Abstract
Background
Dupilumab, a first-in-class therapy targeting the two key cytokines involved in the persistent underlying inflammatory pathway in atopic dermatitis (AD), is approved for treatment of moderate-to-severe AD in Europe, USA, Japan and several other countries.
Objective
To assess dupilumab effects on SCORing Atopic Dermatitis (SCORAD) and component scores (objective and subjective SCORAD) over time in adults with moderate-to-severe AD.
Methods
This post hoc analysis included 2,444 patients in four placebo-controlled, double-blind, randomized, phase 3 trials. SOLO 1 and 2 (NCT02277743; NCT02277769) evaluated 16 weeks of dupilumab monotherapy against placebo. CAFÉ (NCT02755649) and CHRONOS (NCT02260986) evaluated dupilumab with concomitant topical corticosteroids (TCS) against TCS alone for 16 and 52 weeks, respectively.
Results
2,444 patients randomized to treatment in SOLO 1 and 2 (N = 1,379), CAFÉ (N = 325) and CHRONOS (N = 740) were analyzed. Dupilumab treatment significantly improved overall SCORAD and individual components as early as Week 1 or 2, with significant and clinically meaningful differences vs. control through end of treatment (p < .0001). These results occurred irrespective of dupilumab regimen, 300 mg subcutaneously weekly or every 2 weeks.
Conclusions
In four large phase 3 trials in adults with moderate-to-severe AD, dupilumab treatment with or without concomitant TCS resulted in rapid and sustained improvements in all SCORAD outcomes vs. placebo or TCS alone.
© 2020 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group
Keywords:
Introduction
Atopic dermatitis (AD) is a complex, chronic relapsing skin disease characterized by underlying type 2 inflammation, with recent evidence suggesting it is a systemic disease (Citation1–5). Diagnosis is based on clinician assessment of signs, including erythema, edema, oozing, lichenification and xerosis that can be localized or affect a widespread body surface area. Moderate-to-severe AD is highly symptomatic and can have a profound multidimensional burden on patients (Citation6–8). Frequent, intense pruritus and sleep loss are the most recognized and impactful symptoms contributing to reduced quality of life (QoL) and worse psychological and overall health status among patients (Citation1,Citation9–16).
Numerous clinical tools have been developed to assess AD severity. These tools are used primarily in clinical trials but can also be used for clinical monitoring and to guide treatment decisions (Citation17). The most commonly used and validated scoring systems are the Eczema Area and Severity Index (EASI), SCORing Atopic Dermatitis (SCORAD) and Patient-Oriented Eczema Measure (POEM) (Citation18–21).
Developed and validated in the 1990s by the European Task Force on AD (ETFAD), SCORAD is recommended by European AD guidelines and evaluates investigator-reported affected body surface area and severity of signs (objective component) as well as patient-reported symptoms of pruritus and sleep loss (subjective component) (Citation22–24). Established severity strata reflect mild, moderate and severe disease, with thresholds differing depending on the specific population (Citation25,Citation26). The current ETFAD guidelines on diagnosis and treatment of AD and the ETFAD position paper on AD recommend using SCORAD for overall disease severity and have anchored therapy step-up/step-down to SCORAD levels of 25 and 50, respectively (Citation5,Citation24,Citation27). The ETFAD has also proposed a grading scale to assess disease severity based on the objective component of SCORAD only (o-SCORAD), namely the extent of disease and intensity of signs (Citation23).
EASI and o-SCORAD/SCORAD have several key differences (Table S1). EASI gives a higher weighting to the area of involvement (∼60% of the score) and o-SCORAD and SCORAD to intensity of signs (∼75% and ∼60%, respectively), making o-SCORAD/SCORAD more suitable for measuring localized, severe AD than EASI (Citation28). EASI evaluates area and severity in four different body regions, whereas SCORAD uses overall body surface calculation using the rule of nines, with severity graded in a target lesion. Finally, EASI assesses four signs (erythema/edema/lichenification/excoriation), whereas SCORAD also assesses oozing/crusting and xerosis in nonlesional skin, and subjective patient assessment of pruritus and sleep loss. Thus, EASI and SCORAD provide different assessments of disease severity.
