Abstract
Purpose
To compare the short-term cost and effectiveness of calcipotriol/betamethasone dipropionate (Cal/BD) cutaneous foam against nonbiologic systemics in psoriasis patients for whom oral systemic or topical therapy is considered appropriate in seven European countries.
Methods
Matching-adjusted indirect comparisons of four-week PASI-75 responses of Cal/BD foam were performed versus 12-week responses of methotrexate, acitretin, fumaric acid esters (FAE) and 16-week responses of apremilast. Analyses took a payer perspective and included drug, physician visit and monitoring costs.
Results
In all countries, Cal/BD foam generated the lowest cost per responder (CPR). Against methotrexate, apremilast and acitretin, Cal/BD foam generated response for less than €190 in Italy, €195 in Portugal, €216 in Greece, £218 in the United Kingdom, €250 in Belgium, €319 in Spain, and €359 in the Netherlands. Relative to treatment with FAE, Cal/BD foam resulted in response for less than €298, €430, €382 and £262 in Belgium, the Netherlands, Spain and the United Kingdom, respectively. For Cal/BD foam, apremilast and FAE, total costs were driven by drug costs; for methotrexate and acitretin, by monitoring.
Conclusions
Driven by its lower costs and high response rates, Cal/BD foam is likely to be a cost-effective option over the short-term in the investigated psoriasis population.
Introduction
Psoriasis is a chronic, immune-mediated inflammatory condition primarily manifesting on the skin and is estimated to affect 2–3% of the world’s population (Citation1). Plaque psoriasis symptoms negatively impact patient quality of life and are associated with a considerable economic burden (Citation2,Citation3). Treatment aims to reduce the severity of skin symptoms and to improve patient quality of life.
Topical therapies are usually considered the first-line option in mild-to-moderate psoriasis, while phototherapies or oral systemic therapies are generally offered for moderate-to-severe disease or after failure of topical therapy. Systemic therapies are often associated with greater costs to the healthcare system than topical treatments, driven by costs of medication and monitoring (Citation3). Individual patient characteristics and preferences determine the choice of treatment prescribed by physicians.
A frequently used first-line topical psoriasis treatment is the fixed-dose combination of calcipotriol (Cal, 50 μg/g) and betamethasone dipropionate (BD, 0.5 mg/g). The Cal/BD combination formulation has a comparable safety profile and is more effective in treating psoriasis compared to monotherapy with either calcipotriol or betamethasone dipropionate (Citation4–7). A novel cutaneous foam developed for the fixed-dose Cal/BD combination has demonstrated significantly improved efficacy with a comparable safety profile when compared to Cal/BD gel and ointment (Citation8,Citation9), a foam vehicle (Citation10) and Cal or BD foams alone (Citation11).
A recent matching-adjusted indirect comparison (MAIC) (Citation12), showed Cal/BD foam to have better effectiveness compared to apremilast, methotrexate and acitretin and to have comparable effectiveness versus fumaric acid esters (FAE) in patients eligible for either topical or nonbiologic systemic treatment. Methotrexate and acitretin are associated with relatively low acquisition costs but require regular doctor visits and monitoring for adverse events. By comparison, treatments such as apremilast and FAE are associated with higher acquisition costs, and potentially less frequent monitoring in the case of apremilast. Cal/BD foam requires no ongoing monitoring and could be prescribed in general practice. Pharmaco-economic data on whether Cal/BD foam offers better value for money in patients who are eligible for both topical and systemic therapy is essential for optimal decision making in restricted health care budget systems.
The objective of this study is to compare the costs and effectiveness of Cal/BD foam with that of nonbiologic systemic therapies in patients eligible for topical and nonbiologic systemic treatment in seven European countries: Belgium, Greece, Italy, the Netherlands, Portugal, Spain and the United Kingdom.
Materials and methods
Responder rates
Response was defined as a 75% reduction in the Psoriasis Area and Severity Index score (PASI-75). The proportion of patients responding to Cal/BD foam and oral systemic therapies were derived from a recently published MAIC (Citation12). MAIC is a methodological approach for indirect comparisons where no appropriate common comparator is available and individual patient data (IPD) are available for at least one intervention. Here, IPD from 4 Cal/BD foam randomized controlled trials (RCTs) were matched to average study population characteristics from published studies of nonbiologic systemic therapies.
