Abstract
Objective
To evaluate the cost-effectiveness of risankizumab versus other biologic treatments (adalimumab, infliximab, ustekinumab, secukinumab, brodalumab, ixekizumab, and guselkumab) of moderate-to-severe psoriasis in Japan.
Methods
A Markov cohort-level model was constructed to estimate quality-adjusted life years (QALYs) and costs for each treatment over a lifetime horizon. The model simulated patients’ transition through one line of active biologic therapy followed by best supportive care and death. Transition probabilities were informed by network meta-analyses of Psoriasis Activity and Severity Index responses and adverse event-related discontinuation in clinical trials, as well as published real-world evidence and national mortality rates. Costs were evaluated from the health system, societal, and patient out-of-pocket perspectives.
Results
Risankizumab was expected to provide 0.30–0.89 additional QALYs versus comparator biologics. Under the health system perspective, incremental cost-effectiveness ratios (ICERs) of risankizumab ranged from ¥2,545,812/QALY versus ustekinumab to ¥6,077,134/QALY versus adalimumab. Societal ICERs were lower, ranging from ¥921,770/QALY to ¥4,350,879/QALY. From the patient perspective, risankizumab was estimated to be cost-saving versus four comparators and was associated with ICERs of <¥500,000/QALY versus the remaining comparators.
Conclusion
Risankizumab was associated with higher QALYs and, based on typical willingness-to-pay benchmarks (¥5-6.7 million/QALY), considered cost-effective versus other biologics for the treatment of psoriasis in Japan.
Introduction
Psoriasis is a chronic autoimmune disease of the skin that affects approximately 0.34% of the population in Japan (Citation1,Citation2). Presenting as erythematous, scaled skin plaques that can be painful and disfiguring, psoriasis adversely impacts the physical, mental, and social well-being of patients (Citation3). The extent of impairment to health-related quality of life is reported to be comparable to that of other major chronic diseases, including heart failure, type 2 diabetes, and depression (Citation4,Citation5). Psoriasis has also been found to cause significant loss of work productivity, with high correlations between disease severity and missed work (i.e. absenteeism) and reduced productivity while working (i.e. presenteeism) (Citation6,Citation7).
Initial treatments of psoriasis typically include topical medications, followed by phototherapy or systemic therapies in patients refractory to topical agents (Citation8). Conventional non-biologic systemic therapies such as methotrexate and ciclosporin continue to have a role in the management of psoriasis but are often insufficient to achieve high skin clearance in patients with moderate to severe disease (Citation9). Since 2010, several different biologic systemic therapies have been approved in Japan for the treatment of psoriasis, including inhibitors of tumor necrosis factor-alpha (TNFα) (adalimumab, infliximab and certolizumab), interleukin (IL)−12/23 (ustekinumab), IL-17 (secukinumab, brodalumab, and ixekizumab), and IL-23 (guselkumab and risankizumab) (Citation10). Clinical guidelines in Japan recommend treatment with biologics in patients who either: have not adequately responded to standard systemic therapies or phototherapy, with an affected body surface area (BSA) of least 10%; or have refractory skin or joint symptoms that are intractable to standard systemic therapies and significantly impaired quality of life (e.g. Dermatology Life Quality Index [DLQI] of 10 or more) (Citation11,Citation12).
Risankizumab is a fully humanized monoclonal antibody with a high affinity for the p19 component of IL-23, a cytokine that contributes directly to the pathogenesis of psoriasis (Citation13). Data from four large, multi-national, phase 3 randomized controlled trials (UltIMMa-1/NCT02684370, UltIMMa-2/NCT02684357, IMMvent/NCT02694523, and IMMhance/NCT02672852) showed significantly greater efficacy with risankizumab versus placebo (Citation14–16), ustekinumab (Citation14), and adalimumab (Citation15) as measured by relative improvements from baseline in the Psoriasis Area and Severity Index (PASI). Across the trials, 72.4–75.3% of patients randomized to risankizumab achieved ≥90% improvement in PASI (i.e. PASI 90) at week 16 (Citation14–16), with 75.7–81.9% achieving PASI 90 following longer-term maintenance therapy with risankizumab at week 44 (Citation15) or 52 (Citation14). Risankizumab is the latest anti-IL-23 biologic approved in Japan (approval date: March 26, 2019) for the treatment of psoriasis in patients who have not responded sufficiently to conventional therapies (Citation17).
The advent of biological therapies has improved the standard of care for moderate to severe psoriasis and levels of treatment satisfaction and efficacy compared with conventional systemic therapies (Citation13–15), but at a higher cost of treatment (Citation18). Cost-effectiveness analyses conducted from different perspectives can help guide consensus building among decision-makers regarding the economic value of different treatment options (Citation19). Few economic evaluations of biologic treatments for psoriasis have been conducted in the Japanese health care setting (Citation18,Citation20–22), as cost-effectiveness analyses historically did not have a formal role in the appraisal of new health care interventions in Japan. Economic evaluation has increasingly gained traction in Japan as a potential tool to promote efficient health care spending, with the establishment of a pilot program in 2016 and subsequent development of guidelines for cost-effectiveness methods by the newly created Center for Outcomes Research and Economic Evaluation for Health (CORE2-Health) (Citation23,Citation24). In Japan, interventions may be considered cost-effective if associated with an incremental cost-effectiveness ratio (ICER) below a willingness-to-pay of ¥5 million to ¥6.7 million per quality-adjusted life year (QALY) gained (Citation25,Citation26).