Treatment options are limited for adults with moderate-to-severe AD uncontrolled with currently approved topical and/or systemic treatments. Long-term use of systemic immunosuppressants is not recommended due to unfavorable benefit-risk profiles. Dupilumab, a fully human VelocImmune®-derived (Citation29,Citation30) monoclonal antibody, blocks the shared receptor subunit for interleukin (IL)-4 and IL-13, thus inhibiting signaling of both IL-4 and IL-13. Dupilumab is approved for patients with type 2 inflammatory diseases, including AD, asthma, and chronic rhinosinusitis with nasal polyps (Citation31,Citation32).
Four randomized, double-blind, placebo-controlled, phase 3 trials involving 2,444 adult patients with moderate-to-severe AD have demonstrated efficacy and safety of dupilumab with or without concomitant topical corticosteroids (TCS) (Citation33–35). The objective of this manuscript is to report the effects of dupilumab on SCORAD outcomes in adults with moderate-to-severe AD included in these dupilumab phase 3 studies, either when used as a monotherapy or in combination with concomitant TCS.
Methods
Study design
This post hoc analysis includes data from four randomized, double-blind, placebo-controlled, parallel-group, phase 3 clinical trials that evaluated dupilumab treatment in 2,444 adults with moderate-to-severe AD. Patients in these trials received dupilumab monotherapy (300 mg weekly [qw] or every 2 weeks [q2w]; LIBERTY AD SOLO 1 [NCT02277743] and LIBERTY AD SOLO 2 [NCT02277769], data pooled in this analysis) (Citation33) or dupilumab with concomitant TCS (300 mg qw or q2w; LIBERTY AD CAFÉ [NCT02755649](Citation34) and LIBERTY AD CHRONOS [NCT02260986]) (Citation35).
Key study details for SOLO 1 and 2, CAFÉ and CHRONOS are provided in Table S2. Detailed methodology and primary efficacy and safety results have been reported previously (Citation33–35). Of note, the CAFÉ study included a population with more severe disease unresponsive, intolerant or contraindicated to ciclosporin A.
These trials were approved by their respective institutional review boards and conducted in accordance with the ethical principles outlined in the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice guideline and applicable regulatory requirements.
Outcomes assessed
SCORAD includes three components (Citation22) (Table S3): Part A, the extent of disease (investigator-assessed); Part B, the intensity of signs of disease (investigator-assessed); and Part C, symptoms (patient-assessed). Total SCORAD is calculated as: A/5 + 7B/2 + C, with a maximum possible score of 103; higher scores indicate worse disease. The extent of disease (Part A), the intensity of signs (Part B) and symptoms (Part C) account for 19.4, 61.1, and 19.4% of the total score, respectively. o-SCORAD includes the objective components of SCORAD (Parts A and B mentioned previously) (Citation23), with area and signs accounting for 24% and 76% of the total score, respectively. o-SCORAD is calculated as: A/5 + 7B/2, for a maximum possible score of 83; higher scores indicate worse disease. The within-patient clinically meaningful changes have been estimated as 8.7 and 8.2 for SCORAD and o-SCORAD, respectively (Citation36). Subjective assessment of pruritus and sleep loss is recorded by the patient or relative on a visual analog scale (VAS) based on an average value over the past 3 days or nights: 0: no pruritus or sleep loss;10: worst imaginable pruritus or sleep loss.
SCORAD was assessed at screening, baseline, Weeks 1, 2, 4, 6, 8, 12, and 16 in all studies, and every 4 weeks thereafter through Week 52 in CHRONOS. The following severity bands were used in these analyses: for SCORAD (Citation26), clear (0–9.9), mild (10.0–28.9), moderate (29.0–48.9) and severe (49.0–103); for o-SCORAD (Citation26) clear (0–7.9), mild (8.0–23.9), moderate (24.0–37.9) and severe (38.0–83); for SCORAD pruritus VAS (Citation37) and sleep loss VAS: none (0), mild (>0 to <4), moderate (4 to <7), severe (≥7 to 9) and very severe pruritus (≥9).
This analysis includes results over time for each study as SCORAD, o-SCORAD, SCORAD-50 (≥50% improvement from baseline in total SCORAD), percentage of patients achieving the clinically meaningful change for SCORAD and o-SCORAD, SCORAD component scores (Parts A, B, and C), percent change from baseline in total SCORAD, categorical changes in SCORAD severity according to the severity strata outlined previously (Citation26) categorical changes in individual signs severity over time, the percent reduction in Parts A, B, and C, change in absolute values from baseline to Week 16 or 52 for SCORAD, o-SCORAD, Part A, Part B (erythema, edema/papulation, excoriation, lichenification, oozing/crusting and dryness on nonlesional areas) and individual symptoms (pruritus VAS and sleep loss VAS).
