Abstract
Background
The presence of Actinic Keratoses (AKs) represent the most important warning sign of subclinical ultraviolet radiation. Currently, the regular use of sunscreens is considered essential for the prevention of the development of AKs.
Aim
We evaluated the effectiveness of a new class I Medical Device (MD) for the prevention and treatment of AKs vs traditional sunscreen alone (SPF 100+).
Methods
We conducted a randomized controlled prospective study in 90 Caucasian patients: 62 immunocompetent and 28 Organ Transplant Recipients (OTRs). We randomly assigned subjects to the MD group or sunscreen alone in a 1:1 assignment ratio. The patients have been reevaluated after three and six months.
Results
In immunocompetent patients treated with MD, at the end of the study the reduction of the mean number of AKs was 54.7 vs. 9.43% with photoprotector. In OTRs, the global reduction was of 36.7% after MD use compared to 14.3% with the sunscreen. The prevalence of NMSCs, in the patients treated with MD, was 11.11 and 17.18 with sunscreen; the incidence was 19.7 in patients treated with MD and 32.1 in those treated with sunscreen.
Conclusion
The MD has demonstrated good efficacy in the reduction of visible AKs, encouraging its use also in high-risk category, like OTRs.
Introduction
Actinic Keratoses (AKs) represent the most frequent ‘in situ’ neoplasms in Caucasian subjects, with more than 20% of the diagnosis in Dermatology settings (Citation1). In Italy, the prevalence of AKs has been reported of 27.4% (Citation2). They are considered a precursor of squamous cell carcinoma (SCC) and their presence is the most important warning sign of subclinical ultraviolet radiation (UVR) induced damage, accumulated over the time (Citation3–5). UVR-induced photodamage is known as field cancerization (FC) that is a cutaneous area with erythematous, atrophic, telangiectatic, hypo/hyperpigmented skin, presence of clinically visible AKs and subclinical cell alterations (histologic atypia), that represent a substrate for new lesions appearance (Citation6–9). It has been advanced the hypothesis that the number of subclinical AKs in FC can surpass the number of clinically AKs by ten-fold (Citation10).
In addition to UVR-exposure, the main risk factors for the AKs development are fairy phototype (I–II–III), age over 50 years, male gender, beta-HPV infection, and immunosuppression. Particularly, the immunosuppression, like in Organ Transplant Recipients (OTRs), can increase the risk of AKs of 250 times compared to immunocompetent subjects (Citation11).
The regular use of sunscreens is crucial to prevent AKs development and to reduce the risk of SCC in the long-term period (Citation12). Moreover, the early treatment of all clinically visible lesions and of FC is very important since it is not possible to predict which AKs will progress only based on clinical classification (AK I-II-III) or histopathological features (KIN ‘Keratinocyte Intraepithelial Neoplasia’ I-II-III, low or high-grade intraepithelial neoplasia) (Citation13) . Indeed, Figueras has recently demonstrated that the transformation into invasive SCC can occur directly from AKs with involvement limited to the basal layer and that clinical classifications do not correlate with histology (Citation13).
This fact must be kept in mind when we decide the treatment, besides cost-effectiveness and patient-related factors (Citation13–17).
In the last few years, some studies have shown that the addition of xenogenic DNA repair enzymes (i.e. photolyase) to traditional sunscreens may reduce UVR-induced cyclobutane pyrimidine dimers (CPDs) formation more than the sunscreens alone (Citation18–20). CPDs represents the first cause of alterations in the structure of DNA and then of mutations in skin tumorigenesis (Citation21,Citation22).
In the present study, we evaluated the effectiveness of a new class I Medical Device (MD, ®Rilastil AK Repair 100+) for the prevention and coadjuvant treatment of AKs vs. standard sunscreen (SPF100). Furthermore, the effectiveness was compared in a group of immunocompetent patients and a group of OTRs.
Materials and methods
Setting
We conducted a randomized controlled prospective study in our Dermatologic Unit, in Novara (Italy). Continuous enrollment took place between October 2017 and June 2018.
The study protocol was approved by the Local Ethical Committee and was conducted following the Declaration of Helsinki.
Patients
The study population comprised of 90 Caucasian patients: 62 immunocompetent and 28 OTRs. Inclusion criteria were age >50 years and clinical evidence of AKs (grade I and II) on the face and scalp; exclusion criteria were represented by the incapacity to apply independently the products or by genetic disorders conditioning the development of Non-Melanoma Skin Cancer (i.e. Gorlin-Goltz syndrome, Xeroderma Pigmentosum, Epidermodysplasia Verruciformis). OTRs group included patients with kidney’s transplant and immunosuppressive treatment for at least 5 years.