This study aimed to evaluate the cost-effectiveness of risankizumab versus other biological treatments (specifically adalimumab, infliximab, ustekinumab, secukinumab, brodalumab, ixekizumab and guselkumab at the time of the analysis in May 2019) of moderate to severe psoriasis in Japan. Costs were evaluated from three different perspectives: the health system perspective, which included total direct health care costs incurred by the national payer or the patient; the societal perspective, encompassing both direct health care costs and indirect costs of work loss associated with psoriasis severity and treatments; and the patient perspective, which focused on copayments for health care that are incurred out-of-pocket by the patient. The societal perspective is relevant to the decision problem given the reported impact of psoriasis on work productivity (Citation6). The patient perspective is also important to evaluate, as drug copayment has been found to influence patient preferences for psoriasis treatments in Japan (Citation27), and is described in clinical guidelines as a factor that should be considered when selecting biologics for psoriasis (Citation11,Citation28).
Methods
Model overview
A decision-analytic model was implemented in Excel 2016 (Microsoft Corporation, Redmond, WA) to examine the cost-effectiveness of biologic treatments for moderate to severe psoriasis. A lifetime horizon (defined as 100 years minus the starting age of the model cohort) was adopted in order to comprehensively capture relevant differences in costs and health effects between the comparators (Citation24,Citation29). The model used a 4-week cycle length, an interval that was sufficiently short to precisely model variable dosing schedules and timing of response assessment for different treatments, without the need for half-cycle correction. Outcomes included QALYs, costs (in 2019 JPY), and ICERs. Annual discounting by 2% was used for costs and health effects (Citation24).
Parameter inputs for the model were obtained from existing data sources, including summary-level trial results and other published literature, publicly available databases, and de-identified, retrospective claims data. Therefore, no ethical review was required.
Target population
According to clinical guidelines in Japan, biologics are positioned for use after systemic non-biologic therapies (Citation11,Citation12). The model, therefore, considered adults (age 20 years or older) with moderate to severe plaque psoriasis for whom conventional systemic treatment or phototherapy is inadequately effective, not tolerated or contraindicated. Based on the intention-to-treat population of the phase 2/3 SustaIMM (NCT03000075) trial of risankizumab in Japan, patients entering the model were 51.9 years old, with a mean weight of 74 kg and with females comprising 16.4% of the population (Citation30).
Intervention and comparators
Risankizumab was evaluated based on the label-approved dosage of 150 mg administered via subcutaneous injection at weeks 0 and 4, followed by every 12 weeks (Q12W) thereafter. Comparator treatments were similarly modeled according to licensed dosing schedules (Citation28) and clinical guidelines (Citation11,Citation12) and included biologics approved for the treatment of psoriasis in Japan: adalimumab (80 mg at week 0, then 40 mg every 2 weeks [Q2W]), infliximab (5 mg/kg at weeks 0, 2, and 6, then every 8 weeks [Q8W] thereafter), secukinumab (300 mg at weeks 0, 1, 2, 3, and 4, then every 4 weeks [Q4W]), ixekizumab (160 mg at week 0, then 80 mg Q2W until week 12 and Q4W thereafter), ustekinumab (45 mg at weeks 0 and 4, then Q12W, with potential dose escalation to 90 mg in case of inadequate response), brodalumab (210 mg at weeks 0, 1, and 2, then Q2W thereafter), and guselkumab (100 mg at weeks 0 and 4, then Q8W thereafter).
Model structure
A Markov cohort-level model was constructed to simulate the treatment status and survival of patients over time. The model structure comprised four mutually exclusive treatment-related states (): primary response period; subsequent maintenance period; best supportive care (BSC); and death.
Figure 1. Model structure schematic. Transitions to death may occur from any health state. Arrows to death are omitted from the diagram for simplicity. The primary response period consists of up to four 4-week tunnel states, depending on the recommended timing of response assessment for the biologic received. Patients are assumed to continue treatment until the end of the primary response period, unless they transition to death within this timeframe.
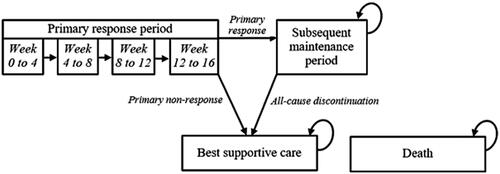
At model entry, patients newly initiated a line of biologic therapy at the start of the primary response period, which ranged from 10 to 16 weeks depending on the recommended timing of response assessment for the specific treatment received () (Citation31). Each primary response period is modeled as a series of up to four 4-week tunnel states, depending on the length of the primary response period for a given treatment. All patients were assumed to continue treatment throughout the primary response period (unless death occurs within this state). At the end of this period, patients who did not meet the specified minimum response threshold (PASI 75, i.e. ≥75% improvement in PASI score, in the base case) were assumed to discontinue treatment at end of the primary response period. Patients who met the minimum response threshold were classified as responders who would continue to the subsequent maintenance period with the same therapy. To account for potential loss of efficacy or tolerability over time, the model applied an annual risk of discontinuation to patients receiving maintenance therapy. Patients were assumed to maintain their initial level of PASI response only until discontinuation of maintenance therapy due to any cause.
Table 1. Probabilities of PASI 50, 75, 90, and 100 response by treatment, based on a network meta-analysis of randomized controlled trials.
Upon discontinuing biologic therapy, patients transitioned to BSC, a state representing a mix of non-biologic supportive medications. Patients who entered the BSC state were assumed to remain in this state until death. In each model cycle, transitions to death could occur from any of the other states. All-cause mortality risks were assumed to be unaffected by the choice of treatment for psoriasis or by the condition itself.
Utility was determined based on the treatment-specific distribution of PASI response across five levels, each defined by percentage improvements in PASI scores from baseline: PASI 100 (100% improvement), PASI 90–99 (90–99% improvement), PASI 75–89 (75–89% improvement), PASI 50–74 (50–74% improvement), and PASI <50 (<50% improvement). The base case assumed that utility gains increase linearly over the course of the primary response period, and remain constant for the duration of time spent in the subsequent maintenance period. Sensitivity analyses assuming zero or immediate utility gains in the primary response period were also tested. Patients’ utility was assumed to revert to baseline levels upon transitioning to BSC.
