Abstract
Purpose
To compare PASI outcomes of approved biologics and apremilast after 1 year of treatment.
Methods
A systematic review identified RCTs and long-term extensions reporting PASI 75, 90, and 100 responses in adults with moderate-to-severe psoriasis. Data for analysis were modeled using a Bayesian multinomial likelihood model with probit link.
Results
Twenty-eight studies reporting PASI responses were included in the network meta-analysis. Differences in study design led to a stepwise approach to synthesis, consisting of two analyses. The primary analysis included nine RCTs investigating comparative efficacy at 1 year. Results indicated risankizumab, brodalumab, and guselkumab were the most effective therapies, followed by ixekizumab and secukinumab; all demonstrated superiority to ustekinumab and etanercept. The secondary analysis extended the primary analysis with 19 further studies comparing active interventions to placebo outcomes extrapolated from induction. The interventions generating the highest PASI response were the same as the primary analysis. These therapies were more effective than apremilast, ustekinumab, adalimumab, certolizumab, etanercept, and infliximab.
Conclusions
This NMA demonstrated that evaluated IL-17 and IL-23 inhibitors outperformed other biological therapies after 1 year. Risankizumab had a higher probability of achieving PASI outcomes than all other biologics, except brodalumab and guselkumab, where no significant difference could be concluded.
Introduction
Psoriasis is a chronic inflammatory systemic disorder which affects approximately 100 million people worldwide (Citation1). Psoriasis symptoms can present on both skin and nails in varying severities and a quarter of patients suffer from moderate-to-severe disease. The most characteristic skin lesions are localized, demarcated, red papules, and plaques often covered with white or silver scales (Citation2).
Due to the chronic nature of the disease, most patients require long-term therapy (Citation3). In clinical trials for psoriasis there are two defined treatment periods: a short 10- to 16-week induction phase, followed by maintenance phase therapy (Citation4). Studies evaluating treatments beyond induction use a variety of different study designs (Citation5,Citation6). In many trials, some or all patients from the induction phase placebo arm switch to the active therapy and can no longer provide a suitable comparison for the patients receiving a licensed dose throughout the induction and maintenance phases. The maintenance phases of these trials are usually non-randomized and may be considered single-arm studies. In another group of trials, referred to as randomized withdrawal trials, treatment responders from one or more induction phase arms are re-randomized to continue active therapy or to receive placebo. Therefore, such a randomized withdrawal design compares continuous versus interrupted therapy, rather than the comparative efficacy of maintained interventions.
For clinical decision making, the most useful studies are those that compare interventions head to head from baseline through to a long-term endpoint, though there are not many of these, especially for older treatments. However, most clinical studies focus on short-term induction periods (Citation7).
In recent years, the number of treatments available for moderate-to-severe psoriasis has increased, enabling dermatologists to optimize therapy for patients to achieve better outcomes (Citation8); however, choosing between therapies can be challenging. Numerous network meta-analyses (NMAs) that have investigated psoriasis therapies over the first 10 to 16 weeks of treatment have been published (Citation9–14) and yet only two reviews have quantitatively assessed the long-term efficacy of psoriasis therapies: one at 6 months (Citation15) and another at 1 year (Citation16). Since these studies were published, four new treatments have been licensed for psoriasis – certolizumab pegol, guselkumab, tildrakizumab, and risankizumab. In addition, new long-term studies of previously approved biologics have been published (Citation17). As new therapies become licensed and new evidence emerges, it is important to evaluate the available therapies to inform treatment decisions.
The objective of this systematic literature review (SLR) and NMA was to update the study by Sawyer et al. (Citation16) with the inclusion of new evidence and recently licensed interventions. This study aimed to compare the efficacy of approved targeted therapies (brodalumab, ixekizumab, secukinumab, guselkumab, risankizumab, tildrakizumab, ustekinumab, adalimumab, certolizumab pegol, etanercept, infliximab, apremilast) for moderate-to-severe psoriasis after around 1 year of therapy. Efficacy was measured using the Psoriasis Area and Severity Index (PASI) which captures both severity of psoriatic lesions and area coverage. The PASI score ranges between 0 and 72, and response is usually measured with percentage improvement in PASI score, with values of 50, 75, 90, and 100% frequently cited in the literature (Citation18,Citation19).
Materials and methods
An SLR was conducted to identify randomized controlled trials (RCTs) and long-term extensions that assessed the long-term efficacy of biologic therapies and apremilast in adult patients with moderate-to-severe chronic plaque psoriasis. The SLR was performed in accordance with a protocol developed prior to the commencement of the review.