Study design
Baseline assessments were undertaken by a dermatologist before randomization. At baseline visit (T0) all patients signed an informed consent and we collect information about their personal data, risk factors and previous treatment for AKs, the number and site of visible lesions, which were reported in medical records and photographed for comparison in following visits. At each visit photographic documentation was performed.
We randomly assigned subjects to MD group (SPF 100+ plus ®DNA repair complex) or standard sunscreen (SPF 100+ alone) in a 1:1 assignment ratio. Randomization was done separately for immunocompetent patients and OTRs. The participants were instructed to apply the study products over the photo-damaged areas twice daily (morning and early afternoon) for 6 months. The MD was provided to patients free of charge by the manufacturer (Rilastil Laboratori Milano). This MD consists of physical and chemical UVA-UVB filters (corresponding to SPF 100+) and active ingredients with antioxidant and repairing action, among which the most important is ®DNA repair complex (a complex of amino acids, acetyl-tyrosine and proline, ATP and vegetable protein hydrolyzate) (Citation23).
Outcome
The patients were reevaluated after three (T3) and six (T6) months of products application. The primary outcome of our study was the number of AKs reported in medical records still visible at T3 and T6 visit (lesions clearance); the secondary outcome was the eventual appearance of NMSC at the same time points.
Statistical analysis
In immunocompetent patients, we expected to observe a reduction of lesions number at T6 for subjects treated with the new device; whereas, we assumed that the AKs number would remain constant in patients treated with the sunscreen alone. In OTR patients, we supposed a constant number of lesions at T6 in the first group, and a possible increasing of lesions number in the second.
The analysis for the primary outcome was done according to an intention-to-treat principle.
Descriptive statistic was performed at baseline and the number of lesions for each risk factor was evaluated using N (%) and mean ± standard deviation. Median (interquartile range) of the number of lesions for each level of the categorical variables was also reported. We performed a non-parametric test (Wilcoxon Rank–Mann Whitney and Kruskal–Wallis) and a p-value less than .05 was considered statistically significant.
To evaluate the presence of baseline differences between immunocompetent and OTR patients for the risk factors Chi-square or Exact (Fisher) association tests were performed.
Before doing the analysis that accounts for the repeated measurements within subject, we calculated the average response to the different treatment over time. We reported only the mean ± standard deviation of the number of AKs and the % change from baseline at different times separately for type of patients and treatment.
For analysis of the number of lesions, we used a model with patient type (immunocompetent vs. OTR), time (6 months vs. 3 months), the interaction between patient type and time as a factor, and the logarithmic of the baseline lesions as a continuous covariate. A Poisson model and an unstructured covariance matrix were assumed, and we reported the estimate Relative Risks (RRs) and their 95% Confidence Intervals (CIs).
We performed the incidence and prevalence of SCC alone and NMSCs (Squamous or Basal Cell Carcinomas). We defined prevalence as the total number of subjects with SCC (and NMSC) divided by the total of subjects included in the study (population at risk); while incidence was defined as the number of new cases identified divided by the person time (the months at risk) and the value was reported per 1000 people per month.
We performed the analysis of the two outcomes (incidence and prevalence) distinguishing two-time period: the first 3 months and the entire follow up (6 months). To calculate the 95% CIs for the prevalence and incidence rates, we used methods based on Fisher’s exact test; this approach is appropriate for the fairly small number of cases identified.
All analyses were performed using the software SAS 9.3 and OpenEpi.
Results
Clinical characteristics
Among the 90 patients enrolled in this study, 65 were males (72.22%) and 25 females (27.78%), with a mean (± standard deviation) age of 75.98 ± 7.52. Sixty-two patients were immunocompetent and 28 were OTRs with a median time of immunosuppression of 11.43 years (range 5–35).
In general, baseline characteristics were similar between immunocompetent and OTRs patients (); however, there were slight differences in gender (p-value=.0210) and use of MD analogs (p-value .0033): the OTR patients were more frequently males (89.29 vs. 64.52%) and MD analogs users (42.86 vs. 14.52%). Also, OTRs used more frequently sunscreens (67.86 vs. 48.39%), even if this variable is not statistically significant (p-value .0860).
