Abstract
Objectives
To compare the cost-effectiveness of tildrakizumab with other commonly used biologics and apremilast as the first-line treatment for moderate-to-severe plaque psoriasis from a US health plan’s perspective.
Methods
A 10-year cost-effectiveness model was developed to compare the incremental cost per extra month with a Psoriasis Area and Severity Index (PASI) 75 response. Patients were assumed to receive one of the treatments evaluated as their first-line treatment at the outset of the analysis. Nonresponders (PASI <75) discontinued their current treatment; 25% went on to receive a mix of topical therapies, phototherapies, and other systemic therapies, while 75% received a second-line therapy before receiving a mix of topical therapies, phototherapies, and other systemic therapies. Direct medical costs were calculated based on drug acquisition, administration, and monitoring costs.
Results
The incremental cost per extra month a patient had a PASI 75 response was lowest for brodalumab ($3,685), infliximab ($4,102), apremilast ($4,770), and tildrakizumab ($5,150), followed by risankizumab ($5,319), secukinumab ($5,675), guselkumab ($5,784), ixekizumab ($5,900), adalimumab ($5,943), ustekinumab ($6,131), etanercept ($6,618), and certolizumab pegol ($13,476).
Conclusion
Tildrakizumab was among the most cost-effective first-line treatments for moderate-to-severe psoriasis and was more cost-effective than risankizumab, secukinumab, guselkumab, ixekizumab, adalimumab, ustekinumab, etanercept, and certolizumab pegol.
Introduction
Psoriasis is a chronic, relapsing, and systemic inflammatory skin disease that affects approximately 7.4 million adults in the United States (US) (Citation1), with plaque psoriasis accounting for more than 80% of cases (Citation2). According to an estimation based on data from the US National Health and Wellness Survey from 2007 to 2012, 1.7 million insured US patients have moderate-to-severe plaque psoriasis (Citation3). Moderate-to-severe psoriasis is associated with a considerable disease burden, affecting patients’ physical and psychological wellbeing and resulting in reduced quality of life and work productivity (Citation4–7). It also incurs a substantial financial burden on both patients and society, requiring significant long-term medical resource use, out-of-pocket costs, and indirect costs (Citation2,Citation8,Citation9).
Over the last 15 years, many biologic and small-molecule systemic therapies have obtained approval from the US Food and Drug Administration (FDA) for moderate-to-severe plaque psoriasis. Using the Psoriasis Area and Severity Index (PASI), a recent large meta-analysis of randomized controlled trials demonstrated that in general biologic and small-molecule therapies are highly effective for the treatment of moderate-to-severe psoriasis (Citation10). Newer biologic and small-molecule therapies such as interleukin-17 (IL-17) and IL-23 inhibitors have better efficacy and safety compared with other systemic therapies (Citation9–11). However, the comparative cost-effectiveness of these drugs has not been fully established. Although previous publications have compared the cost-effectiveness of systemic therapies for psoriasis in the US (Citation10–13), none has fully assessed the currently available treatment options, including newly approved treatments such as the IL-23 inhibitor tildrakizumab. The purpose of the current study was to examine the cost-effectiveness of tildrakizumab and the other systemic treatments for moderate-to-severe plaque psoriasis from the perspective of a US health plan.
As moderate-to-severe plaque psoriasis is a chronic and relapsing disease, maintenance of high levels of skin clearance over time is a crucial clinical outcome when considering treatment efficacy. Thus, several previous studies have examined cost-effectiveness in terms of average cost to achieve a PASI 75 response (i.e. at least 75% improvement in PASI from baseline) over time (Citation11,Citation12,Citation14). In the current study, we aimed to evaluate the incremental cost per extra month with a PASI 75 response among apremilast and all of the biologics approved for psoriasis, including the latest additions of tildrakizumab, risankizumab, and certolizumab pegol.