In the base case, patients were assumed to be treated by a single line of biologic therapy followed by BSC. In an alternative model scenario, patients who discontinued the first-line biologic were instead assumed to transition through two additional lines of biologic treatment before BSC. The supplemental material includes an alternative model schematic (Figure S1) and details on the treatment sequencing assumptions (Table S1) used this scenario analysis.
Model parameters
Clinical efficacy
Measures of treatment effectiveness in the model included the proportions of patients achieving ≥50%, ≥75%, ≥90%, and 100% relative improvement in PASI scores from baseline. Response rates based on relative change in PASI score are consistently reported as outcomes in randomized controlled trials, which facilitated evidence synthesis through network meta-analysis (NMA).
PASI response rates used to inform the model were obtained from a Bayesian NMA using a random effects ordinal model, which adjusted for reference arm response (Citation32). The NMA included data reported at 10–16 weeks from the four multi-national phase 3 clinical trials of risankizumab (Citation14–16) and from phase 2 or 3 trials of comparators identified through a systematic literature review. presents the estimated probabilities of PASI 50, 75, 90, and 100 response at the end of the primary response period.
In a scenario analysis, PASI response rates were instead estimated based on a meta-analysis of long-term (44- to 60-week) trial results, conducted using the DerSimonian and Laird meta-analytic method (Table S2 of the Supplemental material) (Citation32).
Transition probabilities
From primary response period to subsequent maintenance
At the end of the primary response period, assessment of whether patients achieved a minimum response of PASI 75 determined whether they transitioned to subsequent maintenance or BSC state. The probability of continuing on to subsequent maintenance therapy, therefore, corresponded to the probability of PASI 75 response (). Alternative PASI response criteria (PASI 50, PASI 90, and PASI 100) were also tested in scenario analyses.
From subsequent maintenance to BSC
Among primary responders who entered the subsequent maintenance period, a treatment-specific annual dropout probability was applied during this period to represent discontinuation due to any cause, including loss of efficacy and safety concerns. The annual discontinuation probability was converted into a 4-week discontinuation probability based on an assumption of constant hazards. The resulting probability was applied in each 4-week model cycle as the transition probability from maintenance therapy to BSC.
The annual discontinuation risk for each treatment represented the sum of annual discontinuation risks due to adverse events (AEs) and other cases. Risks of AE-related discontinuation were estimated using comparative evidence from an NMA of AE-related discontinuation reported from randomized controlled trials in moderate to severe psoriasis (Citation33). Specifically, the NMA-based odds ratio of AE-related discontinuation for each treatment vs. adalimumab was applied to the reported percentage of patients who discontinued adalimumab due to AEs by 1 year (i.e. 7%) in a large, real-world registry study by Warren et al. (Citation34), resulting in estimates of 1-year AE-related discontinuation risk for each treatment (Table S3 of the Supplemental material). Adalimumab was the treatment with the largest available sample size in Warren et al. and was therefore used as the reference treatment for these calculations. For all treatments, 1-year discontinuation risk due to reasons other than AEs was assumed to be equivalent to that reported for adalimumab in Warren et al. (i.e. 14%).
Mortality from all other health states
Age-dependent all-cause mortality rates were based on national life tables for Japan (Citation35). The mortality rate at each age was calculated as a weighted average of gender-specific mortality rates, based on the gender distribution of the target population.
Utility inputs
Valuation of health effects for each treatment in the model was based on the change in utility from baseline associated with different levels of PASI response (). The utility gain corresponding to each PASI response level was assumed to be the same irrespective of treatment. Utility inputs were obtained from the York Model, developed by the technology assessment group at the University of York on behalf of the National Institute for Health and Care Excellence (NICE) for a multiple technology appraisal of psoriasis treatments (Citation36).
Table 2. Summary of cost and utility inputs.
Cost inputs
Drug acquisition and administration costs
Drug acquisition costs were calculated in the model as a function of unit drug costs and dosing schedules for the included treatments (). Unit drug costs as of May 2019 were retrieved from the Ministry of Heath, Labor and Welfare (MHLW) (Citation37). Weight-based dosing requirements for infliximab were calculated using the mean baseline weight of patients in the target population, with the assumption of no vial-sharing. Based on guideline recommendations (Citation11,Citation12), a proportion of patients receiving ustekinumab were expected to dose-escalate to the 90 mg dosage during the subsequent maintenance period ().
Drug administration costs were dependent on the route of administration in Japan, categorized as provider-administered intravenous infusion (infliximab), provider-administered subcutaneous injection (risankizumab, ustekinumab, guselkumab), or self-administered subcutaneous injection (all other comparators). Unit costs of visits to administer or prescribe treatments were obtained from the MHLW () (Citation38). Administration costs were applied at intervals corresponding to licensed dosing schedules for provider-administered biologics (Citation28), or the observed average interval between prescription fills for each self-administered biologic within the Japan Medical Data Center (JMDC) Claims Database (Table S4 of the Supplemental material).
Monitoring costs
Routine monitoring requirements during biologic treatment, including routine outpatient visits and laboratory tests, were derived from the Japanese Dermatological Association (JDA) guidelines (Citation11). Unit costs of monitoring services were obtained from the MHLW ().
Adverse event costs
The model considered medical management costs for the following serious AEs: non-melanoma skin cancer, malignancy other than non-melanoma skin cancer, and severe infections (). These AEs were selected for inclusion based on data availability and the expectation that each event would result in hospitalization and significant medical expenditure.
