Abstract
Background
Patients with psoriasis experience decreased health-related quality of life due to physical and psychological burdens.
Objective
To assess the effect of a highly effective psoriasis treatment (secukinumab) on domains of the 3-level EuroQol 5 Dimensions questionnaire (EQ-5D-3L) in patients with moderate-to-severe psoriasis who reported problems at baseline.
Methods
This pooled analysis of four phase 3 clinical trials (ERASURE [NCT01365455], FIXTURE [NCT01358578], FEATURE [NCT01555125], and JUNCTURE [NCT01636687]) included patients with moderate-to-severe psoriasis randomized to receive placebo or secukinumab 300 mg and who reported problems in the EQ-5D-3L domains of mobility, self-care, usual activities, pain/discomfort, or anxiety/depression at baseline. Percentage of patients reporting problems in each domain were compared at Weeks 4, 8, and 12 between patients receiving secukinumab 300 mg and placebo.
Results
At baseline, 570 patients receiving secukinumab 300 mg and 579 receiving placebo reported ≥1 problem in the EQ-5D-3L domains. Patients receiving secukinumab 300 mg reported improvements in all 5 domains at Weeks 4, 8, and 12 compared with placebo (all p < .0001).
Conclusion
These findings provide additional evidence of the quality-of-life impairment in patients with moderate-to-severe psoriasis and highlight the improvement across all EQ-5D-3L domains among patients treated with secukinumab.
Keywords:
Introduction
Psoriasis is a chronic, systemic, inflammatory disorder of the skin that affects >7.4 million people in the United States, with an estimated prevalence of 2% to 4% (Citation1). Beyond skin manifestations, patients experience decreased health-related quality of life due to physical and psychological burdens (Citation2). Skin pain and discomfort have been reported in ≈40% of patients with psoriasis (Citation3,Citation4) and anxiety and depression in ≈20% of patients in clinical practice. Due to the multifaceted burden associated with psoriasis, patients require a treatment with feature attributes that holistically manage the physical and psychological symptoms of psoriasis (Citation5).
Secukinumab is a fully human monoclonal antibody that selectively neutralizes interleukin 17A and has long-lasting efficacy and safety in the treatment of the complete spectrum of psoriasis manifestations—including nail, scalp, and palmoplantar psoriasis—and psoriatic arthritis (PsA) (Citation6–11). Limited information is available from clinical trials on the effect of secukinumab treatment on the domains of the 3-level version of the EuroQol 5 Dimensions questionnaire (EQ-5D-3L) in patients with moderate-to-severe psoriasis.
The aim of this study was to assess the effect of a highly effective treatment, secukinumab, on the domains of the EQ-5D-3L in patients with moderate-to-severe psoriasis who reported problems at baseline.
Patients and methods
Patient population
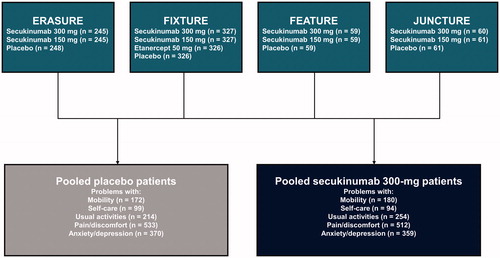
This pooled analysis of four phase 3 clinical trials (ERASURE, FIXTURE, FEATURE, and JUNCTURE) included patients with moderate-to-severe psoriasis who were randomized to receive placebo or secukinumab 300 mg and who reported any problems in the EQ-5D-3L domains of mobility, self-care, usual activities, pain/discomfort, or anxiety/depression at baseline ().
Study variables and data analysis
The EQ-5D-3L is a general health instrument with 5 domains (mobility, self-care, usual activities [e.g. work, study, housework, family or leisure activities], pain/discomfort, and anxiety/depression), each scored at 3 levels (Citation12). For the purpose of this study, “some problems” and “extreme problems” were grouped as “any problems with,” resulting in scoring at 2 levels.
Demographic characteristics (age, sex, race, and body weight), treatment history, and clinical characteristics (Psoriasis Area and Severity Index [PASI]; body surface area; Investigator’s Global Assessment modified 2011 categories; mobility, self-care, usual activity, pain/discomfort, and anxiety/depression domains of the EQ-5D-3L; Dermatology Life Quality Index [DLQI]; and concurrent PsA) were examined at baseline for patients with and without problems in any of the EQ-5D-3L domains.