MEDLINE, MEDLINE In-Process, Embase, and the Cochrane Library were searched from the January 01, 2000 to September 23, 2019 for articles published in English. Search strategies included index and text terms for psoriasis, the relevant interventions and RCTs. The full search strategies can be found in the Supplementary Table 1. Supplementary searches were also performed. These included checking the reference lists of included studies and searches in disease-specific and health economic and outcomes research congresses. All identified titles and abstracts were assessed for inclusion by one reviewer, with a second reviewer independently performing a 50% check. Full texts were then further reviewed in the same manner by both reviewers. Discrepancies were resolved by discussion or, if necessary, by a third reviewer.
Long-term extensions and RCTs comparing European Medicines Agency (EMA)-approved interventions of interest at their licensed doses – adalimumab, apremilast, brodalumab, certolizumab pegol, etanercept, guselkumab, infliximab, ixekizumab, risankizumab, secukinumab, tildrakizumab, and ustekinumab – with any comparator including placebo, another biologic therapy of interest or a non-biologic systemic therapy were eligible for inclusion. Trials had to include an intervention regimen that was administered at a licensed dose throughout the entire induction to maintenance phase. The outcome of interest was improvement in PASI score (PASI 75, PASI 90, and PASI 100) at or around 52 weeks. PASI 50 was not included as it was reported in very few studies. The full set of inclusion criteria can be found in the Supplementary Material S2.
For studies meeting the eligibility criteria, characteristics regarding the study design, patient population, interventions, outcomes, and PASI results were extracted. The methodological quality of studies was assessed using the Cochrane Risk of Bias Tool.
Analysis
The between-study heterogeneity was assessed by comparing features including treatment, outcome, study design, and patient characteristics. Studies were combined using a hierarchical Bayesian NMA of PASI responses using an ordered probit model to estimate the probabilities of achieving each level of PASI response. PASI 75 responses of the reference arm in included studies were utilized to inform baseline event rates. Prior exposure to biological therapies varies across trials in psoriasis and is thought to be a potential effect modifier (Citation20,Citation21); therefore, a sensitivity analysis was carried out excluding studies which included <5% of patients with prior experience of biologics or which did not report this information.
Due to differences in study design for maintenance phase data, a stepwise approach to synthesis was undertaken, with a primary and secondary analysis. The primary analysis (analysis 1) included studies with comparative evidence at 52-weeks. In order to include more studies and interventions, a second analysis was conducted (analysis 2). This analysis included long-term extension data for active therapies and induction-phase PASI results for patients receiving placebo who, in long-term extensions had switched from placebo to active treatment. In these studies, the induction phase data from the placebo arms were compared with the maintenance phase data from the active therapy arms, assuming placebo responses during induction persist into maintenance. In other words, placebo induction phase responses were carried forward to the end of maintenance.
Both fixed and random-effects models were used to generate results. Model-fit statistics including deviance information criterion (DIC) and the total residual deviance indicated that the random-effects model was the best-fit for the data, therefore, only these results are presented in this study. Inconsistency between direct and indirect estimates of effect was assessed for any loops in the evidence network using the two stage Bucher method (Citation22).
WinBUGS version 1.2 statistical software was used to perform all analyses, using non-informative priors. After an initial burn-in of at least 20,000 simulations, convergence was confirmed through visual inspection the Brook-Gelman-Rubin diagnostic and history plots. Sampled parameters were then estimated using 50,000 simulations on three chains. Results were calculated as the absolute probabilities of response for each treatment and as risk ratios (RRs) for every pairwise comparison at each level of PASI response. Point estimates reflecting the median value are presented, along with 95% credible intervals (95% CrIs), reflecting the range of true effects with 95% probability. Statistically significant differences have been interpreted as a 95% CrI which does not include the null value, 1. Numbers needed to treat (NNT) are also presented for the achievement of complete clearance (PASI 100). A numeric presentation of each treatment’s rank distribution, called the surface under the cumulative ranking (SUCRA) curve, is also presented. The SUCRA takes into account the effect sizes and accompanying uncertainty. If a treatment always ranks first, then the SUCRA is equal to 100%; if a treatment always ranks last, then the SUCRA equals 0%.
Results
Literature search results
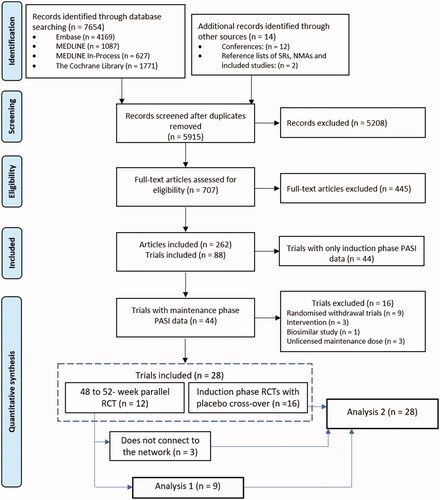
Electronic database searches identified 7654 articles, and a further 14 articles were identified through supplementary searches. A total of 5915 records were screened at title and abstract stage, of these, 707 full text records were assessed. The SLR included 88 articles consisting of both induction and maintenance phase data ().