Table 1. Characteristics of study population.
AKs at baseline
At baseline, a greater number of lesions with a statistically significant difference was found for patients with an outdoor job (p-value=.0053), previous treatment for AKs (p-value .0177) and lesions located on the scalp (p-value .0074). The number of visible AKs at T0 was greater in immunocompetent patients (5.37 ± 3.76) than in OTRs (4.96 ± 5.26) but the variable failed to reach statistical significance (p-value .0853). The results are shown in .
Table 2. Association between risk factors and number of AKs at T0.
AKs after treatment
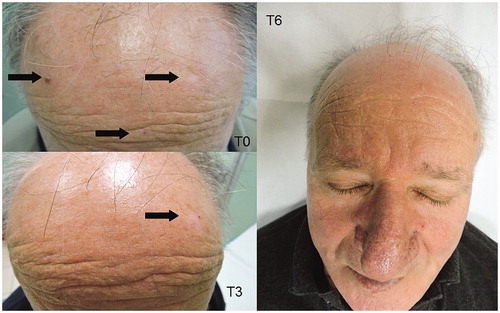
In immunocompetent patients treated with MD, the mean number of AKs at T3 was 4.10 ± 2.96 (31% reduction; at T0 5.97 ± 3.81); instead, patients assigned to use of standard sunscreen showed a mean number of 4.32 ± 3.30 (9.43% reduction; at T0 4.77 ± 3.67). At T6, the mean number of AKs in the treatment group was 2.70 ± 2.07 (54.7% reduction) while in patients who only applied sunscreen it remains unchanged.
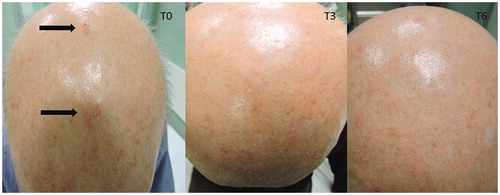
In OTRs, at T3 the mean AKs number was 5.50 ± 5.61 (14.5% reduction) in the MD group and 3.69 ± 3.82 (5.4% increase) in the standard sunscreen group; at T6, the mean number of lesions after MD use shows a reduction of 36.7% compared to 14.3% with the sunscreen alone.
In and are shown two patients with an evident AKs reduction.
The results of the interaction models are shown in . The covariate with the greater estimate in this study is the number of lesions at baseline: subjects who had a great number of AKs at T0 had a statistically significant risk of development AKs (RR 2.64, CI95% 2.37–2.95). The time influences the number of AKs and also treatment and type of subject had an effect just significant at 5%.
Table 3. Rate of AKs development in immunocompetent and OTRs treated with MD.
Table 4. Prevalence (per 100) and incidence (per 1000 patient months) of NMSC during the treatment period.
Immunocompetent treated with MD have a reduction of the risk of AK development of 36% (RR 0.64, CI 95% 0.55–0.75), compared to immunocompetent non-treated. A lower value of estimate was showed for the OTR patients: we observed an RR of 1.04 (CI 95% 0.89–1.21) and 0.84 (CI 95% 0.69–1.04), respectively for OTRs treated and OTRs non-treated compared with the reference category (immunocompetent non-treated) .
Non-melanoma skin cancer
We have also evaluated the prevalence and incidence of non-melanoma skin cancer (NMSC) during the observation period of six months.
The prevalence of SCC in patients treated with MD was 2.22 at T3 and T6 and this value was the same in the group treated with the standard sunscreen at T3, but it was twice at 6 months (4.44) without reaching the statistical significance (p-value >.9999)
The incidence data for BCC considering the overall information (6 months) was 3.37 per 1000 people per month in the MD group and 7.58 per 1000 people per month in the control group: the difference was not significant (p-value .9958).
If we consider all the NMSCs (squamous or basal cell carcinomas), the prevalence, in the patients treated with MD, was 6.67 at T3 and 11.11 at T6. In patients that only applied sunscreen, this value was the same as T3 but increased to 17.18 at T6.
The incidence (per 1000 people per month) of NMSCs at T6 was 19.7 in patients treated with MD and 32.1 in those treated with standard sunscreen, without statistical significance (p-value .5587)
Discussion
In the present study, we report our experience with the use of a new topical MD for the prevention and treatment of AKs, in a cohort of both immunocompetent and immunosuppressed patients.