The percentages of patients receiving placebo or secukinumab 300 mg reporting problems in the EQ-5D-3L mobility, self-care, usual activities, pain/discomfort, or anxiety/depression domains were compared between baseline and Weeks 4, 8, and 12. An exploratory analysis of the proportion of patients reporting problems in EQ-5D-3L domains at Week 12 was performed among patients who achieved PASI 100 vs those who did not. Due to the small number of patients receiving placebo who achieved PASI 100 responses, this analysis was limited to patients receiving secukinumab 300 mg. All analyses were for hypothesis generation only.
Results
Patient demographics and clinical characteristics
Of 2403 patients who were randomized to the four phase 3 clinical trials (ERASURE, FIXTURE, FEATURE, and JUNCTURE), 691 patients were treated with secukinumab 300 mg and 694 patients were treated with placebo. Of these, 570 patients treated with secukinumab 300 mg and 579 patients treated with placebo met the inclusion criteria of reporting an issue in ≥1 EQ-5D-3L domain. Among these 570 and 579 patients with problems in EQ-5D-3L domains who received secukinumab 300 mg and placebo, respectively, the mean ages were 44.7 and 44.9 years, 66.7% and 67.9% were men, and the mean times since psoriasis diagnosis were 16.8 and 17.2 years (). Overall, respectively, 20.9% and 22.8% of those who received secukinumab 300 mg and placebo had a concurrent PsA diagnosis, and 20.7% and 23.0% had prior biologic use. Overall, patients who reported problems in any EQ-5D-3L domain were more likely than those without any problems to be men, have a shorter time to psoriasis diagnosis, and have a concurrent PsA diagnosis and prior systemic psoriasis therapy.
Table 1. Baseline demographics and treatment history of the pooled population of patients with psoriasis who received secukinumab 300 mg or placebo stratified by patients who reported any vs no problems in the EQ-5D-3L domains.
In the secukinumab 300-mg and placebo groups, respectively, 31.6% and 29.7% of patients reported any problems with mobility, 17.4% and 16.2% with self-care, 44.6% and 37.0% with usual activities, 89.8% and 92.1% with pain/discomfort, and 63.0% and 63.9% with anxiety/depression at baseline (). As expected, patients who reported problems in any EQ-5D-3L domain tended to have more severe disease and worse quality of life at baseline than did those who did not report any problems.
Table 2. Baseline clinical characteristics of the pooled population of patients with psoriasis who received secukinumab 300 mg or placebo stratified by patients who reported any vs no problems in the EQ-5D-3L domains.
Change in EQ-5D-3L domains
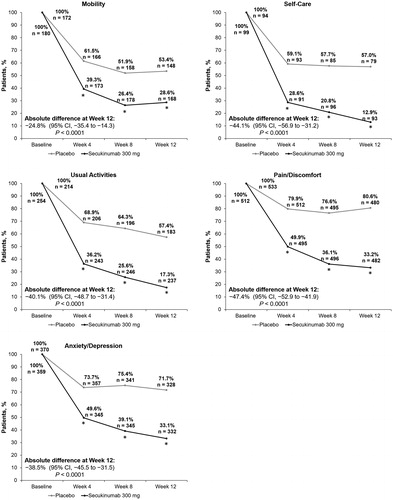
Across all EQ-5D-3L domains, treatment with secukinumab 300 mg was more effective than placebo from baseline to Week 4, continuing through Weeks 8 and 12 (p < .0001 for all domains and time points) (). The percentage of patients with problems in mobility at Week 4 was lower with secukinumab 300 mg than with placebo (39.3% vs. 61.5%; absolute difference, −22.1% [95% CI, −35.5 to −11.8]). Similar trends were observed at Weeks 8 (26.4% vs. 51.9%; absolute difference, −25.5% [95% CI, −35.6 to −15.4]) and 12 (28.6% vs. 53.4%; absolute difference, −24.8% [95% CI, −35.4 to −14.3]). The percentage of patients with problems in self-care was lower with secukinumab 300 mg than with placebo at Week 4 (28.6% vs. 59.1%; absolute difference, −30.6% [95% CI, −44.2 to −16.9]) and at Weeks 8 (20.8% vs. 57.7%; absolute difference, −36.8% [95% CI, −50.1 to −23.5]) and 12 (12.9% vs. 57.0%; absolute difference, −44.1% [95% CI, −56.9 to −31.2]). For problems with usual activities, a lower percentage of patients treated with secukinumab 300 mg (36.2%) than with placebo (68.9%) reported problems at Week 4 (absolute difference, −32.7% [95% CI, −41.5 to −24.0]). The respective results for problems with usual activities at Weeks 8 (25.6% vs. 64.3%; absolute difference, −38.7% [95% CI, −47.3 to −30.0]) and 12 (17.3% vs. 57.4%; absolute difference, −40.1% [95% CI, −48.7 to −31.4]) were similar. The percentage of patients with pain/discomfort at Week 4 was lower with secukinumab 300 mg than with placebo (49.9% vs. 79.9%; absolute difference, −30.0% [95% CI, −35.6 to −24.4]), which continued until Weeks 8 (36.1% vs. 76.6%; absolute difference, −40.5% [95% CI, −46.1 to −34.8]) and 12 (33.2% vs. 80.6%; absolute difference, −47.4% [95% CI, −52.9 to −41.9]). Lastly, the percentage of patients who had problems with anxiety/depression at Week 4 was also lower with secukinumab 300 mg than with placebo (49.6% vs. 73.7%; absolute difference, −24.1% [95% CI, −31.1 to −17.1]). Similar respective trends were observed at Weeks 8 (39.1% vs. 75.4%; absolute difference, −36.2% [95% CI, −43.1 to −29.4]) and 12 (33.1% vs. 71.7%; absolute difference, −38.5% [95% CI, −45.5 to −31.5]).