For risankizumab, rates of these AEs (in terms of events per patient-year) were based on pooled data from the UltIMMa-1, UltIMMa-2, IMMvent, and IMMhance trials. To pool the AE rates across trials, the sum of the number of events in the four trials was divided by the sum of the total patient-years contributed by the four trials. For other treatments, rates of each AE were extracted from summary of product characteristic labels (Citation39–41), phase 3 trial publications (Citation42–45), and large registry studies (Citation46,Citation47).
BSC costs
A recurring cost per cycle was applied within the BSC state to capture the health care resource use of patients with moderate to severe psoriasis who are managed without biologic therapy. The monthly cost of BSC incorporated costs associated with: conventional systemic therapies or phototherapy; hospitalization for a subset of patients; and routine monitoring, prescription fees, and physician visits for all patients ().
Indirect costs
Under the societal perspective, indirect costs associated with work productivity impairment were also considered. Indirect costs were estimated based on the absenteeism and presenteeism associated with different levels of PASI response (Citation7), as well as the expected loss of additional work hours due to provider visits to administer or prescribe treatment (Citation48,Citation49). Details are provided in Table S5 of the Supplemental material.
Patient copayments
Under the patient perspective, copayments for biologic treatment costs (including drug acquisition, administration, and monitoring) were calculated based on Japan’s National Health Insurance (NHI) copayment requirements under the High-Cost Medical Expense Benefit (HCMEB) for general NHI members (Citation50,Citation51), described in Table S6 of the Supplemental material.
Sensitivity analyses
Sensitivity analyses were conducted to assess the robustness of the cost-effectiveness results under the health system perspective. To identify parameters with a strong influence on the results, deterministic sensitivity analyses (DSAs) were conducted in which one model input was varied at a time. PASI response probabilities and odds ratios of AE-related discontinuation were varied between the lower and upper limits of their 95% credible intervals from the posterior distributions of the NMAs (Citation32,Citation33). Unit drug costs were varied by ±2% to account for small potential fluctuations in drug prices. Mean patient weight, the annual rate of discontinuation, and cost inputs were varied above and below the mean by 20%. Additionally, scenario analyses were conducted to test the influence of specific model settings and assumptions, including the time horizon, discount rates for costs and QALYs, primary response criterion, source of efficacy inputs, treatment sequences, and timing of utility gains during the primary response period.
To characterize uncertainty in the model results, a probabilistic sensitivity analysis (PSA) was undertaken in which key model parameters were simultaneously varied based on specified distributional assumptions over 1,000 model iterations. Where available, the standard error of a distribution was obtained from the same data source used to inform the base-case input value; otherwise, the standard error was assumed to be equal to the mean value divided by 4. Probabilities of PASI response and odds ratios of AE-related discontinuation were varied according to the posterior distribution produced by the NMAs (Citation32,Citation33). Normal distributions were used to represent uncertainty in the utility gains associated with each level of PASI response. Gamma distributions were used for cost parameters, which are constrained to be greater than zero. Beta distributions were used for other probabilities to reflect their allowable range between zero and one.
Results
Base-case results
Health system perspective
Over the lifetime model horizon, total QALYs gained were (in order of magnitude): 1.84 for risankizumab, 1.54 for guselkumab, 1.29 for brodalumab, 1.29 for secukinumab, 1.17 for ustekinumab, 1.14 for adalimumab, 1.06 for ixekizumab, and 0.95 for infliximab (). For each treatment, total QALYs gained are measured relative to baseline and thus represent the additional QALYs that accrue above and beyond the scenario in which patients had remained at their baseline utility level throughout the model horizon.
Table 3. Base-case cost-effectiveness results under the health system, societal, and patient perspectives.
Under the health system perspective, which focused on direct health care costs only, total costs were ¥16,325,662 for risankizumab and ranged from ¥12,054,076 for adalimumab to ¥15,008,768 for guselkumab among the comparators (). Cost differences were largely attributable to biologic drug acquisition and administration costs (¥10,027,108 for risankizumab versus ¥5,215,073–¥8,499,879 for comparators); these costs were generally higher for biologics associated with longer total treatment duration (as determined by probabilities of PASI 75 response and risks of AE-related discontinuation). Costs of medical management and supportive medications in the BSC state were lowest for risankizumab (¥6,079,057) and highest for infliximab (¥6,813,386).
The resulting base-case ICERs of risankizumab versus comparators from the Japanese health system perspective ranged from ¥2,545,812/QALY versus ustekinumab to ¥6,077,134/QALY versus adalimumab ().
Societal perspective
When considering a societal perspective, total costs (i.e. including both direct health care costs and indirect costs of work productivity loss) were ¥25,538,283 for risankizumab and between ¥22,480,073 and ¥24,913,367 for comparators (). Indirect costs were lowest for risankizumab (¥9,212,621), with an indirect cost savings of ¥504,539 to ¥1,452,278 relative to comparator treatments. The resulting ICERs of risankizumab versus comparators from the societal perspective ranged from ¥921,770/QALY versus ustekinumab to ¥4,350,879/QALY versus adalimumab ().
Patient perspective
Based on patient cost-sharing requirements in Japan, risankizumab was estimated to reduce out-of-pocket copayments by ¥7,027–¥236,154 relative to four comparators (adalimumab, brodalumab, guselkumab, and secukinumab), and was, therefore, a cost-saving strategy versus these comparators from a patient perspective. Risankizumab was expected to increase copayments by ¥88,493–¥326,747 versus the remaining three comparators, with resulting ICERs of ¥112,421/QALY–¥481,961/QALY under the patient perspective.
DSA and scenario analysis results
Tornado diagrams present the fifteen most influential one-way DSAs and scenario analyses in comparisons of risankizumab versus IL-23 or IL-12/23 inhibitors () and versus other comparators (Figure S2 of the Supplemental material) under the health system perspective.