Evidence network
Of the 88 studies included in the SLR, 44 included maintenance phase data between 40 and 64 weeks. Studies were compared for potential sources of heterogeneity and the evidence considered relevant for synthesis further refined. Sixteen studies were excluded from the NMA due to differences in study design and the comparisons made. This included data from nine responder-enriched randomized withdrawal maintenance phase studies (Citation23–31) and one responder-enriched re-randomized dose comparison study of tildrakizumab (Citation32). Three studies comparing different doses or dosing regimens of comparators listed in the eligibility criteria were excluded (Citation33–35) as was a study comparing a biosimilar etanercept to the originator product (Citation36). One study was excluded due to protocol-driven dose intensifications (Citation37) and another was excluded due to a very small sample size (Citation38). These exclusions were made to reduce heterogeneity in the network which could give rise to biased estimates of relative treatment effects. Therefore, a total of 28 studies were eligible for quantitative synthesis. presents the network diagram highlighting the available evidence for analysis 1 and analysis 2.
Figure 2. Network diagram of PASI responses - analysis 1 and analysis 2.
ADA: adalimumab; APR: apremilast; BRO: brodalumab; CZP: certolizumab pegol; ETN: etanercept; GUS: guselkumab; INF: infliximab; IXE: ixekizumab; PBO: placebo; SEC: secukinumab; RIS: risankizumab; UST WBD: ustekinumab weight-based dose.
contains baseline characteristics of the included studies which were broadly similar. The mean age of the participants ranged from 42 (Citation43) to 51 (Citation49) years, and 49% (Citation50) to 91% (Citation56, Citation60) were male. Previous conventional systemic therapy and phototherapy was reported in 0% (Citation49) to 95% (Citation55) and 31% (Citation23) to 82% (Citation60) patients, respectively. The proportion who had received a previous biological therapy ranged from 0% (Citation48,Citation49) to 60% (Citation46).
Table 1. Baseline characteristics of studies included in the primary analysis and the secondary analysis.
Risk of bias
Most studies were rated as being low risk of bias, but there was some heterogeneity between the included studies (Supplementary Figure 1). Of the 28 included RCTs, four (14%) reported an inadequate randomization method while 25 (89%) supplied sufficient information to assess whether allocation concealment was properly ensured. In four studies, the blinding of participants and personnel was insufficient as the long-term extension was open label (Citation45,Citation46,Citation51,Citation54). In all studies, the risk of attrition bias was low, as incomplete outcome data were sufficiently addressed. The risk of reporting bias was low in most of the studies. The risk of bias for each study is presented in Supplementary Figure 2.
Primary analysis
Twelve studies included head-to-head data on maintenance phase outcomes. Nine of these studies (Citation17,Citation39–42,Citation44,Citation56) connected to form an evidence network comparing eight interventions (brodalumab, secukinumab, ixekizumab, risankizumab, guselkumab, ustekinumab, adalimumab, and etanercept) for PASI 75, 90, and 100 at 48 to 52 weeks. The other three studies (Citation47,Citation50) did not share a common comparator with the nine studies and were therefore disconnected from the network and could not be synthesized. The nine connected studies, including 5054 patients, were included in analysis 1.
Results indicate that treatment with risankizumab, brodalumab, and guselkumab provide the highest levels of PASI 75, 90, and 100 response (). The probabilities of achieving complete clearance ranged from 61.1% with risankizumab, 54.5% for brodalumab, 54.2% for guselkumab, 46.1% for ixekizumab, 40.9% for secukinumab, 28.9% for ustekinumab, 26.4% for adalimumab, and 16.8% for etanercept. Risankizumab was found to be significantly superior to all therapies except for brodalumab and guselkumab at achieving all levels of PASI response. Brodalumab and guselkumab were both found to be significantly more efficacious than secukinumab, ustekinumab, adalimumab, and etanercept (). Based on mean values, treatment with brodalumab or guselkumab is expected to result in slightly more PASI responders at all levels than treatment with ixekizumab, though the differences are not statistically significant. Ixekizumab and secukinumab were superior to ustekinumab and adalimumab and all therapies were significantly better than etanercept. The relative treatment effects for all interventions are presented in . The NNT for PASI 100 compared to ustekinumab was four for risankizumab, five for brodalumab and guselkumab, seven for ixekizumab, and nine for secukinumab. The NNT for ustekinumab versus etanercept was 9 and ustekinumab versus adalimumab was 17. The SUCRA percentage indicated that risankizumab was the most efficacious therapy in 97.3% of Bayesian iterations with a median rank of 1 (CrI 1 − 3) and brodalumab was second with a SUCRA of 78.8% and rank of 2 (CrI 1 − 4). This was followed by guselkumab (SUCRA 78.2%, median rank 3 [CrI 1 − 4]), ixekizumab (57.1%, 4 [2 − 5]), secukinumab (45.7%, 5 [4 − 5]), ustekinumab (24.3%, 6 [6 − 7]), adalimumab (18.4%, 7 [6 − 7]), and etanercept (0.1%, 8 [8 − 8]). The upper and lower bounds of the 95% credible interval for etanercept are the same which indicates a low level of uncertainty in its rank as the least effective therapy evaluated in this analysis.