Exploratory analysis among patients who achieved skin clearance
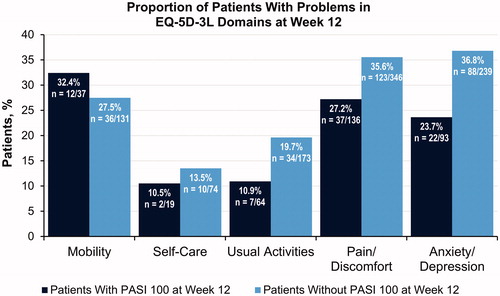
An exploratory analysis of the proportion of patients reporting problems in EQ-5D-3L domains at Week 12, between patients treated with secukinumab who achieved PASI 100 (n = 153) and those who did not (n = 383), was performed to assess whether achievement of skin clearance was associated with improved activities of daily living relative to the total population (). We observed that the proportion of patients who experienced problems in the self-care, usual activities, pain/discomfort, and anxiety/depression domains of the EQ-5D-3L was lower among those with PASI 100 at Week 12, suggesting that achievement of skin clearance may lead to additional improvements in these aspects of daily living—particularly for pain/discomfort and anxiety/depression. A higher proportion of patients who achieved PASI 100 responses experienced problems in the mobility domain at Week 12 compared with those who did not (32.4% vs. 27.5%), indicating that achievement of skin clearance may not have translated to better mobility for some patients.
Figure 3. Exploratory analysis of the proportion of patients reporting problems in the EQ-5D-3L domains at Week 12 among patients treated with secukinumab 300 mg who achieved PASI 100 responses vs those who did not. EQ-5D-3L, 3-level version of the EuroQol 5 Dimensions questionnaire; PASI, Psoriasis Area and Severity Index.
Discussion
In this pooled analysis of four phase 3 clinical trials of patients with moderate-to-severe psoriasis, secukinumab improved the EQ-5D-3L mobility, self-care, usual activities, pain/discomfort, and anxiety/depression domains from baseline as early as 4 weeks. The improvements were persistent through Weeks 8 and 12.
Secukinumab 300 mg is more rapidly and consistently effective across all body regions compared with placebo in patients with moderate-to-severe plaque psoriasis in the same trials used for this study (Citation13,Citation14). The time to a 50% mean reduction in PASI subscore was observed at 2 weeks for the head and neck and at 3–4 weeks for the trunk, upper limbs, and lower limbs. In ERASURE and FIXTURE, the improvement in PASI scores with secukinumab also reflected greater reductions in the patient-reported symptoms of itching, pain, and scaling and in the DLQI subscales when compared with placebo (Citation15–17). Disease clearance occurred rapidly on highly visible areas of the skin, as did alleviation of patient-reported symptoms; however, data to explain how this translates into physical, psychological, and emotional improvement for patient quality of life are limited.
In this study, health-related quality of life related to impaired movement, physical disability, and psychological burdens improved in all patients. Approximately 77% of patients with available EQ-5D-3L data had a problem with pain/discomfort and 53% with anxiety/depression, suggesting that the majority of patients with moderate-to-severe plaque psoriasis have additional quality-of-life complications that need to be managed in addition to their disease symptoms. By Week 12 in this study, 66.8% of patients who reported problems at baseline and were treated with secukinumab 300 mg reported no problems with pain/discomfort and 66.9% reported no problems with anxiety/depression. Similar results were observed in the other EQ-5D-3L domains: 71.4% of patients reported no problems with mobility, 87.1% no problems with self-care, and 82.7% no problems with usual activities at Week 12.