Materials and methods
Study design
A 10-year cost-effectiveness model was developed from the perspective of a US health plan to compare the incremental cost per extra month with a PASI 75 response among patients with moderate-to-severe psoriasis treated with biologic or small-molecule therapies. The analysis included all of the biologic and small-molecule systemic therapies that were available in the US up to September 2019 (except biosimilars): adalimumab, apremilast, brodalumab, certolizumab pegol, etanercept, guselkumab, infliximab, ixekizumab, risankizumab, secukinumab, tildrakizumab, and ustekinumab.
Patients were assumed to receive one of the therapies examined as their first-line treatment at the start of the analysis. Nonresponders (PASI <75) discontinued their current treatment; 25% went on to receive a mix of topical therapies, phototherapies, and other systemic therapies, while 75% received a second-line therapy treatment (a mix of equally weighted treatments that were included in this analysis) before receiving a mix of topical therapies, phototherapies, and other systemic therapies (Citation11–13). Responders to first- or second-line treatment could withdraw at treatment-specific discontinuation rates over time, and death could occur at any time (Citation11–13).
Model inputs
PASI efficacy data were derived from the 2018 network meta-analysis conducted by the Institute for Clinical and Economic Review (Citation11). Second-line treatment was modeled as a mix of all of the included treatments, but consistent with other recent cost-effectiveness analyses we assumed that they would have reduced efficacy when used in the second line (Citation11–13). Therefore, efficacy was based on the average PASI response of the treatments included in the first line, assuming that 10% fewer patients achieved a PASI 75 response. Treatment discontinuation rates were derived from published literature (Citation15,Citation16). For treatments lacking available data, discontinuation rates were assumed to be the same as that for ustekinumab (Citation12). The probability of death was based on US general population age-specific mortality rates (Citation17).
The 2019 wholesale acquisition cost of each included treatment was obtained from RedBook, and the corresponding dosing regimens were based on the prescription information for each treatment () (Citation18–24). Drug administration costs and laboratory test costs were also estimated () (Citation25). In addition, the analysis accounted for four clinic visits per year for all patients.
Table 1. Key model inputs: drug acquisition costs.
Table 2. Key model inputs: laboratory monitoring costs.
Incremental cost per time in PASI 75
The total direct healthcare cost of each included treatment to a US health plan was estimated and included drug acquisition and administration costs, laboratory test costs, and clinic visit costs. The cumulative months with a PASI 75 response over 10 years were estimated. Both total direct healthcare costs and total months with a PASI 75 response were discounted at 3% per year. The incremental cost-effectiveness ratio (ICER) for each included treatment compared with a mix of topical therapies, phototherapies, and other systemic therapies was estimated as the incremental cost per extra month with a PASI 75 response.
Scenario analyses
Multiple scenario analyses were conducted to determine the effects of varying the model parameters on the ICER of each of the treatments examined. The evaluated model parameters included time horizon, treatment pathway, and costs (). The impact of varying the time horizon was examined in three scenarios in which the time horizon was changed to 3 years, 5 years, and lifetime, respectively. One scenario varied the treatment pathway by assuming that fewer patients received a second-line treatment after they withdrew from first-line treatment (i.e. 50% of nonresponders received a second-line treatment, while the remaining 50% received a mix of topical therapies, phototherapies, and other systemic therapies). Four scenarios were examined to evaluate the impact of varying the cost inputs. One scenario included adverse event (AE) costs, based on the estimated healthcare resource use required for treating severe infection, nonmelanoma skin cancer, and malignancies other than nonmelanoma skin cancer. Unit inpatient costs were derived from the National and State Summaries of Inpatient Charge Data (Citation27). AE rates were derived from prescribing information and the secukinumab submission to the United Kingdom National Institute for Health and Care Excellence; the AE rates for tildrakizumab were assumed to be the same as those for ustekinumab (Citation19–24,Citation26). A second cost scenario included the cost associated with productivity gain in patients with a PASI 75 response, with unit cost estimates based on the analysis carried out by the Institute for Clinical and Economic Review in 2016 (Citation12) and adjusted to 2018 US dollars. Finally, two additional scenarios varied the drug cost for the mix of topical therapies, phototherapies, and other systemic therapies by ± 20%.