Figure 3. Results of predicted percentage PASI 75, 90, and 100 responses for evaluated interventions (Analysis 1).
Q2W: every 2 weeks; Q4W: every 4 weeks; WBD: weight-based dose.
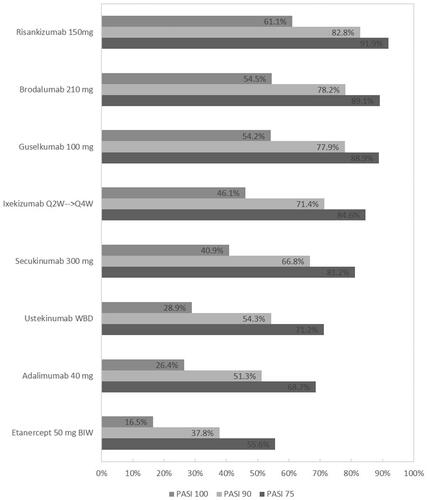
Figure 4. Analysis 1: Relative treatment effect for all interventions. Relative effects are plotted as the median difference in response on the probit scale, where positive values indicate greater efficacy for the intervention and negative values indicate greater efficacy for the comparator.
ADA: adalimumab; BRO: brodalumab; ETN: etanercept; GUS: guselkumab; IXE: ixekizumab; SEC: secukinumab; RIS: risankizumab; UST: ustekinumab weight-based dose.
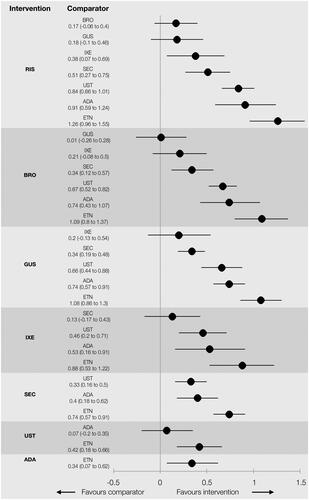
Table 2. NMA of 52-week active therapy RCTs (Analysis 1): results for PASI response.
Secondary analysis
Unlike the studies eligible for the primary analysis, the remaining 16 studies presented maintenance phase data for active therapies but included long-term extensions where patients switched from placebo to active treatment. To form a connected network, and to augment analysis 1, the induction phase data from the placebo arms were compared with the maintenance phase data from the active therapy arms. This assumption allowed for the inclusion of data for adalimumab, apremilast, certolizumab pegol, guselkumab, and infliximab, as well as additional data for etanercept, secukinumab and ustekinumab (45 and 90 mg). This analysis also allowed for studies that could not be connected to the network in analysis 1 to be included via their induction-phase placebo arm (Citation43,Citation50). Therefore, a total of 28 studies, including 9940 patients, were included in analysis 2.
Results of analysis 2 showed that all therapies were significantly more effective than placebo (). Probabilities of achieving all levels of PASI response were highest for risankizumab and brodalumab, followed by guselkumab, ixekizumab, and secukinumab (). The lowest probabilities were generated by apremilast and etanercept. Similar to analysis 1, risk ratios indicate treatment with risankizumab resulted in significantly more PASI responders than any other intervention apart from brodalumab and guselkumab. Brodalumab and guselkumab were found to be superior to apremilast, adalimumab, certolizumab pegol, etanercept, infliximab, secukinumab, and ustekinumab, but similar in efficacy to ixekizumab (). Overall, results of analysis 2 were consistent with analysis 1.
Figure 5. Results of predicted percentage PASI 75, 90, and 100 responses for evaluated interventions (Analysis 2).