A separate MAIC was available for Cal/BD foam versus each nonbiologic systemic therapy. In each comparison, the IPD from pooled Cal/BD foam trials were re-weighted such that the average baseline characteristics from the Cal/BD foam treatment cohort matched those from each comparator study (Supplementary Table 1). As a result, the absolute responder rate of Cal/BD foam is unique for each matched comparison. The PASI-75 endpoint of the Cal/BD foam trials at week 4 were compared to the PASI-75 endpoints of the comparator trials, which were measured at week 12 for methotrexate, acitretin and FAE, and week 16 for apremilast. Results from a sensitivity analysis comparing the week 12 outcomes for Cal/BD foam (only reported in the PSO-ABLE study) (Citation8) to the week 12 or week 16 outcomes for the nonbiologic systemic therapies were also available (Citation12).
presents the MAIC results from Bewley et al., which fed into the present cost per responder analysis. These results indicate that, among patients considered for either topical or nonbiologic systemic treatment, Cal/BD foam for either 4 weeks or 12 weeks is expected to generate significantly more PASI-75 responders than 12 weeks of treatment with acitretin or methotrexate or 16 weeks of treatment with apremilast. Compared to 12 weeks of therapy with FAE, the absolute responder rate for Cal/BD foam is lower over 4 weeks (42.4% vs. 47.0%) and higher over 12 weeks of treatment (58.5% vs. 47.0%), though the differences are not statistically significant at either time point. No comparison could be made with ciclosporin due to a lack of data (Citation12).
Table 1. Proportion of PASI-75 responders with Cal/BD foam versus nonbiologic systemic therapies.
Costs and health care resource use
The analysis took a payer perspective for each of the European countries and considered both drug and monitoring costs based on local databases and national guidelines. Not all nonbiologic systemic therapies are available or used in all countries. For example, neither apremilast nor FAE are currently available in Portugal; FAE are also not available in Greece. Methotrexate administered via injection is rarely used in the United Kingdom, Greece, Belgium and the Netherlands and was therefore not evaluated in these countries.
Drug costs were calculated by combining the total dose received during treatment with local drug costs obtained from published sources (Supplementary Table 2). For Belgium and the Netherlands, the pharmacy selling price was used. In Portugal and Spain, drug costs were based on their wholesale purchase price, and in Greece, they were based on the reimbursed prices. In Italy, the ex-factory wholesale purchase price was used with mandatory discounts (−5%; −5%) included. Finally, list prices in the United Kingdom were taken from the National Health Service drug tariff.
The Cal/BD foam dose was estimated as a weighted average of 4-week consumption across the included Cal/BD foam RCTs (Citation8–11). Nonbiologic systemic therapy doses were determined according to European Medicines Agency label or the trial included in the analysis and calculated for treatment duration.
The consumption of methotrexate could not be derived from the observational study synthesized in the MAIC (Citation13), so was calculated based on the label posology (Citation14) and a titration period validated by clinical experts. Patients were assumed to start on 7.5 mg of methotrexate per week and escalate to 10 mg in weeks 2 and 3, to 15 mg in weeks 4 to 6, to 20 mg in weeks 7 to 10 and to 25 mg in weeks 11 and 12. This amounts to an average weekly dose of 16.9 mg for the first 12 weeks. The dose of acitretin is determined by patient weight and was derived from Chiricozzi et al. (Citation15), the observational study synthesized in the MAIC. The mean dosage in this study was 25.01 mg (SD ± 6.79) corresponding to a mean of 0.31 mg/kg. Multiplied by the mean patient weight from the PSO-ABLE study (87 kg), the mean daily dosage was 27 mg. The dose of apremilast was 30 mg twice daily following a 5-day titration. Dimethyl fumarate was given at a daily dose of 240 mg three times daily after a nine-week titration schedule.