Table 3. Summary of the inputs applied in the scenario analyses.
Results
Total health plan costs over 10 years
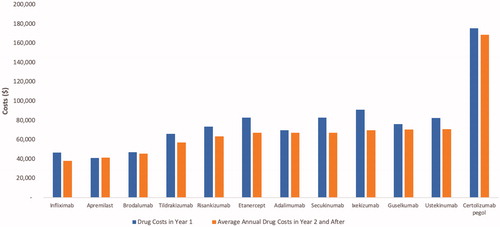
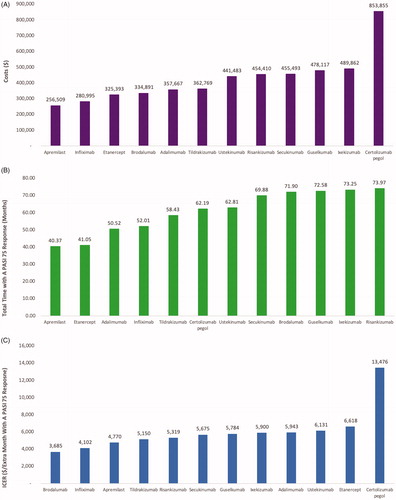
The average annual drug costs after year 1 were lowest for infliximab and highest for certolizumab pegol (). The total costs to a health plan per patient treated with apremilast, infliximab, etanercept, brodalumab, adalimumab, tildrakizumab, ustekinumab, risankizumab, secukinumab, guselkumab, ixekizumab, and certolizumab pegol were estimated to be $256,509 $280,995 $325,393 $334,891 $357,667 $362,769 $441,483 $454,410 $455,493 $478,117 $489,862 and $853,855 over 10 years (. These costs were driven mostly by annual drug wholesale acquisition costs.
Cumulative time in PASI 75 over 10 years
The cumulative time a patient would spend with a PASI 75 response over a 10-year period was estimated to be 40 months, 41 months, 51 months, 52 months, 58 months, 62 months, 70 months, 72 months, 73 months, 73 months, and 74 months for apremilast, etanercept, adalimumab, infliximab, tildrakizumab, certolizumab pegol, ustekinumab, secukinumab, brodalumab, guselkumab, ixekizumab, and risankizumab, respectively (.
Incremental cost per extra month with a PASI 75 response
Based on the cost-effectiveness analysis, the incremental cost per extra month with a PASI 75 response was lowest for brodalumab ($3,685), infliximab ($4,102), apremilast ($4,770), and tildrakizumab ($5,150), followed by risankizumab ($5,319), secukinumab ($5,675), guselkumab ($5,784), ixekizumab ($5,900), adalimumab ($5,943), ustekinumab ($6,131), etanercept ($6,618), and certolizumab pegol ($13,476) (.
Scenario analyses
The rankings of the treatments evaluated via ICER were similar across all of the scenario analyses (). Varying the time horizon to 3 years and including productivity gain costs had the greatest impact on the ICERs, varying by 9%, and −8%, respectively. Including medical costs associated with AEs, varying the treatment pathway, and changing the time horizon to 5 years had relatively small impacts on the results.
Figure 3. Scenario analyses of incremental cost per extra month with a PASI 75 response when the (A) time horizon, (B) treatment pathway, and (C) costs were varied.
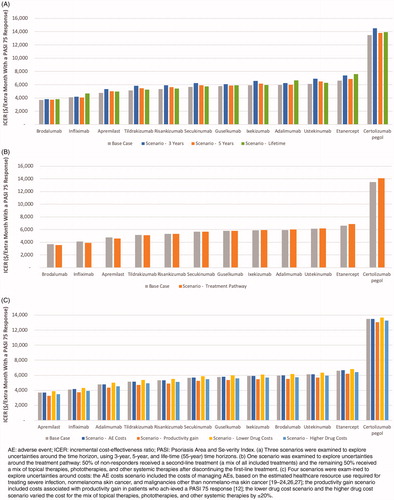
Across all scenarios, the ICER of using tildrakizumab as the first-line treatment varied from −8% to 14%. The ranking was stable for brodalumab, certolizumab pegol, etanercept, infliximab, and tildrakizumab across all of the scenarios. Changes in ranking occurred only among adalimumab, apremilast, risankizumab, secukinumab, guselkumab, ixekizumab, and ustekinumab, which mostly occupied the middle ranks (fifth to tenth place.)