Q2W: every 2 weeks; Q4W: every 4 weeks; WBD: weight-based dose
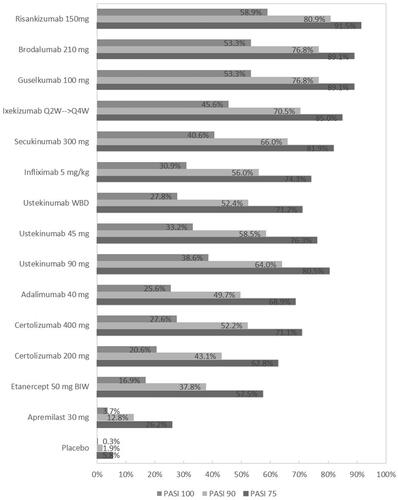
Table 3. NMA of 52-week RCTs using induction phase placebo control (Analysis 2): results for PASI responses.
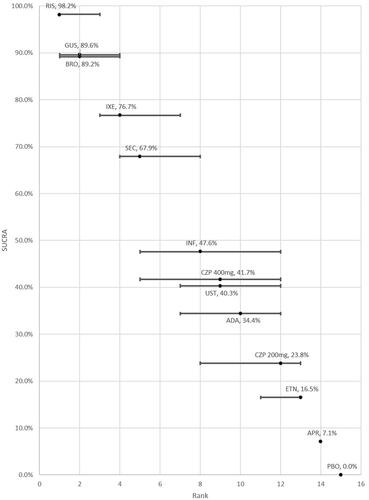
The SUCRAs for each therapy are also presented in along with their median rank and associated uncertainty. The highest SUCRAs, indicating best rank, are reported for risankizumab (98.2%) and guselkumab (89.6%), followed by brodalumab (89.2%), ixekizumab (76.7%), and secukinumab (67.9%). In contrast, apremilast and etanercept were ranked the least efficacious and had the lowest SUCRA scores of all the active therapies.
Figure 6. SUCRA and ranking with error bars indicating the 95% credible interval (Analysis 2).
ADA: adalimumab; BRO: brodalumab; ETN: etanercept; GUS: guselkumab; IXE: ixekizumab; SEC: secukinumab; RIS: risankizumab; UST: ustekinumab weight-based dose
Sensitivity analysis: exclusion of studies with less than 5% prior biologic exposure
Eight studies (Citation45,Citation48,Citation49,Citation51–53,Citation55,Citation60) reported less than 5% of patients with prior biologic exposure (including all apremilast trials) and were excluded in the sensitivity analysis. All of these studies were included in analysis 2 only; therefore, this sensitivity analysis was only conducted for analysis 2. Overall, the relative ranks and statistical significance of treatment effects were consistent with results of the base-case analysis 2 (Supplementary Table 4).
Discussion
This SLR and NMA compared licensed systemic therapies for moderate-to-severe psoriasis. Tildrakizumab was included among the comparators of interest, but because there was no comparable maintenance data available at 1 year, it could not be included in the NMA. The synthesis of evidence from nine RCTs reporting PASI outcomes at week 48 to 52 in the primary analysis, showed risankizumab, brodalumab, and guselkumab to be associated with the highest likelihood of response, followed by ixekizumab and secukinumab. Results indicated that risankizumab was statistically superior to ixekizumab but not statistically different from brodalumab or guselkumab. Brodalumab and guselkumab produced greater PASI responders than ixekizumab, but this was not statistically significant. Analysis 1 showed all therapies except etanercept and adalimumab were found to be superior to ustekinumab, a result consistent with the findings of the head to head RCTs included in the analysis. In the secondary analysis, studies from the first analysis were supplemented with the inclusion of long-term extensions of placebo controlled RCTs reporting maintenance phase outcomes for other licensed therapies, including certolizumab pegol, infliximab and apremilast. Results were consistent across both analyses and suggested the superiority of IL-17s and IL-23s over other systemic therapies after 1 year of treatment.
The results of this NMA are consistent with our original synthesis of long-term data (Citation16), although further interventions are included in the current analysis (ixekizumab, risankizumab, guselkumab, and certolizumab pegol). Additionally, our results are broadly in line with the results of previously published NMAs (Citation9–14) on induction phase data, which show proportions of PASI responders are similar from induction to maintenance phase.
The off-treatment durability of PASI response could be investigated with randomized withdrawal trials. However, such trials, which may be considered responder-enriched, were excluded. Compared to maintenance phase studies without responder enrichment, this design may introduce bias in favor of the active intervention. Additionally, these studies varied in their definition of responders and consequently on the eligibility for re-randomization and were therefore not comparable.
Another source of long-term evidence are registry studies which can provide evidence of therapies in the real world (Citation61). However, these observational studies are not always useful in addressing questions of efficacy, as treatment choice is influenced by factors such as patient characteristics, or healthcare coverage (Citation62). In addition, the data collection is not sufficiently consistent to allow the combination of data in an NMA (Citation61). Such data may serve to supplement the efficacy assessment presented here with real world evidence on drug survival and tolerability.