The type and frequency of general practitioner (GP) visits, specialist dermatologist visits and monitoring tests for each therapy were based on published guidance, where available, and clinical expert opinion (Supplementary Table 3). Baseline tests were also included as these differed across treatments. Unit costs of health care visits and laboratory tests were combined with country-specific estimates of resource use to estimate the total cost of physician visits and monitoring (Supplementary Table 4). Liver ultrasound and biopsies for methotrexate were excluded as a very small percentage of patients are assumed to require this and such testing falls outside the first 12 weeks of therapy. All health care resource use and cost inputs were validated by local clinical experts.
presents the total and disaggregated costs for the treatment period by country.
Table 2. Drug consumption and healthcare costs in seven European countries.
Scenario analysis
The primary analysis used four-week costs and outcomes for Cal/BD foam and compared them to the 12- or 16-week costs and outcomes for nonbiologic systemic therapies. Differences in time points reflect the use of each therapy within its label and correspond to the primary endpoints in the included trials.
Two scenarios were tested to increase comparability between topical and nonbiological systemic treatment duration. In the first scenario, the results of the MAICs using the PASI-75 responder rate from week 12 for Cal/BD foam from the PSO-ABLE (Citation8) study were combined with the mean consumption at week 12 (236.4 g) and compared to the week 12 or 16 endpoint for systemic therapies. In the second, resource use and costs for all comparators were amended to reflect only the first four weeks of treatment, including early follow-up visits and drug titration where applicable. Responder rates were unchanged from the main analysis. That is, costs reflect the first 4 weeks of treatment, but responder rates reflect 12 to 16 weeks of nonbiologic systemic treatment and 4 weeks of Cal/BD foam. For inputs to these scenario analyses, see Supplementary Table 5.
Results
Among patients analyzed in this study, Cal/BD foam is associated with a lower cost per PASI-75 responder than the non-biologic systemic therapies included in the analysis (). Note that the absolute PASI-75 response rate of Cal/BD foam depends on the matched comparison; therefore, its cost per response differs depending on the comparator.
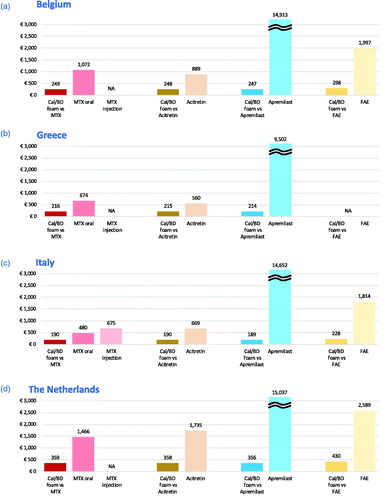
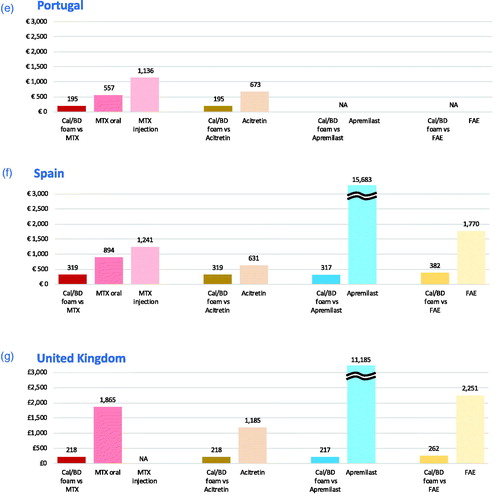
Figure 1. Cost per PASI-75 responder for 4-week Cal/BD foam compared to methotrexate (12 weeks), apremilast (16 weeks), acitretin and fumaric acid esters (12 weeks) in the seven European countries. (a) Belgium, (b) Greece, (c) Italy, (d) the Netherlands, (e) Portugal, (f) Spain, (g) United Kingdom. NOTES: Apremilast cost-per-responder bars exceed scale, value is indicated on top of bar. Analyses carried out versus comparators recommended for use in each country, as reflected in graphic. Cost-per-responder for Cal/BD foam varies due to differences in the individual analyses carried out per pairwise comparison. Acitretin was identified as a comparator at the time of the analysis for Greece (b) but is now temporarily not available: the results are still displayed for completeness. Cal/BD: calcipotriol and betamethasone dipropionate; FAE: fumaric acid esters; MTX: methotrexate; NA: comparison not applicable as comparator not recommended for use in country.