Figure 2. Tornado diagrams based on DSAs and scenario analyses of risankizumab versus: (a) guselkumab; and (b) ustekinumab. AE: adverse event; BSC: best supportive care; CrI: credible interval; DSA: deterministic sensitivity analysis; ICER: incremental cost-effectiveness ratio; NMA: network meta-analysis; OR: odds ratio; PASI: Psoriasis Area and Severity Index; QALY: quality-adjusted life year.
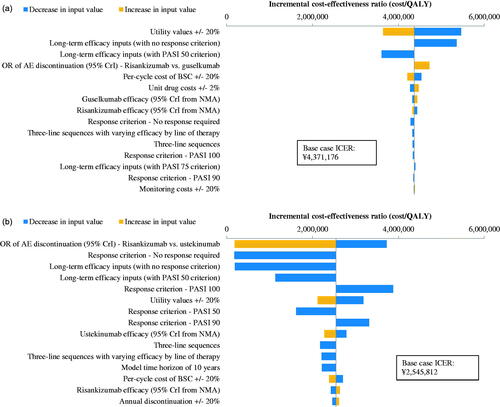
Across all one-way sensitivity analyses, ICERs (in terms of incremental costs per QALYs gained) were within a range of ¥3,609,391–¥5,463,970 versus guselkumab, ¥180,068–¥3,878,932 versus ustekinumab, ¥5,064,278–¥8,567,721 versus adalimumab, ¥4,547,657–¥13,213,457 versus brodalumab, ¥2,313,479–¥5,648,838 versus infliximab, ¥3,676,702–¥5,515,053 versus ixekizumab, and ¥3,948,023–¥6,171,337 versus secukinumab. The ICERs of risankizumab versus all comparators varied inversely with the size of utility gains associated with PASI response. When changing parameters or assumptions that affect treatment duration, the impact on the ICER differed across comparators; for example, the highest ICER versus brodalumab (¥13,213,457/QALY) and the lowest ICER versus ustekinumab (¥180,068) occurred when varying the odds ratio of AE-related discontinuation for risankizumab versus the comparator to the upper limit of its 95% credible interval.
The ICERs showed small to moderate variation in scenarios using: three-line treatment sequences (with or without variation in efficacy by line of therapy); alternative criteria to define primary response (i.e. PASI 50, 90, or 100 instead of PASI 75); and long-term efficacy inputs (with or without also changing the primary response criterion). Relative to the base-case ICERs (reflecting a lifetime model horizon), ICERs were similar when using 10- or 20-year timeframes. The cost-effectiveness results also were not sensitive to characteristics of the model cohort (with the exception of patient weight in comparisons with infliximab); BSC, drug administration, or monitoring costs; assumptions of zero or immediate utility gains in the primary response period; annual discount rates for costs and QALYs; or small fluctuations in unit drug prices.
PSA results
Probabilistic ICERs, which are based on the averages of total costs and total QALYs gained over 1,000 PSA simulations, were consistent with base-case ICERs under the health system perspective (Table S7, Supplemental material).
The cost-effectiveness acceptability curves in Figure S3a show the probability of each biologic being the most cost-effective treatment from a health system perspective over a range of different willingness-to-pay thresholds. Based on a willingness-to-pay threshold of ¥6.7 million/QALY, the probability of being the most cost-effective treatment was 52% for risankizumab, 32% for adalimumab, 13% for brodalumab, and <2% for all other comparators. At the lower bound of willingness-to-pay in Japan (¥5 million/QALY), the highest probability of cost-effectiveness was estimated for adalimumab (80%), followed by risankizumab (11%), brodalumab (8%), and other comparators (all <1%).
Under the societal perspective, the probability of cost-effectiveness was highest for risankizumab at willingness-to-pay thresholds at or above ¥4.7 million/QALY, and ranged from 48.9% at ¥5 million/QALY to 77.5% at ¥6.7 million/QALY (Figure S3b, Supplemental material).
Discussion
The present study sought to evaluate the cost-effectiveness of risankizumab compared with other biologics approved for the treatment of moderate to severe psoriasis in Japan at the time of the analysis in May 2019, using three complementary perspectives (health system, societal, and patient) for the estimation of costs.
Results from this economic evaluation suggest that risankizumab is a cost-effective strategy for achieving higher levels of skin clearance within the biologic-eligible target population. Under base-case assumptions, risankizumab was expected to provide an additional 0.30–0.89 QALYs relative to comparator biologics, at an incremental cost of ¥1.3 million–¥4.3 million (11,864–38,483 United States dollars [USD] (Citation52)) to the health system or ¥0.6 million–¥3.1 million (5,630–27,551 USD) to society. Across the comparators, base-case ICERs were ¥2.5 million–¥6.1 million/QALY (22,935–54,749 USD/QALY) under the health system perspective or ¥0.9 million–¥4.4 million/QALY (8,304–39,197 USD/QALY) under the societal perspective. The main driver of increased cost with risankizumab was drug acquisition and administration costs, as the model was designed to predict longer treatment durations for biologics associated with higher probabilities of PASI 75 achievement and lower risks of AE-related discontinuation. Sensitivity analyses showed that the ICERs versus some comparators were sensitive to parameters determining treatment duration (e.g. annual discontinuation risks). Because changes to treatment duration had a simultaneous competing influence on both costs and QALYs, the direction of the ICER impact varied by comparator.
In a cost-effectiveness framework, ICERs are interpreted in relation to a region-specific willingness-to-pay benchmark, representing the maximum ICER at which a treatment is considered cost-effective. Based on evidence from population-based discrete choice experiments, willingness-to-pay in Japan has been estimated within a range of ¥5 million to ¥6.7 million per QALY gained (Citation25,Citation26), equating to approximately 45,045 to 60,360 USD per QALY gained (Citation52). By comparison, when applying the global willingness-to-pay benchmark adopted by the World Health Organization (i.e. three times gross domestic product per capita) (Citation53), the threshold for Japan increases to ¥12,882,459/QALY (or 116,058 USD/QALY) (Citation54). Under each perspective considered, the base-case ICERs of risankizumab versus other biologics fell within or below the range of acceptable thresholds. In probabilistic simulations based on the health system perspective, risankizumab had the first- or second-highest probability of being the most cost-effective biologic within the willingness-to-pay range of ¥5 million/QALY–¥6.7 million/QALY. Based on probabilistic simulations under the societal perspective, risankizumab had the highest probability of being the most cost-effective biologic throughout this willingness-to-pay range.