Strengths and limitations
This study included a comprehensive and up to date SLR of English language studies, which allows recently approved therapies to be included. The study also presents the first long term indirect comparison with IL-23s, apart from tildrakizumab for which there is no comparable evidence.
The number of studies reporting on the maintenance phase outcomes of interventions in psoriasis is limited, especially considering the volume of RCT evidence evaluating induction phase efficacy and safety. In addition to their relative scarcity, variations in their study design make quantitative comparisons challenging. The primary analysis, which relies on largely 52-week parallel RCTs, is the most robust comparison of biologic therapies for moderate-to-severe psoriasis over the longer-term; however, it is limited by the number of comparisons it can make.
The secondary analysis aims to extend the comparisons that can be made, but it is limited by the uncertainty of an assumed placebo response. Due to the treatment switching in these studies, the missing maintenance data were imputed using induction phase responses. A plateau in placebo response between induction and 24 weeks has been noted in four RCTs (Citation53,Citation63–65), which lends some support to the assumption that placebo responses do not improve with time. Carrying forward the placebo responses from induction fails to account for any losses of efficacy between 10 and 16 weeks and 1 year, thereby making the assumption quite conservative.
This study did not investigate adverse events or health-related quality of life at 1 year due the heterogenous reporting between trials. To enable a full evaluation of therapies for moderate-to-severe plaque psoriasis, future long-term studies should investigate these outcomes and report these in a more uniform way, enabling a formal comparison.
Additionally, a potential limitation of analyses 1 and 2 is that it may not be directly relevant to clinical practice as patients normally would discontinue treatment at the end of induction if the response is insufficient or receive higher doses than recommended. Since this study only included clinical trials and not real-world evidence, only licensed doses of interventions were included. Several studies have been conducted which report that dose adjustments are used routinely in clinical practice (Citation66,Citation67).
Conclusion
This NMA of maintenance phase RCT evidence indicates that high levels of PASI can be achieved and maintained at around 1 year. Results demonstrated that the evaluated IL-17 and IL-23 inhibitors outperformed other biological therapies in terms of the proportion achieving all levels of PASI response after 48–52 weeks of treatment. Risankizumab had a higher probability of achieving PASI outcomes than all other biologics, except brodalumab and guselkumab, where no significant difference could be concluded. Further long-term trials with head to head evidence in which patients receive a licensed therapy for the entire induction and maintenance periods are needed to validate these conclusions. Further real-world studies may also help to validate the findings and provide evidence on the long-term performance of these treatments in clinical practice.
Supplemental Material
Download Zip (834.2 KB)Acknowledgments
The authors thank Jana Tillotson for graphic design support during the preparation of this article.
Disclosure statement
Najeeda Yasmeen and Laura Sawyer are Symmetron employees, as was Kinga Malottki when the work was undertaken; all were consultants to LEO Pharma for this study. Eydna Didriksen Apol is a LEO Pharma employee. Lars-Åke Levin has received consulting fees from LEO Pharma and Gregor B Jemec has received honoraria from AbbVie, Coloplast, Galderma, Inflarx, LEO Pharma, MSD, Pierre-Fabre, UCB, and his department received grants from AbbVie, Actelion, Janssen-Cilag, LEO Pharma, Novartis, Regeneron, Serono and UCB for his participation as an investigator.
Additional information
Funding
References
- Girolomoni G, Strohal R, Puig L, et al. The role of IL-23 and the IL-23/TH 17 immune axis in the pathogenesis and treatment of psoriasis [Review]. J Eur Acad Dermatol Venereol. 2017;31(10):1616–1626.
- World Health Organization. Global report on psoriasis. World Health Organization; 2016 [Last accessed March 2020]. Available from: https://apps.who.int/iris/handle/10665/204417
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet (London, England). 2007;370(9583):263–271.
- Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303(1):1–10.
- Taylor GJ, Wainwright P. Open label extension studies: research or marketing? BMJ. 2005;331(7516):572–574.
- Buch MH, Aletaha D, Emery P, et al. Reporting of long-term extension studies: lack of consistency calls for consensus. Ann Rheum Dis. 2011;70(6):886–890.
- Naldi L, Svensson A, Zenoni D, et al. Comparators, study duration, outcome measures and sponsorship in therapeutic trials of psoriasis: update of the EDEN Psoriasis Survey 2001-2006. Br J Dermatol. 2010;162(2):384–389.
- Ronholt K, Iversen L. Old and new biological therapies for psoriasis. Int J Mol Sci. 2017;18(11): 2297.
- Hendrix N, Ollendorf DA, Chapman RH, et al. Cost-effectiveness of targeted pharmacotherapy for moderate to severe plaque psoriasis. J Manag Care Spec Pharm. 2018;24(12):1210–1217.