Methotrexate has the lowest drug costs but is among the treatments associated with the highest monitoring costs. The drug costs for acitretin are higher than Cal/BD foam in the United Kingdom, Belgium, the Netherlands and Portugal and lower in Spain, Greece and Italy; however, physician visit and monitoring costs are higher across all countries. Monitoring costs for FAE are higher than for acitretin and lower than for methotrexate (except in Italy), but FAE has a considerably higher drug cost. Apremilast is the systemic with the lowest monitoring costs, but the drug itself costs more than twice as much as any other therapy over a 12- to 16-week period. Cal/BD foam has the lowest total costs, because it requires no laboratory testing at baseline and few, if any, follow-up visits. Even if all Cal/BD foam treated patients are assumed to have two specialist visits in the first 4 weeks of treatment, it remains the option with the lowest total cost across all countries.
In comparison with methotrexate, apremilast and acitretin, the cost per response for Cal/BD foam ranged from €216 (€109.54/0.508) in Greece to €359 (€182.16/0.508) in the Netherlands. The cost per PASI-75 response of oral methotrexate, on the other hand, ranged from €480 (€160.83/0.335) in Italy to £1,865 (£624.90/0.335) in the United Kingdom. In Italy, Portugal and Spain, where methotrexate is available in injection form, the costs per response were €675, €1,136 and €1,241, respectively. For acitretin, the cost per response was as low as €560 (€177.55/0.317) in Greece and up to €1,735 (€549.84/0.317) in the Netherlands. Apremilast was universally associated with the highest cost per responder, ranging from approximately €9500 (€2,052.52/0.216) in Greece to more than €15,600 (€3387.53/0.216) in Spain. Cal/BD foam had a higher cost per response when compared with FAE (from €228 [€96.76/0.424] in Italy to €382 [€162.17/0.424] in Spain) than when compared to other oral systemic treatments due to the lower expected responder rate generated in the MAIC. However, the cost to generate a PASI-75 responder is substantially greater for FAE than Cal/BD foam, ranging from around €1800 (€852.46/0.470) in Italy to nearly €2600 (€1216.75/0.470) in the Netherlands.
In the first scenario analysis, presented in , where Cal/BD foam was assumed to be used for 12 weeks instead of four, both total costs and the proportions of responders increased, though at different rates. The costs increased proportionally more than the responder rate, resulting in an increase to the cost per PASI-75 response for Cal/BD foam; however, it remained substantially lower than all comparator nonbiologic systemic therapies in all markets.
Table 3. Cost per PASI-75 responder for Cal/BD foam compared to nonbiologic systemic therapies when treatment duration is varied in alternative scenarios 1 and 2.
In the second scenario, the total costs for nonbiologic systemic therapies decreased substantially when drug and health care resource use estimates were restricted to just the first four weeks of treatment. In this conservative scenario, Cal/BD foam treatment was less costly than all comparators and remained the treatment with the lowest cost per PASI-75 response in all markets. Neither scenario showed the conclusions of the main analysis to be sensitive to reasonable variation in cost or efficacy assumptions.
Discussion
This study compared the relative effectiveness and costs of treatment with Cal/BD foam versus non-biologic systemic therapies in seven European countries. The cost per responder analysis applied country-specific costs and resource use and combined it with PASI-75 response rates from a recently published MAIC analysis, which evaluated Cal/BD foam and nonbiologic systemic therapies in a population of patients eligible for topical and nonbiologic systemic therapies (Citation12).
There is a clear distinction between patients with mild and severe psoriasis in terms of both symptoms and treatment received: topical therapy is the preferred treatment for mild patients, while the latter require systemic treatment. Additionally, there is an ill-defined group of psoriasis patients that falls in the middle of this spectrum, which is the target population for our study. This patient population is clinically heterogenous, and there is no consensus on their treatment. Determining the best value treatment for these patients, while tailoring care to individual patient needs and preferences, may lead to the consideration of a number of treatments and modalities. This cost per responder analysis demonstrated that Cal/BD foam is consistently associated with better or comparable response rates at a lower total cost than methotrexate, acitretin, apremilast and FAE.