We performed an exploratory analysis between patients treated with secukinumab who achieved and did not achieve PASI 100 to evaluate whether achievement of skin clearance tracked with improvements in different aspects of daily living. Patients who achieved skin clearance at Week 12 were less likely than the overall study population to have problems in the self-care, usual activities, pain/discomfort, and anxiety/depression domains; the most pronounced differences were observed for pain/discomfort and anxiety/depression. In contrast, this trend was not observed for the mobility domain, suggesting that skin symptoms may be less likely to affect this domain than the other domains. However, the sample size for the number of patients with mobility issues who achieved PASI 100 was small; therefore, a difference of 1 or 2 patients reporting or not reporting problems with this domain could greatly alter the proportions. It is also possible that when patients achieve skin clearance, they want to be more socially active and are more bothered by impairments or limitations in mobility. Lastly, because secukinumab reduces inflammation of the joints, it is likely that the effect on the joints is large and overshadows any effect that achievement of PASI 100 has on mobility. Because joints improve so much, there may be a large change in mobility relative to baseline among both patients who achieve and do not achieve PASI 100, so that the difference in mobility between these subgroups is small relative to that in patients who receive placebo.
Because psoriasis is a heterogeneous disease that can pose significant clinical burden beyond skin symptoms, assessment of how treatments improve quality of life and health utility scores is of great importance. A previous analysis of patients enrolled in the ERASURE and FIXTURE trials evaluated the correlation between greater response in skin clearance and quality of life; a higher proportion of patients with PASI 90–100 than with PASI 75–89 responses at Week 12 achieved a DLQI 0/1 response (69.4% vs. 47.1%; p < .001), which was sustained through Week 52 (74.0% vs. 56.7%; p < .001) (Citation18). However, few studies have examined relationships between skin clearance and improvements in EQ-5D and/or EuroQol–visual analog scale scores, particularly in the United States (Citation19–24). In the Swedish Psoriasis Registry, marginal changes in PASI were associated with a nonlinear response in EQ-5D-3L (Citation19), and EQ-5D-3L scores correlated weakly with PASI (r = −0.25; p < .001) (Citation20). Similarly, in real-world studies from Japan and Iran, there was little or no correlation between EQ-5D scores and PASI (Citation21,Citation22), while in a cross-sectional study there was only a moderate correlation (r = −0.43; p < .05) (Citation23). Based on randomized controlled trials of ixekizumab, the generic 5-level version of the EQ-5D does not differentiate between the highest PASI response levels (PASI > 90–100 vs. PASI 100) as effectively as a psoriasis-specific utility score (EQ-PSO) (Citation24). Although exploratory in nature and performed for hypothesis generation only, data from this study provide dermatologists with useful information on how treating skin symptoms with secukinumab improves health utility scores.
Limitations
Because the patients with moderate-to-severe psoriasis included in this study were from secukinumab clinical trials, the results may not be generalizable to all patients with psoriasis. No adjustments for any potential confounding variables were conducted using multivariate analysis, possibly introducing bias. This analysis was limited to patients who reported any problems in mobility, self-care, usual activities, pain/discomfort, and/or anxiety/depression at baseline. Furthermore, patients were not randomized with respect to EQ-5D scores or impairment; therefore, there may have been imbalances in impairment across treatment arms at baseline.
Conclusions
In all EQ-5D-3L domains, similar trends were observed between the secukinumab 300 mg and placebo groups. Secukinumab 300 mg resulted in greater improvements compared with placebo at Week 4, which continued through Weeks 8 and 12. These findings provide additional evidence of the quality-of-life impairment in patients with moderate-to-severe psoriasis and highlight the improvement across all domains of the EQ-5D-3L among patients treated with secukinumab.
Acknowledgments
Support for third-party writing assistance for this manuscript, furnished by Meaghan Paganelli, PhD, and Eric Deutsch, PhD, CMPP, of Health Interactions, Inc, was provided by Novartis Pharmaceuticals Corporation, East Hanover, NJ.
Disclosure statement
Dr Feldman has received consulting, speaking, and/or research support from Novartis, AbbVie, Celgene, Sun Pharma, Janssen, Lilly, and Ortho Dermatologics. Dr Gomez and Dr Meng are employees of Novartis. Dr Germino was an employee of Novartis Pharmaceuticals Corporation at the time of this study and is a current employee of Pfizer.
Additional information
Funding
References
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70(3):512–516.
- Coates LC, Kavanaugh A, Mease PJ, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68(5):1060–1071.