AKs are a skin condition caused by chronic sun exposure; in patients with multiple and/or subclinical lesions, the main need is to reduce field cancerization in order to prevent the development of novel AKs and decrease the risk of their malignant transformation in SCC (Citation18). Although multiple treatment modalities are currently available, the incidence of AKs continues to increase and it represents a public health concern, making new methods of prevention and treatment necessary (Citation24,Citation25).
It is now common opinion that cosmetic sunscreen products are an essential element of UV protection strategies, but they are not adequate when damage is already evident (Citation25). In 2015 a group of six German Dermatologist met to discuss the prevention and treatment strategies available for AKs and developed an adjuvant treatment algorithm for various risk levels; particularly, for patients with low and moderate risk, standard sunscreen is recommended, but for groups with high and very high risk (immunocompetent subjects with presence and history of AKs and NMSC, OTRs and immunosuppressed patients) is necessary a very high photoprotection with photo-repair action (DNA repair enzymes) in MD products all year round (Citation25).
In the recent literature (Citation18,Citation26), many studies have demonstrated the effectiveness of a topically applied MD containing photolyase and UV filters with very high SPF (>100).
Our study instead, demonstrates the efficacy of a new product containing UVA-UVB filters with the ‘DNA repair complex’ (a complex of amino acids, acetyl-tyrosine and proline, ATP and vegetable protein hydrolyzate), Epigallocatechin Gallate and vitamin E. These molecules have demonstrated in vitro antioxidant, reparative, and photoprotective actions (Citation27–35).
At baseline, we enrolled a homogeneous population, composed by immunocompetent and OTRs patients presenting the known risk factors for AKs development. From our data emerges that OTRs at T0 already used MD analogs (e.g. MD with photolyase) more than immunocompetent patients. This means that OTRs since they represent a high-risk population, are more sensitized to the use of effective preventive measures.
Furthermore, from our results emerges that at T0 patients with an outdoor job, previous treatment for AKs, and AKs of the scalp had a great number of clinical lesions. Accordingly, in 2006, Naldi et al. have demonstrated that subjects who spent less than 2 h a week outdoors had AKs prevalence of 1.1%, while who spent at least 5 days a week outdoors, had a prevalence of 1.8% (Citation36). The great number of AKs on the scalp is explained by the fact that it is often bald, particularly in the male population with age-related alopecia.
Moreover, at T0 the number of visible AKs was greater in immunocompetent patients than in OTRs due to the different mean age (78.9 versus 69). In fact, in literature, the increase in age is one of the major risk factors for AKs development (Citation37).
In the present study, MD has demonstrated good efficacy in the reduction of clinical visible AKs (lesion clearance); at T6, in immunocompetent patients, the overall reduction was of 54.7% (versus 9.43% with the standard sunscreen). In 2017 Stoddard et al. demonstrated a 46.6% decrease in AKs patients treated with photolyase, compared to 32.7% in the placebo group after 8-weeks treatment (Citation24). Importantly, our work proposes for the first time the use of this MD in OTRs population with an overall reduction of visible AKs of 36.7% (versus 14.3% of standard sunscreen). The lower efficacy in the OTRs could be explained by the minor surveillance of the immune system against the photocarcinogenesis in these subjects (Citation38–40).
Giustini et al. in 2014 reported that MD with photolyase reduced the incidence of new BCC and SCC of 56 and 100%, respectively (Citation26); our results confirmed that the incidence and the prevalence of NMSC decrease with the use of MD, compared to sunscreen only.
This study may have been altered by adherence to therapy because the MD was applied at home and provided a regular and daily application. Moreover, these formulations often determine unesthetic effects (whitish halo on the skin) and in literature is demonstrated a low adherence to these therapies (Citation41–43). Our MD is an oily emulsion with a rapid absorption without leaving marks on the skin and with high compliance by patients. Furthermore, in our study, no adverse events or intolerance were reported by all treated patients.
In Italy, the devices for prevention of NMSC are purchased by the patient without reimbursement and this may represent another problem for the adhesion to therapy (in our study MD was released free to patients who had to return the used vials at the end, but standard sunscreen was purchased by the patient).
Another limitation of the present study could be represented by the fact that it was not a double-blind study and that the outcome was only determined visually and not confirmed histologically; however, to contain the bias, clinical evaluations were performed by a group of trained physicians.
For the future, it would be interesting to expand the follow-up period (up to 12 months), the number of patients enrolled (especially other OTRs categories) and use a measurable marker of FC for microscopic evaluation (i.e. confocal microscopy) of actinic damage reduction.