Older non-biologic systemic therapies (methotrexate, acitretin and FAE) are associated with high rates of discontinuation, driven largely by adverse events and the inconvenience of treatment monitoring (Citation16–18). Psoriasis patients are a group with significant comorbidities (Citation19) and the risk of hepatotoxicity with methotrexate therapy is increased among patients with obesity, diabetes or preexisting liver conditions (Citation20). Topical treatment alternatives such as Cal/BD foam minimize the risk of additional comorbidities associated with conventional systemic therapies that have costs that were not accounted for in this analysis. Of course, the presence of a comorbidity such as psoriatic arthritis would favor systemic therapy over topical treatments regardless of the severity of plaque psoriasis. Hence, the optimal type of treatment (e.g. topical, phototherapy or systemic treatment) will vary by individual, and the prescribing physician will need to consider disease activity, comorbidities and patient preference, among others.
Frequent monitoring visits for systemic therapies are not only inconvenient but can cost the patient a great deal in terms of travel and time away from work or family. The payer perspective adopted in this analysis does not capture these costs and does not reflect differences in access observed in the countries included. For example, in an archipelagic country like Greece, patients living on small islands may not have access to specialist or laboratory care and might need to travel to seek such services.
Furthermore, while some studies assessing patients’ compliance report that relative to systemic treatments, topical therapies are associated lower treatment adherence (Citation21–23), others report the opposite (Citation6,Citation24). Both patient- and drug-related factors contribute to nonadherence to topical therapy, including but not limited to, side effects, time for application and ‘messiness’ of the drug (Citation6). Cal/BD foam is greatly preferred to topical ointments and creams due to its ease of application and lack of mess (Citation25).
Guidelines for psoriasis tend to group ill-defined moderate psoriasis patients with the more severe population, and this group can receive any form of therapy as first line, including phototherapy, biologics, and systemics (Citation26). However, patients may have contra-indications to systemic agents or may be reluctant to initiate systemic therapy. Given the enhanced efficacy of the novel Cal/BD foam formulation at a relatively lower cost, it may be beneficial for physicians to consider Cal/BD foam prior to starting a systemic therapy in patients who are eligible for both topical and systemic therapy. For this category of psoriasis patients, optimal use of an effective topical treatment can be of clinical value, reducing or delaying escalation to potentially more aggressive treatments, such as systemic therapy (Citation27). Finally, our study findings support that Cal/BD foam may represent a cost-effective solution ensuring pharmacoeconomic value in addition to clinical benefit.
Strengths and limitations
The study and its results should be interpreted in the context of certain strengths and limitations. This is the first analysis to compare the costs and effectiveness of Cal/BD foam to nonbiologic systemic therapies in a population of patients who could be considered for either. This was enabled by a published MAIC analysis, which took advantage of patient-level data to account for differences in populations between Cal/BD foam clinical trials and studies of nonbiologic systemic therapies, thus enabling comparisons not yet made in head-to-head trials or in network meta-analyses (Citation12). Though data were available for methotrexate (Citation13), acitretin (Citation15), apremilast (Citation28) and FAE (Citation29), no studies provided sufficient data about ciclosporin, and therefore, it could not be included in the cost per responder comparison presented here. The studies in the MAIC were not all RCTs, and thus, the results could be confounded by unobserved differences between patient populations. Indeed, MAIC methods can only account for known confounders or differences in patient populations that are measured and reported. Only a well-controlled head-to-head RCT can avoid this unobserved confounding.
The main limitation of this study is the comparison across different time points, though these are in line with the label usage for each treatment. Cal/BD foam is recommended for a four-week flare treatment, while systemic therapies are intended for longer-term treatment and they take a longer time (12–16 weeks) to achieve maximal effect. Though the main analysis compared the four-week costs and outcomes of Cal/BD foam to 12- and 16-week costs and outcomes of nonbiologic systemic therapies, the results were not sensitive to variation in the duration of the treatment period. Cal/BD foam was still associated with the lowest cost per PASI-75 response when the treatment period was extended to 12 weeks, though this technically falls outside of its label usage. The analysis has focused on short-term effectiveness as data on the use of Cal/BD foam over the long term, that is beyond 12 weeks, is limited.