Statistical analysis
Efficacy analyses were performed on the full analysis set, which included all randomized patients. For continuous outcomes, patients missing an assessment or who received rescue treatment were considered ‘nonresponders’ (censoring) and imputed using the multiple imputation method; p values were assessed using an analysis of covariance model with baseline measurement as a covariate and the treatment, region, baseline Investigator’s Global Assessment (IGA) strata, and study identifier (for SOLO studies only) as fixed factors. The p values for categorical endpoints were derived by a Cochran-Mantel-Haenszel (CMH) test stratified by region and baseline IGA strata, with a study identifier added as an additional factor for the SOLO pooled analysis. For categorical outcomes, patients who received rescue treatment were considered nonresponders after rescue treatment use (nonresponder imputation). Significance values were considered nominal for all analyses. For Sankey plots of changes in SCORAD severity over time, values were set to missing after rescue treatment, and missing total SCORAD scores were set to the highest category (severe).
Results
Patients
All 2,444 patients randomized to treatment in SOLO 1 and 2 (N = 1,379), CAFÉ (N = 325) and CHRONOS (N = 740) were included in this analysis (). Detailed baseline demographics and characteristics for each study have been published previously (Citation33–35) and were similar across studies. Median age was 34.0–40.5 years for each treatment group across studies, and the median disease duration was 25.0–32.0 years ().
Table 1. Baseline demographics and clinical characteristics: SOLO 1 and 2 (pooled data), CAFÉ, CHRONOS.
Baseline SCORAD details are provided in . Median scores in each treatment group were similar across studies and ranged between 64.1–69.7 for total SCORAD, 52.4–57.2 for o-SCORAD, 51.0–58.8 for Part A, 12.0–13.0 for Part B and 10.4–13.7 for Part C.
Efficacy analysis by SCORAD and SCORAD components
As both dupilumab doses had similar results, only those from the approved 300 mg q2w treatment and control (placebo or placebo + TCS) groups are reported in the text for simplicity. Least squares (LS) mean total SCORAD and o-SCORAD scores declined in all treatment groups over time, with mean values significantly lower with dupilumab vs. control in each study, as early as the first timepoint measured (Week 1, ). The proportion of patients achieving SCORAD-50 was greater with dupilumab vs. control in each study (), with a significantly higher proportion observed with dupilumab as early as Week 1 (monotherapy), Week 2 (CHRONOS) and Week 4 (CAFÉ).
Figure 1. LS mean total SCORAD score (score range 0–103), o-SCORAD (score range 0–83) and SCORAD-50 over time: (a) SOLO 1 and 2 (pooled data), 16-week monotherapy; (b) CAFÉ, 16 weeks with concomitant TCS; (c) CHRONOS, 52 weeks with concomitant TCS. *p < .05 vs. placebo/control; **p < .01 vs. placebo/control; ***p < .0001 vs. placebo/control. LS: least squares; o-SCORAD: objective SCORing Atopic Dermatitis; qw: weekly; q2w: every 2 weeks; SCORAD: SCORing Atopic Dermatitis; SCORAD-50: ≥50% reduction from baseline in SCORing Atopic Dermatitis; SE: standard error; TCS: topical corticosteroids.
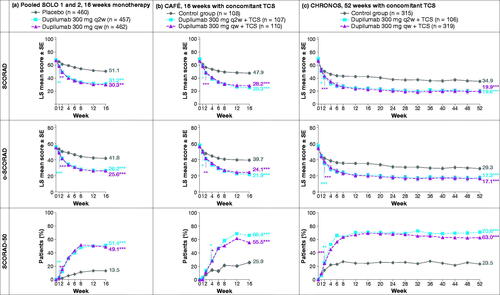
Of all patients treated with dupilumab q2w across all studies, 95.7% achieved a clinically meaningful change for SCORAD (≥8.7-point improvement) and 79.9% for o-SCORAD (≥8.2-point improvement) (Citation36), (p < .0001 vs. control for both).
When looking at individual components, dupilumab treatment resulted in statistically significant improvements as measured by the extent of disease (SCORAD Part A), the intensity of signs (Part B and individual signs) and intensity of symptoms (Part C and individual symptoms of pruritus and sleep loss) vs. the control in each study (). These significant differences were observed as early as the first timepoint measured (Week 1) for most components and continued to improve or were maintained until the end of treatment (16 or 52 weeks).