Acknowledgments
The authors wish to thank Rilastil Laboratori Milano for the support in carrying out the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ferrándiz-Pulido C, Lera-Imbuluzqueta M, Ferrándiz C, et al. Plazas-fernandez MJ; on behalf to EPIQA Study Group. prevalence of actinic keratosis in different regions of Spain: the EPIQA Study. Actas Dermosifilogr. 2018;109(1):83–86.
- Fargnoli MC, Altomare G, Benati E. Prevalence and risk factors of actinic keratosis in patients attending Italian dermatology clinics. Eur J Dermatol. 2017;27(6):599–608.
- Stockfleth E. The paradigm shift in treating actinic keratosis: a comprehensive strategy. J Drugs Dermatol. 2012;11(12):1462–1467.
- Berman B, Amini S. Pharmacotherapy of actinic keratosis: an update. Expert Opin Pharmacother. 2012;13(13):1847–1871.
- Feldman SR, Fleischer AB. Jr. Progression of actinic keratosis to squamous cell carcinoma revisited: clinical and treatment implications. Cutis. 2011;87(4):201–207.
- Figueras Nart I, Cerio R, Dirschka T, et al. Defining the actinic keratosis field: a literature review and discussion. J Eur Acad Dermatol Venereol. . 2018;32(4):544–563.
- Stockfleth E. The importance of treating the field in actinic keratosis. J Eur Acad Dermatol Venereol. 2017;31(2):8–11.
- Torezan LA, Festa-Neto C. Cutaneous Field cancerization: clinical, histopathological and therapeutic aspects. An Bras Dermatol. 2013;88(5):775–786.
- Vanharanta S, Massague J. Field cancerization: something new under the sun. Cell. 2012;149(6):1179–1181.
- Uhlenhake EE. Optimal treatment of actinic keratoses. Clin Interv Aging. 2013;8:29–35.
- Stockfleth E A. k. Cancer treatment and Research. In: Stockfleth E, Ulrich C, editors. Skin cancer after organ transplantation. Berlin, Germany: Springer; 2009. p. 227–239.
- Thompson SC, Jolley D, Marks R. Reduction of solar keratoses by regular sunscreen use. N Engl J Med. 1993;329(16):1147–1151.
- Fernandez Figueras MT. From actinic keratosis to squamous cell carcinoma: pathophysiology revisited. J Eur Acad Dermatol Venereol. 2017;31:5–7.
- Pellacani G, Neri L, Longo C. Comment to: evidence and consensus based (S3) guidelines for the treatment of actinic keratosis. J Eur Acad Dermatol Venereol. 2016;30:e114.
- Hofbauer G, Anliker M, Boehncke WH, et al. Swiss clinical practice guidelines on field cancerization of the skin. Swiss Med Wkly. 2014; 144:w14026.
- Werner RN, Stockfleth E, Connolly SM, et al. Evidence and consensus based (S3) guidelines for the treatment of actinic keratosis – International League of Dermatological Societies in cooperation with the European Dermatology Forum – short version. J Eur Acad Dermatol Venereol. 2015;29(11):2069–2079.
- Peris K, Calzavara-Pinton PG, Neri L, et al. Italian expert consensus for the management of actinic keratosis in immunocompetent patients. J Eur Acad Dermatol Venereol. 2016;30(7):1077–1084.
- Carducci M, Pavone PS, De Marco G, et al. Comparative effects of sunscreens alone vs sunscreens plus DNA repair enzymes in patients with actinic keratosis: clinical and molecular findings from a 6-months, randomized. J Drugs Dermatol. 2015;14(9):986–990.
- Spencer JM, Morgan MB, Trapp KM, et al. Topical formulation engendered alteration in p53 and cyclobutene pyrimidine dimer expression in chronic photodamaged patients. J Drugs Dermatol. 2013;12(3):336–340.
- Berardesca E, Bertona M, Altabas K, et al. Reduced ultraviolet-induced DNA damage and apoptosis in human skin with topical application of a photolyase-containing DNA repair enzyme cream: clues to skin cancer prevention. Mol Med Rep. 2012;5(2):570–574.
- Besaratinia A, Yoon JI, Schroeder C, et al. Wavelenght dependence of ultraviolet radiation-induced DNA damage as determined by laser irradiation suggests that cyclobutene pyrimidine dimers are the principal DNA lesions produced by terrestrial sunlight. FASEB J. 2011;25(9):3079–3091.