Discussion
Among all the biologics and apremilast approved for moderate-to-severe plaque psoriasis in the US, brodalumab, infliximab, apremilast, and tildrakizumab were the most cost-effective first-line treatments as measured by incremental cost per extra month with a PASI 75 response, and were more cost-effective than risankizumab, secukinumab, guselkumab, ixekizumab, adalimumab, ustekinumab, etanercept, and certolizumab pegol for the treatment of moderate-to-severe psoriasis. The better ranking of brodalumab, infliximab, apremilast, and tildrakizumab was largely driven by lower wholesale acquisition costs compared with the other evaluated treatments. The results of this study may help to inform healthcare providers, patients, and payers with regard to treatment decision-making.
The advent of biologic and small-molecule therapies has revolutionized the treatment of moderate-to-severe psoriasis, but the improvement in efficacy has come with high treatment costs. The wide range of treatment options now available and the high costs mean that cost-effectiveness analyses are particularly important for decision-makers in psoriasis. Impressive short-term efficacy data have been published for many high cost new treatments such as ixekizumab and risankizumab, but psoriasis is a life-long chronic disease, so maintenance of outcomes over time is highly clinically relevant. Therefore, in this study, we chose to examine cost-effectiveness in terms of incremental cost per extra month with PASI 75 over a time horizon of 10 years.
Our study is among the first to evaluate the incremental cost per month with a PASI 75 response for all of the currently available therapies for moderate-to-severe psoriasis, including tildrakizumab, risankizumab, and certolizumab pegol. Among these therapies, tildrakizumab was the fourth most cost-effective treatment for moderate-to-severe psoriasis. Risankizumab was the fifth most cost-effective, while certolizumab pegol was the least cost-effective. The results were robust in multiple scenario analyses, with the highest and lowest drug rankings remaining consistent across the analyses.
The cost-effectiveness rankings in our study are consistent with two recent studies that examined cost-effectiveness via incremental cost per month in PASI 75 (Citation11) and cost to achieve a PASI 75 response (Citation14), respectively. In the 2018 psoriasis analysis conducted by the Institute for Clinical and Economic Review, the relative ranking of ICERs, assessed with regard to time in PASI response, was the same as in our study, except for ixekizumab, which had a lower ICER (fourth versus eighth in our analysis), likely due to the discount (44%, the highest of all) applied to the drug acquisition costs in that analysis (Citation11). In a study of the comparative cost-effectiveness of adalimumab, brodalumab, ixekizumab, secukinumab, and ustekinumab, as measured by the cost to achieve a PASI 75 response, brodalumab was the most cost-effective treatment and, similar to our study, this was primarily due to the lower wholesale acquisition cost (Citation14).
Many patients with moderate-to-severe plaque psoriasis are still untreated or undertreated, despite the wide range of available treatments. This cost-effectiveness analysis provides a more up-to-date comparative analysis of the newer systemic treatments for moderate-to-severe plaque psoriasis and can thereby help US stakeholders make informed decisions when managing psoriasis.
Limitations
Due to the lack of long-term comparative PASI response data, our data were derived from a network meta-analysis of clinical trials and patients’ initial responses (assessed at the end of week 10–16) were assumed to persist until treatment discontinuation or death. Treatment responses may be different in real-world settings and over longer time frames. This study did not fully account for treatment sequencing because limited evidence is available to understand the efficacy of second-line treatment or specific treatment sequences. Different treatment sequences in the real world may produce different results. Assumptions were made for parameters that lacked available data; however, these were similar to those used in previous US cost-effectiveness analyses for psoriasis treatments (Citation11–13). Finally, other patient outcomes, such as health-related quality of life and potential impact of treatments on the cost of comorbidities in patients with psoriasis were not included in the analysis.