Indirect costs were estimated to be substantial in the model, comprising between 36% and 46% of the total societal cost burden in each treatment arm. This result is in line with prior reports from cost of illness studies conducted in other country settings; for example, 32–40% of the total cost burden of psoriasis in the United States has been attributed to work productivity loss (Citation55,Citation56). The magnitude of indirect costs, and the reported linkage between PASI and work productivity (as measured by the validated WPAI questionnaire) (Citation6,Citation7), suggests that cost-effectiveness analyses of biologic treatments for psoriasis should consider a broad scope of costs beyond direct health care costs alone. When taking a societal perspective, drug acquisition costs in the risankizumab arm were partially offset by reductions in the indirect costs of productivity loss. These cost savings reflected higher estimated levels of PASI response achievement (resulting in less psoriasis-related absenteeism and presenteeism) and less frequent dosing schedule than most comparators (resulting in less frequent work absences for provider visits to administer or prescribe treatments).
The patient perspective has been proposed as an important consideration in health economic evaluations, particularly for decision problems that are high-stakes with respect to out-of-pocket costs (Citation57,Citation58). Real-world studies among psoriatic patients in Japan have shown that patient copayments over 1 year are substantial in those treated with biologics (Citation18,Citation20), and that dosing convenience and copayment are among the important attributes determining patient preference for biologic treatments (Citation27). Based on insurance benefits including the HCMEB in Japan, copayments in the present model were, all else equal, minimized for treatments with the longest (i.e. Q12W) interval between consecutive administrations or prescription fills. Consequently, despite longer treatment duration, risankizumab resulted in lower total copayments than four of the comparators and was thus considered a cost-saving strategy over these comparators from a patient perspective.
Given the recent introduction of several novel biologic treatments for moderate to severe psoriasis in Japan, rigorous economic evaluations of these treatments are needed to guide the efficient use of health care resources. Prior research describing the cost-effectiveness of biologics in the Japan setting has been restricted to group-level comparisons or a subset of all relevant approved comparators. One recent study used prospective observational data from an outpatient clinic in Japan to evaluate the cost-effectiveness of biologics relative to topical corticosteroids and conventional systemic therapies among patients with psoriasis (Citation20). The study concluded that biologics may be considered cost-effective based on an ICER of ¥6,366,769/QALY versus topical therapy, but did not comparatively assess the cost-effectiveness of different biologics and was restricted to a 1-year observation period (Citation20). Another study used a decision-analytic modeling approach to evaluate the cost-effectiveness of secukinumab over a 10-year timeframe; however, the comparator set was limited to the older biologics adalimumab, infliximab, and ustekinumab (Citation22).
Key strengths of this analysis include the lifetime horizon and consideration of all biologic drug classes currently approved for the treatment of psoriasis in Japan. This study also provides the first report of cost-effectiveness from a societal perspective in this setting. Nevertheless, the cost-effectiveness model is subject to limitations, including those associated with the data sources used for parameter estimation. In particular, the NMA used to inform efficacy inputs may be impacted by cross-trial difference in protocols and patient characteristics that may modify the treatment effect. The adjustment for reference arm response in the NMA should reduce, but does not eliminate, the potential for confounding due to cross-trial heterogeneity.
The NMA included short-term (10- to 16-week) data from clinical trials, corresponding to the randomized controlled portion of the included clinical trials. To explore the impact of this limitation, a scenario analysis was conducted using PASI response probabilities estimated from a meta-analysis of long-term trial data (Citation32). ICERs from this scenario analysis were comparable to those estimated in the base case.
Based on data availability, the utility gain corresponding to each level of PASI response was assumed to be the same irrespective of treatment. The model thus did not incorporate any variation in utility values that may occur due to differences in patient preferences for different modes of administration or less-frequent dosing intervals (Citation27), or due to differences in the conditional distribution of PASI responses within each PASI response level.
The model incorporated the costs of three serious AEs of special interest based on their high expected cost of management per event. However, the health-related quality of life impact of AEs was not modeled in this economic evaluation due to uncertainty regarding the mean duration of the disutility impact. The influence of AE-related disutility on the model results was expected to be small given the infrequency of malignant AEs and the acute nature of serious infections.
The analysis adopted global efficacy data given the larger sample size of multi-national trials and unavailability of Japan-specific trial data for some comparators. Epidemiologic studies have shown differences in the mean age and gender distribution of patients with psoriasis in Japan versus Western countries, and it is possible that these differences could impact the generalizability of global trial results to a Japan-specific population. However, the results for risankizumab 150 mg in the SustaIMM trial in Japan were consistent with findings from four global phase III trials, with 74.5% achieving PASI 90 at week 16 (Citation30) (compared with 72.4–75.3% in the global studies (Citation14–16)). Further research is warranted to comparatively assess the effectiveness of different biologic therapies in Japan as additional data become available.
Conclusions
Risankizumab was estimated to increase costs relative to other biologics approved for the treatment of psoriasis in Japan, with high probabilities of PASI response and sufficient incremental QALY gains over comparators to be considered cost-effective from the health system and societal perspectives. Based on the structure of insurance benefits in Japan, risankizumab was expected to yield savings or minimal increases in out-of-pocket copayments versus comparators under the patient perspective.