- Yamaguchi Y, Coelho SG, Zmudzka BZ, et al. Cyclobutane pyrimidine dimer formation and p53 production in human skin after repeated UV irradiation. Exp Dermatol. 2008;17(11):916–924.
- Dell’Acqua G, Schweikert K. A DNA repair complex to decrease erythema and UV-induced CPD formation. Cosmetics Toilet. 2008;123(5).
- Stoddard M, Herrmann J, Moy L, et al. Improvement of actinic keratoses using topical DNA repair enzymes: a randomized placebo-controlled trial. J Drugs Dermatol. 2017;16(10):1030–1034.
- Krutmann J, Berking C, Berneburg M, et al. New strategies in the prevention of actinic keratosis: a critical review. Skin Pharmacol Physiol. 2015;28(6):281–289.
- Giustini S, Miraglia E, Berardesca E, et al. Preventive long-term effects of a topical film -forming medical device with ultra-high UV protection filters and DNA repair enzyme in xeroderma pigmentosum: a retrospective study of eight cases. Case Rep Dermatol. 2014;6(3):222–226.
- Morozova OB, Kiryutin AS, Sagdeev RZ, et al. Electron transfer between guanosine radical and amino acids in aqueous solution. 1. Reduction of guanosine radical by tyrosine. J Phys Chem B. 2007;111(25):7439–7448.
- Milligan JR, Aguilera JA, Ly A, et al. Repair of oxidative DNA damage by amino acids. Nucleic Acids Res. 2003;31(21):6258–6263.
- Katiyar SK, Perez A, Mukhtar H. Green tea polyphenol treatment to human skin prevents formation of ultraviolet light B-induced pyrimidine dimers in DNA green tea polyphenol treatment to human skin prevents formation of ultraviolet light B-induced pyrimidine. Clin Cancer Res. 2000;6(10):3864–3869.
- Vayalil PK, Mittal A, Hara Y, et al. Green tea polyphenols prevent ultraviolet light-induced oxidative damage and matrix metalloproteinases expression in mouse skin. J Invest Dermatol. 2004;122(6):1480–1487.
- Yusuf N, Irby C, Katiyar SK, et al. EC. Polyphenols, photoprotective effects of green tea. Photoderm Photoimm Photomed. 2007;23(1):48–56.
- Zingg JM. Modulation of signal transduction by vitamin E. Mol Aspects Med. 2007;28(5–6):481–506.
- McMillan DC, Talwar D, Sattar N, et al. The relationship between reduced vitamin antioxidant concentrations and the systemic inflammatory response in patients with common solid tumours. Clin Nutr. 2002;21(2):161–164.
- Thiele JJ, Ekanayake-Mudiyanselage S. Vitamin E in human skin: organ-specific physiology and considerations for its use in dermatology. Mol Aspects Med. 2007;28(5–6):646–667.
- Jiang Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic Biol Med. 2014;72:76–90.
- Naldi L, Chatenoud L, Piccitto R, et al. Prevalence of actinic keratoses and associated factors in a representative sample of the Italian adult population: results from the prevalence of actinic keratoses Italian study, 2003-2004. Arch Dermatol. 2006;142(6):722–726.
- Frost C, Williams G, Green A. High incidence and regression rates of solar keratoses in a queensland community. J Invest Dermatol. 2000;115(2):273–277.
- Pascual M, Theruvath T, Kawai T, et al. Strategies to improve long-term outcomes after renal transplantation. N Engl J Med. 2002;346(8):580–590.
- Gutierrez-Dalmau A, Campistol JM. Immunosuppressive therapy and malignancy in organ transplant recipients: a systematic review. Drugs. 2007;67(8):1167–1198.
- Ingham AI, Weightman W. The efficacy and safety of topical 5% 5-fluorouracil in renal transplant recipients for the treatment of actinic keratoses. Australas J Dermatol. 2014;55(3):204–208.
- Moloney FJ, Almarzouqi E, O’Kelly P, et al. Sunscreen use before and after transplantation and assessment of risk factors associated with skin cancer development in renal transplant recipients. Arch Dermatol. 2005;141(8):978–982.
- Mahe E, Morelon E, Fermanian J, et al. Renal-transplant recipients and sun protection. Transplantation. 2004;78(5):741–744.
- Donovan JCH, Shaw JC. Compliance with sun protection following organ transplantation. Arch Dermatol. 2006;142(9):1231–1233.