Conclusion
Among the newer systemic treatments, brodalumab, infliximab, apremilast, and tildrakizumab had the lowest incremental costs per extra month with a PASI 75 response. Tildrakizumab was more cost-effective than risankizumab, secukinumab, guselkumab, ixekizumab, adalimumab, ustekinumab, etanercept, and certolizumab pegol. Multiple scenario analyses demonstrated the robustness of these results.
Author contributions
XJ, YZ, JC, and THB were responsible for conception and design of the research. Economic modeling was carried out by XJ, JC, and THB; acquisition of data was carried out by YZ, XJ, and JC; XJ, YZ, JC, and THB were responsible for development of the draft manuscript; all authors were responsible for critical revision of the manuscript for important intellectual content.
Acknowledgements
The authors thank Clare Byrne, PhD, for writing assistance. Part of this work was presented in poster format at the 2018 Nexus Meeting of the Academy of Managed Care Pharmacy, October 22–25, Orlando, FL, the 2019 American Academy of Dermatology, March 1, Washington DC, and the 2019 International Society for Pharmacoeconomics and Outcomes Research, May 21, New Orleans, LA.
Disclosure statement
YZ, AM, and SL are employees of Sun Pharma Inc. XJ, JC, and THB are employees of RTI Health Solutions, all of whom were contracted by Sun Pharma Inc. to support the study. SF received research, speaking and/or consulting support from Galderma, GSK/Stiefel, Almirall, Alvotech, Leo Pharma, BMS, Boehringer Ingelheim, Mylan, Celgene, Pfizer, Ortho Dermatology, Abbvie, Samsung, Janssen, Lilly, Menlo, Merck, Novartis, Regeneron, Sanofi, Novan, Qurient, National Biological Corporation, Caremark, Advance Medical, Sun Pharma, Suncare Research, Informa, UpToDate and National Psoriasis Foundation. He is founder and majority owner of www.DrScore.com and founder and part owner of Causa Research, a company dedicated to enhancing patients’ adherence to treatment. AWA has served as a research investigator or consultant to Leo, AbbVie, UCB, Janssen, Lilly, Novartis, Ortho Dermatologics, Sun, Dermavant, BMS, Sanofi, Regeneron, Dermira, and Modmed; non-promotional speaker for AbbVie.
Additional information
Funding
References
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70(3):512–516.
- Luba KM, Stulberg DL. Chronic plaque psoriasis. Am Fam Physician. 2006;73(4):636–644.
- Armstrong AW, Koning JW, Rowse S, et al. Under-treatment of patients with moderate to severe psoriasis in the United States: analysis of medication usage with health plan data. Dermatol Ther (Heidelb). 2017;7(1):97–109.
- Han C, Lofland JH, Zhao N, et al. Increased prevalence of psychiatric disorders and health care-associated costs among patients with moderate-to-severe psoriasis. J Drugs Dermatol. 2011;10(8):843–850.
- Strober B, Karki C, Mason M, et al. Characterization of disease burden, comorbidities, and treatment use in a large, US-based cohort: results from the Corrona Psoriasis Registry. J Am Acad Dermatol. 2018;78(2):323–332.
- Lewis-Beck C, Abouzaid S, Xie L, et al. Analysis of the relationship between psoriasis symptom severity and quality of life, work productivity, and activity impairment among patients with moderate-to-severe psoriasis using structural equation modeling. Patient Prefer Adherence. 2013;7:199–205.
- Korman NJ, Zhao Y, Pike J, et al. Increased severity of itching, pain, and scaling in psoriasis patients is associated with increased disease severity, reduced quality of life, and reduced work productivity. Dermatol Online J. 2015;21(10):13030.
- Yu AP, Tang J, Xie J, et al. Economic burden of psoriasis compared to the general population and stratified by disease severity. Curr Med Res Opin. 2009;25(10):2429–2438.