Cal/BD cost was also the lowest in a conservative scenario where the costs of nonbiologic systemic therapies were restricted to the first 4 weeks, but the effects were held at rates observed after the full 12- or 16-week period. The main properties of Cal/BD foam, namely that it could be effective in eligible patients, requires no monitoring and can be prescribed in primary care, outweigh the high monitoring and follow-up costs for methotrexate and acitretin and the high acquisition costs for apremilast and FAE.
Though there have been many advances in psoriasis treatment made over the last few decades, many systemic therapies are costly and leave some needs unmet. This cost per responder analysis demonstrated that Cal/BD foam, used over 4–12 weeks, can generate more PASI-75 responders than apremilast, acitretin and methotrexate and a comparable proportion of responders to FAE at a lower total cost in patients eligible for either topical or systemic treatment. These results indicate a potential economic benefit of Cal/BD foam and supports its use before considering a switch to oral systemic therapies in suitable patients.
Supplemental Material
Download PDF (495.4 KB)Acknowledgements
The authors thank Fatima Salih for editorial support and Jana Tillotson for graphic design support during the preparation of this article.
Disclosure statement
Deepak Balak is a consultant/speaker for AbbVie, Almirall, Celgene, Janssen, LEO Pharma, Novartis, Sanofi-Genzyme, and has received research grants from LEO Pharma. Jose-Manuel Carrascosa has participated as invited speaker, advisor and principal and site investigator in clinical trials for Abbvie, Almirall, Celgene, Gebro, Janssen, LEO Pharma, Lilly, Novartis and Pfizer. Piergiacomo Calzavara-Pinton has served as an advisory board member for Abbvie, Almirall, Galderma, LEO Pharma, Meda, Pierre Fabre, and Sanofi. Stamatis Gregoriou and Joana Antunes have been invited speakers for LEO Pharma. Antony Bewley declared no conflict of interest. Martin Nyeland was an employee of LEO Pharma at the time the analysis was undertaken. Marta Viola and Laura Sawyer are employed by Symmetron Limited, which received funding from LEO Pharma for this research. Lidia Becla is an employee of LEO Pharma.
Additional information
Funding
References
- Goff KL, Karimkhani C, Boyers LN, et al. The global burden of psoriatic skin disease. Br J Dermatol. 2015;172(6):1665–1668.
- de Korte J, Sprangers MA, Mombers FM, et al. Quality of life in patients with psoriasis: a systematic literature review. J Investig Dermatol Symp Proc. 2004;9(2):140–147.
- Feldman SR, Burudpakdee C, Gala S, et al. The economic burden of psoriasis: a systematic literature review. Expert Rev Pharmacoecon Outcomes Res. 2014;14(5):685–705.
- Lambert J, Hol CW, Vink J. Real-life effectiveness of once-daily calcipotriol and betamethasone dipropionate gel vs. ointment formulations in psoriasis vulgaris: 4- and 12-week interim results from the PRO-long study. J Eur Acad Dermatol Venereol. 2014;28(12):1723–1731.
- Lambert J, Hol CW, Vink J. Real-life effectiveness of once-daily calcipotriol and betamethasone dipropionate gel vs. ointment formulations in psoriasis vulgaris: final analysis of the 52-week PRO-long study. J Eur Acad Dermatol Venereol. 2015;29(12):2349–2355.
- Mason AR, Mason J, Cork M, et al. Topical treatments for chronic plaque psoriasis. Cochrane Database Syst Rev. 2013:28(3):CD005028.
- Menter A, Gold LS, Bukhalo M, et al. Calcipotriene plus betamethasone dipropionate topical suspension for the treatment of mild to moderate psoriasis vulgaris on the body: a randomized, double-blind, vehicle-controlled trial. J Drugs Dermatol. 2013;12(1):92–98.
- Paul C, Stein Gold L, Cambazard F, et al. Calcipotriol plus betamethasone dipropionate aerosol foam provides superior efficacy vs. gel in patients with psoriasis vulgaris: randomized, controlled PSO‐ABLE study. J Eur Acad Dermatol Venereol. 2017;31(1):119–126.
- Koo J, Tyring S, Werschler WP, et al. Superior efficacy of calcipotriene and betamethasone dipropionate aerosol foam versus ointment in patients with psoriasis vulgaris–A randomized phase II study. J Dermatolog Treat. 2016;27(2):120–127.