- Geng W, Zhao J, Fu J, et al. Efficacy of several biological therapies for treating moderate to severe psoriasis: a network meta-analysis. Exp Ther Med. 2018;16(6):5085–5095.
- Sawyer L, Fotheringham I, Wright E, et al. The comparative efficacy of brodalumab in patients with moderate-to-severe psoriasis: a systematic literature review and network meta-analysis. J Dermatolog Treat. 2018;29(6):557–568.
- Loos AM, Liu S, Segel C, et al. Comparative effectiveness of targeted immunomodulators for the treatment of moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis. J Am Acad Dermatol. 2018;79(1):135–144.e7.
- Lin PT, Wang SH, Chi CC. Drug survival of biologics in treating psoriasis: a meta-analysis of real-world evidence. Sci Rep. 2018;8(1):16068.
- Cameron C, Druchok C, Hutton B, et al. Guselkumab for the treatment of moderate-to-severe plaque psoriasis during induction phase: a systematic review and network meta-analysis. J Psoriasis Psoriatic Arthritis. 2019;4(2):81–92.
- Nast A, Jacobs A, Rosumeck S, et al. Efficacy and safety of systemic long-term treatments for moderate-to-severe psoriasis: a systematic review and meta-analysis. J Invest Dermatol. 2015;135(11):2641–2648.
- Sawyer LM, Cornic L, Levin LA, et al. Long-term efficacy of novel therapies in moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response. J Eur Acad Dermatol Venereol. 2019;33(2):355–366.
- Paul C, Griffiths CEM, van de Kerkhof PCM, et al. Ixekizumab provides superior efficacy compared with ustekinumab over 52 weeks of treatment: results from IXORA-S, a phase 3 study. J Am Acad Dermatol. 2019;80(1):70–79.e3.
- Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis. 2005;64(suppl_2):ii65–ii68.
- Shikiar R, Bresnahan BW, Stone SP, et al. Validity and reliability of patient reported outcomes used in psoriasis: results from two randomized clinical trials [journal article. Health Qual Life Outcomes. 2003;1(1):53.
- Papp KA, Gordon KB, Langley RG, et al. Impact of previous biologic use on the efficacy and safety of brodalumab and ustekinumab in patients with moderate-to-severe plaque psoriasis: integrated analysis of the randomized controlled trials AMAGINE-2 and AMAGINE-3. Br J Dermatol. 2018;179(2):320–328.
- Ruiz Salas V, Puig L, Alomar A. Ustekinumab in clinical practice: response depends on dose and previous treatment. J Eur Acad Dermatol Venereol. 2012;26(4):508–513.
- Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–691.
- Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet (London, England). 2015;386(9993):541–551.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375(4):345–356.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73(1):37–49.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175(2):273–286.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173(6):1387–1399.
- Lebwohl M, Blauvelt A, Paul C, et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: results through 48 weeks of a phase 3, multicenter, randomized, double-blind, etanercept- and placebo-controlled study (CIMPACT). J Am Acad Dermatol. 2018;79(2):266–276.e5.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76(3):418–431.
- Blauvelt A, Ferris LK, Yamauchi PS, et al. Extension of ustekinumab maintenance dosing interval in moderate-to-severe psoriasis: results of a phase IIIb, randomized, double-blinded, active-controlled, multicentre study (PSTELLAR). Br J Dermatol. 2017;177(6):1552–1561.
- Reich K, Gooderham M, Thaci D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet (London, England). 2019;394(10198):576–586.
- Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173(4):930–939.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376(16):1551–1560.
- Langley RG, Papp K. Efficacy and safety of continuous every-2-week dosing of ixekizumab over 52 weeks in patients with moderate-to-severe plaque psoriasis in a randomized phase III trial (IXORA-P). Br J Dermatol. 2018;178(6):1315–1323.
- Khattri S, Goldblum O, Solotkin K, et al. Early onset of clinical improvement with ixekizumab in a randomized, open-label study of patients with moderate-to-severe plaque psoriasis. J Clin Aesthet Dermatol. 2018;11(5):33–37.
- Griffiths CEM, Thaci D, Gerdes S, et al. The EGALITY study: a confirmatory, randomized, double-blind study comparing the efficacy, safety and immunogenicity of GP2015, a proposed etanercept biosimilar, vs. the originator product in patients with moderate-to-severe chronic plaque-type psoriasis. Br J Dermatol. 2017;176(4):928–938.
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet (London, England). 2008;371(9625):1675–1684.