- Schaefer CP, Cappelleri JC, Cheng R, et al. Health care resource use, productivity, and costs among patients with moderate to severe plaque psoriasis in the United States. J Am Acad Dermatol. 2015;73(4):585–593.
- de Carvalho AV, Duquia RP, Horta BL, et al. Efficacy of immunobiologic and small molecule inhibitor drugs for psoriasis: a systematic review and meta-analysis of randomized clinical trials. Drugs R D. 2017;17(1):29–51.
- Institute for Clinical and Economic Review. Targeted immunomodulators for the treatment of moderate-to-severe plaque psoriasis: condition update. Final Evidence Report. 2018. [cited 2018 Sep 10]. Available from: https://icer-review.org/wp-content/uploads/2017/11/ICER_Psoriasis_Update_Final_Evidence_Report_080118.pdf.
- Institute for Clinical and Economic Review. Targeted immunomodulators for the treatment of moderate-to-severe plaque psoriasis: effectiveness and value. Final Evidence Report. 2016. [cited 2017 Jul 22]. Available from: https://icer-review.org/wp-content/uploads/2016/11/NE_CEPAC_Psoriasis_Evidence_Report_FINAL_012317.pdf.
- Hendrix N, Ollendorf DA, Chapman RH, et al. Cost-effectiveness of targeted pharmacotherapy for moderate to severe plaque psoriasis. J Manag Care Spec Pharm. 2018;24(12):1210–1217.
- Wu JJ, Feldman SR, Rastogi S, et al. Comparison of the cost-effectiveness of biologic drugs used for moderate-to-severe psoriasis treatment in the United States. J Dermatolog Treat. 2018;29(8):769–774.
- Feldman SR, Zhao Y, Navaratnam P, et al. Patterns of medication utilization and costs associated with the use of etanercept, adalimumab, and ustekinumab in the management of moderate-to-severe psoriasis. J Manag Care Spec Pharm. 2015;21(3):201–209.
- Gniadecki R, Bang B, Bryld LE, et al. Comparison of long-term drug survival and safety of biologic agents in patients with psoriasis vulgaris. Br J Dermatol. 2015;172(1):244–252. Jan
- Arias E, Heron M, Xu J. United States Life Tables, 2013. Natl Vital Stat Rep. 2017;66(3):1–64.
- Micromedex: Thomson Reuters. Red Book Online. [cited 2019 May 14]. Available from: http://www.micromedexsolutions.com.
- Celgene Corporation. Apremilast (Otezla) prescribing information. 2017. [cited 2017 Jul 26]. Available from: https://www.celgene.com/content/uploads/otezla-pi.pdf.
- Janssen Biotech. Guselkumab (Tremfya) prescribing information. 2017. [cited 2017 Jul 26]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761061s000lbl.pdf.
- Valeant Pharmaceuticals North America LLC. SILIQ (brodalumab) prescribing information. 2017. [cited 2017 Jul 26]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf.
- Eli Lilly and Company. Ixekizumab (Taltz) prescribing information. 2017. [cited 2017 Jul 26]. Available from: https://pi.lilly.com/us/taltz-uspi.pdf.
- Novartis Pharmaceuticals Corporation. Secukinumab (Cosentyx) prescribing information. 2017. [cited 2017 Jul 26]. Available from: https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/cosentyx.pdf.
- UBC, Inc. Certolizumab pegol (Cimzia) prescribing information. 2017. [cited 2017 Jul 26]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125160s270lbl.pdf.
- Centers for Medicare and Medicaid Services. Physician fee schedule. 2017. [cited 2017 Sep 25]. Available from: https://www.cms.gov/apps/physician-fee-schedule/.
- National Institute for Health and Care Excellence. Single technology appraisal. Secukinumab for treating moderate to severe plaque psoriasis [TA350]. 2015. [cited 2017 Jul 27]. Available from: https://www.nice.org.uk/guidance/ta350.
- Center for Medicare and Medicaid Services. National and State Summaries of Inpatient Charge Data, FY2015. 2017. [cited 2017 Oct 3]. Available from: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Provider-Charge-Data/Inpatient2015.html.