- Leonardi C, Bagel J, Yamauchi P, et al. Efficacy and safety of calcipotriene plus betamethasone dipropionate aerosol foam in patients with psoriasis vulgaris-a Randomized Phase III Study (PSO-FAST)). J Drugs Dermatol. 2015;14(12):1468–1477.
- Lebwohl M, Tyring S, Bukhalo M, et al. Fixed combination aerosol foam calcipotriene 0.005% (Cal) plus betamethasone dipropionate 0.064% (BD) is more efficacious than Cal or BD aerosol foam alone for psoriasis vulgaris: a randomized, double-blind, multicenter, three-arm, phase 2 study. J Clin Aesthet Dermatol. 2016;9(2):34–41.
- Bewley A, Shear NH, Calzavara-Pinton PG, et al. Calcipotriol plus betamethasone dipropionate aerosol foam versus apremilast, methotrexate, acitretin, or fumaric acid esters for the treatment of plaque psoriasis: a matching-adjusted indirect comparison. J Eur Acad Dermatol Venereol. 2019;33:1107–1115.
- Cabello Zurita C, Grau Pérez M, Hernández Fernández CP, et al. Effectiveness and safety of Methotrexate in psoriasis: an eight-year experience with 218 patients. The Journal of Dermatological Treatment. 2017;28(5):401–405.
- Hospira UK Limited. Methotrexate 10 mg tablets: summary of product characteristics. [cited 2019. Available from: https://www.medicines.org.uk/emc/product/6790/smpc.
- Chiricozzi A, Panduri S, Dini V, et al. Optimizing acitretin use in patients with plaque psoriasis. Dermatol Ther. 2017;30:e12453.
- Arnold T, Schaarschmidt ML, Herr R, et al. Drug survival rates and reasons for drug discontinuation in psoriasis. J Dtsch Dermatol Ges. 2016;14(11):1089–1099.
- Davila-Seijo P, Dauden E, Carretero G, et al. Survival of classic and biological systemic drugs in psoriasis: results of the BIOBADADERM registry and critical analysis. J Eur Acad Dermatol Venereol. 2016;30(11):1942–1950.
- Yeung H, Wan J, Van Voorhees AS, et al. Patient-reported reasons for the discontinuation of commonly used treatments for moderate to severe psoriasis. J Am Acad Dermatol. 2013;68(1):64–72.
- Gottlieb AB, Chao C, Dann F. Psoriasis comorbidities. J Dermatolog Treat. 2008;19(1):5–21.
- Maybury CM, Jabbar-Lopez ZK, Wong T, et al. Methotrexate and liver fibrosis in people with psoriasis: a systematic review of observational studies. Br J Dermatol. 2014;171(1):17–29.
- Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(s3):61–67.
- Tan X, Feldman SR, Chang J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9(10):1263–1271.
- World Health Organisation. Global report on psoriasis. Geneva: WHO; 2016.
- Zaghloul SS, Goodfield M. Objective assessment of compliance with psoriasis treatment. JAMA Dermatology. 2004;140(4):408–414.
- Hong C-H, Papp KA, Lophaven KW, et al. Patients with psoriasis have different preferences for topical therapy, highlighting the importance of individualized treatment approaches: randomized phase IIIb PSO-INSIGHTFUL study. J Eur Acad Dermatol Venereol. 2017;31(11):1876–1883.
- Knuckles MLF, Levi E, Soung J. Defining and treating moderate plaque psoriasis: a dermatologist survey. J Dermatolog Treat. 2018;29(7):658–663.
- Duvetorp A, Levin L, Mattsson E, et al. A cost-utility analysis of calcipotriol/betamethasone dipropionate aerosol foam versus ointment for the topical treatment of psoriasis vulgaris in Sweden. Acta Derm Venerol. 2019;99(4):393–399.
- Strober B, Bagel J, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate plaque psoriasis with lower BSA: week 16 results from the UNVEIL Study. J Drugs Dermatol. 2017;16(8):801–808.
- Inzinger M, Weger W, Heschl B, et al. Methotrexate vs. fumaric acid esters in moderate-to-severe chronic plaque psoriasis: data registry report on the efficacy under daily life conditions. J Eur Acad Dermatol Venereol. 2013;27(7):861–866.