Supplemental Material
Download PDF (578.2 KB)Acknowledgements
The authors acknowledge the editing assistance of Natalia Price, an employee of AbbVie Pty Ltd, in the preparation of this manuscript.
The authors also acknowledge the technical writing assistance of Euijin Kim, an employee of Analysis Group Inc., in the preparation of this manuscript.
Disclosure statement
Hidehisa Saeki has served as a paid speaker for and/or accepted a research grant from companies that manufacture drugs used for the treatment of psoriasis including AbbVie, Celgene, Eisai, Kyowa Kirin, Maruho, Mitsubishi-Tanabe, Taiho and Torii.
Kanako Ishii and Isao Kawaguchi are employees of AbbVie GK and may own AbbVie stock.
Avani Joshi is an employee of AbbVie Inc and may own AbbVie stock.
Arielle Bensimon and Hongbo Yang are employees of Analysis Group Inc., which received consultancy fees from AbbVie GK.
Additional information
Funding
References
- Kubota K, Kamijima Y, Sato T, et al. Epidemiology of psoriasis and palmoplantar pustulosis: a nationwide study using the Japanese national claims database. BMJ Open. 2015;5(1):e006450–e006450.
- Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445(7130):866–873.
- Fortune DG, Richards HL, Griffiths CE. Psychologic factors in psoriasis: consequences, mechanisms, and interventions. Dermatol Clin. 2005;23(4):681–694.
- Rapp SR, Feldman SR, Exum ML, et al. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41(3):401–407.
- Moller AH, Erntoft S, Vinding GR, et al. A systematic literature review to compare quality of life in psoriasis with other chronic diseases using EQ-5D-derived utility values. Patient Relat Outcome Meas. 2015;6:167–177.
- Hayashi M, Saeki H, Ito T, et al. Impact of disease severity on work productivity and activity impairment in Japanese patients with psoriasis. J Dermatol Sci. 2013;72(2):188–191.
- Feldman SR, Zhao Y, Gilloteau I, et al. Higher psoriasis skin clearance is associated with lower annual indirect costs in the United States: a post hoc analysis from the CLEAR study. J Manag Care Spec Pharm. 2018;24(7):617–622.
- Feldman SR. Treatment of psoriasis in adults; 2019. [cited 2019 Dec 9]. Available from: https://www.uptodate.com/contents/treatment-of-psoriasis-in-adults/
- Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2017;12:CD011535.
- Pharmaceuticals and Medical Devices Agency. List of approved products. [cited 2019. Dec 1]. Available from: https://www.pmda.go.jp/english/review-services/reviews/approved-information/drugs/0002.html
- Japanese Dermatological Association. [Japanese guidance for use of biologics for psoriasis (the 2018 version)]; 2019. [cited 2019 Nov 11]. Japanese. Available from: https://www.dermatol.or.jp/uploads/uploads/files/news/J20190219_gaid.pdf
- Ohtsuki M, Terui T, Ozawa A, et al. Japanese guidance for use of biologics for psoriasis (the 2013 version). J Dermatol. 2013;40(9):683–695.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2015;136(1):116–124.e7.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392(10148):650–661.
- Reich K, Gooderham M, Thaci D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394(10198):576–586.
- Blauvelt A, Papp KA, Gooderham M, et al. Risankizumab efficacy/safety in moderate-to-severe plaque psoriasis: 16-week results from IMMhance. Acta Derm Venereol. 2018;98(suppl 219):30.
- Pharmaceuticals and Medical Devices Agency. List of approved products. [cited 2019 Dec 1]. Available from: https://www.pmda.go.jp/files/000229856.pdf
- Takahashi H, Satoh K, Takagi A, et al. Economic burden of psoriatic patients in Japan: analysis from a single outpatient clinic. J Dermatol. 2017;44(9):1024–1026.
- Takura T. An evaluation of clinical economics and cases of cost-effectiveness. Intern Med. 2018;57(9):1191–1200.
- Takahashi H, Satoh K, Takagi A, et al. Cost-efficacy and pharmacoeconomics of psoriatic patients in Japan: analysis from a single outpatient clinic. J Dermatol. 2019;46(6):478–481.
- Igarashi A, Kuwabara H, Fahrbach K, et al. Cost-efficacy comparison of biological therapies for patients with moderate to severe psoriasis in Japan. J Dermatolog Treat. 2013;24(5):351–355.
- Igarashi A, Igarashi A, Graham CN, et al. Evaluating the cost-effectiveness of secukinumab in moderate-to-severe psoriasis: a Japanese perspective. J Med Econ. 2018;22(1):7–15.
- Fukuda T, Shiroiwa T. Application of economic evaluation of pharmaceuticals and medical devices in Japan. J Natl Inst Public Health. 2019;68(1):27–33.
- Center for Outcomes Research and Economic Evaluation for Health, National Institute of Public Health (C2H). Guideline for preparing cost-effectiveness evaluation to the Central Social Insurance Medical Council: version 2.0; 2019. [cited 2019 Nov 11]. Available from: https://c2h.niph.go.jp/tools/guideline/guideline_en.pdf
- Ohkusa Y, Sugawara T. Research for willingness to pay for one QALY gain. J Health Care Soc. 2006;16(2):157–165. Japanese.
- Shiroiwa T, Sung Y, Fukuda T, et al. International survey on willingness-to-pay (WTP) for one additional QALY gained: what is the threshold of cost effectiveness? Health Econ. 2010;19(4):422–437.
- Tada Y, Ishii K, Kimura J, et al. Patient preference for biologic treatments of psoriasis in Japan. J Dermatol. 2019;46(6):466–477.
- Saeki H, Terui T, Morita A, et al. Japanese guidance for use of biologics for psoriasis (the 2019 version). J Dermatol. 2020;47(3):201–222. [Epub ahead of print].