- Korman NJ, Zhao Y, Li Y, et al. Clinical symptoms and self-reported disease severity among patients with psoriasis – implications for psoriasis management. J Dermatolog Treat. 2015;26(6):514–519.
- Ljosaa TM, Rustoen T, Mork C, et al. Skin pain and discomfort in psoriasis: an exploratory study of symptom prevalence and characteristics. Acta Derm Venerol. 2010;90(1):39–45.
- Strober B, Karki C, Mason M, et al. Characterization of disease burden, comorbidities, and treatment use in a large, US-based cohort: results from the Corrona Psoriasis Registry. J Am Acad Dermatol. 2018;78(2):323–332.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338.
- Paul C, the JUNCTURE study group, Lacour JP, Tedremets L, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. 2015;29(6):1082–1090.
- Blauvelt A, the FEATURE Study Group, Prinz JC, Gottlieb AB, et al. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015;172(2):484–493.
- Bissonnette R, Luger T, Thaçi D, et al. Secukinumab sustains good efficacy and favourable safety in moderate-to-severe psoriasis after up to 3 years of treatment: results from a double-blind extension study. Br J Dermatol. 2017;177(4):1033–1042.
- Armstrong AW, Vender R, Kircik L. Secukinumab in the treatment of palmoplantar, nail, scalp, and pustular psoriasis. J Clin Aesthet Dermatol. 2016;9(6 Suppl 1):S12–S16.
- Mease P, van der Heijde D, Landewé R, et al. Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: primary results from the randomised, double-blind, phase III FUTURE 5 study. Ann Rheum Dis. 2018;77(6):890–897.
- EuroQol G. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208.
- Kircik L, Fowler J, Weiss J, et al. Efficacy of secukinumab for moderate-to-severe head and neck psoriasis over 52 weeks: pooled analysis of four phase 3 studies. Dermatol Ther (Heidelb). 2016;6(4):627–638.
- Menter A, Cather JC, Jarratt M, et al. Efficacy of secukinumab on moderate-to-severe plaque psoriasis affecting different body regions: a pooled analysis of four phase 3 studies. Dermatol Ther (Heidelb). 2016;6(4):639–647.
- Strober B, Zhao Y, Tran MH, et al. Psychometric validation of the Psoriasis Symptom Diary using Phase III study data from patients with chronic plaque psoriasis. Int J Dermatol. 2016;55(3):e147–e155.
- Strober B, Sigurgeirsson B, Popp G, et al. Secukinumab improves patient-reported psoriasis symptoms of itching, pain, and scaling: results of two phase 3, randomized, placebo-controlled clinical trials. Int J Dermatol. 2016;55(4):401–407.
- Korman NJ, Sofen H, Fretzin S, et al. Secukinumab provides better relief from the impact of psoriasis on daily activities and personal relationships than etanercept: results of two phase 3 placebo-controlled randomized clinical trials in moderate-to-severe psoriasis. J Dermatolog Treat. 2017;28(5):384–389.
- Elewski BE, Puig L, Mordin M, et al. Psoriasis patients with Psoriasis Area and Severity Index (PASI) 90 response achieve greater health-related quality-of-life improvements than those with PASI 75-89 response: results from two phase 3 studies of secukinumab. J Dermatolog Treat. 2017;28(6):492–499.
- Geale K, Henriksson M, Schmitt-Egenolf M. How is disease severity associated with quality of life in psoriasis patients? Evidence from a longitudinal population-based study in Sweden. Health Qual Life Outcomes. 2017;15(1):151.
- Norlin JM, Steen Carlsson K, Persson U, et al. Analysis of three outcome measures in moderate to severe psoriasis: a registry-based study of 2450 patients. Br J Dermatol. 2012;166(4):797–802.
- Masaki S, Tatsukawa R, Uryu M, et al. Treatment satisfaction, willingness to pay and quality of life in Japanese patients with psoriasis. J Dermatol. 2017;44(2):143–146.
- Moradi M, Rencz F, Brodszky V, et al. Health status and quality of life in patients with psoriasis: an Iranian cross-sectional survey. Arch Iran Med. 2015;18(3):153–159.
- Herédi E, Rencz F, Balogh O, et al. Exploring the relationship between EQ-5D, DLQI and PASI, and mapping EQ-5D utilities: a cross-sectional study in psoriasis from Hungary. Eur J Health Econ. 2014;15(S1):S111–S119.
- Pickard AS, Gooderham M, Hartz S, et al. EQ-5D health utilities: exploring ways to improve upon responsiveness in psoriasis. J Med Econ. 2017;20(1):19–27.