Bureau. Hispanic Origin [cited 2019 Aug 8]; [about 2 screens]. Available from: https://www.census.gov/topics/population/hispanic-origin/about.html.