- National Institute for Health and Care Excellence. Guide to the methods of technology appraisal; 2013. [cited 2019 Nov 11]. Available from: https://www.nice.org.uk/process/pmg9/chapter/foreword
- Ohtsuki M, Fujita H, Watanabe M, et al. Efficacy and safety of risankizumab in Japanese patients with moderate to severe plaque psoriasis: results from the SustaIMM phase 2/3 trial. J Dermatol. 2019;46(8):686–694.
- National Institute of Health and Care Excellence. NICE pathway: psoriasis; 2017. [cited 2017 Sep]. Available from: http://pathways.nice.org.uk/pathways/psoriasis
- Armstrong AW, Puig L, Joshi A, et al. Comparison of biologics and oral treatments for plaque psoriasis: a meta-analysis. JAMA Dermatol. 2020;156(3):258.
- Shear NH, Joshi AD, Zhao J, et al. Comparison of safety outcomes for treatments of moderate to severe plaque psoriasis through a network meta-analysis. Poster presented at the 24th World Congress of Dermatology, June 2019. Milan, Italy.
- Warren RB, Smith CH, Yiu ZZ, et al. Differential drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Invest Dermatol. 2015;135(11):2632–2640.
- Ministry of Heath, Labour and Welfare. National life tables; 2017. [cited 2019 Apr 15]. Available from: https://www.mhlw.go.jp/toukei/saikin/hw/life/life17/xls/life17-12.xls
- Woolacott N, Hawkins N, Mason A, et al. Etanercept and efalizumab for the treatment of psoriasis: a systematic review. Health Technol Assess. 2006;10(46):1–258.
- Ministry of Health, Labour and Welfare. 薬価基準収載品目リスト及び後発医薬品に関する情報について(令和元年9月30日まで)[List of drugs and generics by 30 Sep 2019]; 2019. [cited 2019 Dec 23]. Japanese. Available from: https://www.mhlw.go.jp/topics/2018/04/tp20180401-01.html
- Ministry of Heath, Labour and Welfare. 平成30年度診療報酬改定について [2018 Medical Treatment Fee Revision]; 2019. [cited 2019 May 31]. Japanese. Available from: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000188411.html
- Electronic Medicines Compendium. Summary of product characteristics: cosentyx 150 mg solution for injection in pre-filled syringe and pre-filled pen. [cited 2017 Jul]. Available from: https://www.medicines.org.uk/emc/print-document?documentId=29848
- Electronic Medicines Compendium. Summary of product characteristics: humira 40 mg/0.4 ml pre-filled syringe and pre-filled pen. [cited 2017 Jul]. Available from: https://www.medicines.org.uk/emc/print-document?documentId=31860
- Electronic Medicines Compendium. Summary of product characteristics: stelara 45 mg and 90mg, solution for injection (vials) and solution for injection in pre filled syringe. [cited 2017 Jul]. Available from: https://www.medicines.org.uk/emc/print-document?documentId=32569
- Langley RE, Lebwohl M, Reich K, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375(4):345–356.
- Blauvelt A, Papp KA, Griffiths CEM, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: Results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–417.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318–1328.
- Dixon W, Watson K, Lunt M, et al. Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti-tumor necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2006;54(8):2368–2376.
- Reich K, Mrowietz U, Radtke M, et al. Drug safety of systemic treatments for psoriasis: results from The German Psoriasis Registry PsoBest. Arch Dermatol Res. 2015;307(10):875–883.
- Tokunaga M, Nakane N. Study on outpatient’s waiting time during all phases of medical consultation. J Jpn Soc Health Care Manag. 2006;7(3):434–437.
- Ministry of Heath, Labour and Welfare. 平成8年受療行動調査の概要 [Patient’s behavior survey]; 1996. [cited 2019 Jul 9]. Japanese. Available from: https://www.mhlw.go.jp/www1/toukei/h8jyuryo_8/01-03.html
- Guide to Japan’s National Health Insurance (NHI) System; 2017. [cited 2019 Dec 6]. Available from: https://yosida.com/forms/nationalins.pdf
- Ministry of Health, Labour and Welfare. 高額療養費制度の見直しについて [Revision of high-cost medical expense benefit]. 2016. [cited 2019 Dec 23]. Japanese. Available from: https://www.mhlw.go.jp/file/05-Shingikai-12601000-Seisakutoukatsukan-Sanjikanshitsu_Shakaihoshoutantou/0000138069.pdf
- Bank of Japan. Standard foreign exchange rates and arbitrage foreign exchange rates (applicable in May 2019). [cited 2020 Mar 3]. Japanese. Available from: https://www.boj.or.jp/about/services/tame/tame_rate/kijun/kiju1905.htm/
- Bertram MY, Lauer JA, De Joncheere K, et al. Cost–effectiveness thresholds: pros and cons. Bull World Health Organ. 2016;94(12):925–930.
- The World Bank. GDP per capita (constant LCU) – Japan. World Bank national accounts data, and OECD National Accounts data files; 2018. [cited 2019 Dec 18]. Available from: https://data.worldbank.org/indicator/NY.GDP.PCAP.KN?locations=JP
- Vanderpuye-Orgle J, Zhao Y, Lu J, et al. Evaluating the economic burden of psoriasis in the United States. J Am Acad Dermatol. 2015;72(6):961–967.e5.
- Fowler JF, Duh MS, Rovba L, et al. The impact of psoriasis on health care costs and patient work loss. J Am Acad Dermatol. 2008;59(5):772–780.
- Bilvick Tai B, Bae YH, Le QA. A systematic review of health economic evaluation studies using the Patient's Perspective. Value Health. 2016;19(6):903–908.
- Garrison LP, Jr., Pauly MV, Willke RJ, et al. An overview of value, perspective, and decision context-a health economics approach: an ISPOR Special Task Force report [2]. Value Health. 2018;21(2):124–130.