- de Vries AC, Thio HB, de Kort WJ, et al. A prospective randomized controlled trial comparing infliximab and etanercept in patients with moderate-to-severe chronic plaque-type psoriasis: the Psoriasis Infliximab vs. Etanercept Comparison Evaluation (PIECE) study. Br J Dermatol. 2017;176(3):624–633.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318–1328.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet (London, England). 2018;392(10148):650–661.
- Blauvelt A, Reich K, Tsai TF, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol. 2017;76(1):60–69.e9.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–417.
- Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet (London, England). 2019;394(10201):831–839.
- Gordon KB, Langley RG, Leonardi C, et al. Clinical response to adalimumab treatment in patients with moderate to severe psoriasis: double-blind, randomized controlled trial and open-label extension study. J Am Acad Dermatol. 2006;55(4):598–606.
- Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373(2):136–144.
- Ohtsuki M, Okubo Y, Komine M, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in the treatment of Japanese patients with moderate to severe plaque psoriasis: efficacy, safety and tolerability results from a phase 2b randomized controlled trial. J Dermatol. 2017;44(8):873–884.
- Reich K, Gooderham M, Green L, et al. The efficacy and safety of apremilast, etanercept and placebo in patients with moderate-to-severe plaque psoriasis: 52-week results from a phase IIIb, randomized, placebo-controlled trial (LIBERATE). J Eur Acad Dermatol Venereol. 2017;31(3):507–517.
- Stein Gold L, Bagel J, Lebwohl M, et al. Efficacy and safety of apremilast in systemic- and biologic-naive patients with moderate plaque psoriasis: 52-week results of UNVEIL. J Drugs Dermatol. 2018;17(2):221–228.
- Gottlieb AB, Blauvelt A, Thaci D, et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: results through 48 weeks from 2 phase 3, multicenter, randomized, double-blinded, placebo-controlled studies (CIMPASI-1 and CIMPASI-2). J Am Acad Dermatol. 2018;79(2):302–314.e6.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet (London, England). 2006;367(9504):29–35.
- Ohtsuki M, Kubo H. Guselkumab, an anti-interleukin-23 monoclonal antibody, for the treatment of moderate to severe plaque-type psoriasis in Japanese patients: efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. J Dermatol. 2018;45(9):1053–1062.
- Reich K, Nestle FO, Papp K, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet (London, England). 2005;366(9494):1367–1374.
- Menter A, Feldman SR, Weinstein GD, et al. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2007;56(1):31.e1–15.
- Torii H, Nakagawa H. Infliximab monotherapy in Japanese patients with moderate-to-severe plaque psoriasis and psoriatic arthritis. A randomized, double-blind, placebo-controlled multicenter trial. J Dermatol Sci. 2010;59(1):40–49.
- Ohtsuki M, Fujita H, Watanabe M, et al. Efficacy and safety of risankizumab in Japanese patients with moderate to severe plaque psoriasis: results from the SustaIMM phase 2/3 trial. J Dermatol. 2019;46(8):686–694.
- Blauvelt A, Prinz JC, Gottlieb AB, et al. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015;172(2):484–493.
- Lacour JP, Paul C, Jazayeri S, et al. Secukinumab administration by autoinjector maintains reduction of plaque psoriasis severity over 52 weeks: results of the randomized controlled JUNCTURE trial. J Eur Acad Dermatol Venereol. 2017;31(5):847–856.
- Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet (London, England). 2008;371(9625):1665–1674.
- Igarashi A, Kato T, Kato M, et al. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. 2012;39(3):242–252.
- Eissing L, Rustenbach SJ, Krensel M, et al. Psoriasis registries worldwide: systematic overview on registry publications. J Eur Acad Dermatol Venereol. 2016;30(7):1100–1106.
- Dreyer NA, Garner S. Registries for robust evidence. Jama. 2009;302(7):790–791.
- Asahina A, Nakagawa H, Etoh T, et al. Adalimumab in Japanese patients with moderate to severe chronic plaque psoriasis: efficacy and safety results from a Phase II/III randomized controlled study. J Dermatol. 2010;37(4):299–310.
- Gottlieb AB, Matheson RT, Lowe N, et al. A randomized trial of etanercept as monotherapy for psoriasis. Arch Dermatol. 2003;139(12):1627–1632. discussion 1632.
- Krueger GG, Langley RG, Leonardi C, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356(6):580–592.
- Feldman SR, Zhao Y, Zhou H, et al. Economic impact of above-label dosing with etanercept, adalimumab, or ustekinumab in patients with psoriasis. J Manag Care Spec Pharm. 2017;23(5):583–589.
- Esposito M, Gisondi P, Conti A, et al. Dose adjustment of biologic therapies for psoriasis in dermatological practice: a retrospective study. J Eur Acad Dermatol Venereol. 2017;31(5):863–869.