Figure 2. LS mean SCORAD scores for Part A (extent of disease [BSA] score, 0–100), Part B (signs intensity score, 0–18), pruritus VAS score (0–10), and sleep loss VAS score (0–10) over time: (a) SOLO 1 and 2 (pooled data), 16-week monotherapy; (b) CAFÉ, 16 weeks with concomitant TCS; (c) CHRONOS, 52 weeks with concomitant TCS. *p < .05 vs. placebo; †p < .01 vs. placebo; **p < .001; ***p < .0001 vs. placebo. BSA: body surface area; LS: least squares; qw: weekly; q2w: every 2 weeks; SCORAD: SCORing Atopic Dermatitis; SE: standard error; TCS: topical corticosteroids; VAS: visual analog scale.
![Figure 2. LS mean SCORAD scores for Part A (extent of disease [BSA] score, 0–100), Part B (signs intensity score, 0–18), pruritus VAS score (0–10), and sleep loss VAS score (0–10) over time: (a) SOLO 1 and 2 (pooled data), 16-week monotherapy; (b) CAFÉ, 16 weeks with concomitant TCS; (c) CHRONOS, 52 weeks with concomitant TCS. *p < .05 vs. placebo; †p < .01 vs. placebo; **p < .001; ***p < .0001 vs. placebo. BSA: body surface area; LS: least squares; qw: weekly; q2w: every 2 weeks; SCORAD: SCORing Atopic Dermatitis; SE: standard error; TCS: topical corticosteroids; VAS: visual analog scale.](/cms/asset/efbb63a0-7a78-4580-af1b-7c1586b7bc47/ijdt_a_1750550_f0002_c.jpg)
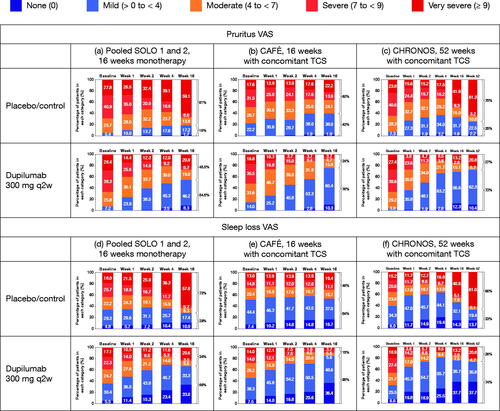
With respect to symptoms, 54.5, 75.7, and 72.7% of patients had mild or no pruritus and 66.3, 85.0, and 73.5% had mild or no sleep loss at end of treatment in SOLO, CAFÉ and CHRONOS, respectively ().
Figure 3. Percentage of patients in each severity category for pruritus over time, by study: (a) SOLO 1 and 2 (pooled data), 16-week monotherapy; (b) CAFÉ, 16 weeks with concomitant TCS; (c) CHRONOS, 52 weeks with concomitant TCS; and percentage of patients in each severity category for sleep loss VAS over time, by study: (d) SOLO 1 and 2 (pooled data), 16-week monotherapy; (e) CAFÉ, 16 weeks with concomitant TCS; (f) CHRONOS, 52 weeks with concomitant TCS. Values were set to missing after rescue treatment; missing total SCORAD scores were set to the highest category (severe). q2w: every 2 weeks; TCS: topical corticosteroids; VAS: visual analog scale.
The percent change in total SCORAD from baseline was greater with dupilumab vs. control at the end of treatment in all studies (p < .0001 for both dupilumab regimens vs. control). At the end of the treatment period, the LS mean percent changes in SCORAD were −54.3, −58.3, and −69.4% with dupilumab vs. −24.0, −29.5, and −47.3% with control in SOLO pooled, CAFÉ and CHRONOS, respectively.
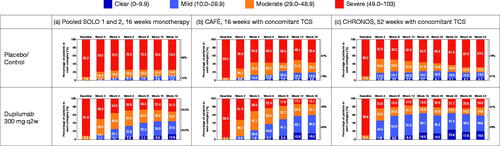
The percentage of patients in each SCORAD severity category shifted over time, with greater movement from ‘severe’ or ‘moderate’ disease at baseline to ‘mild’ disease or ‘clear’ at the end of treatment with dupilumab vs. control (). The majority of patients in the control arms had scores corresponding to ‘severe’ disease by the end of treatment. In contrast, the majority of dupilumab-treated patients had scores corresponding to ‘clear’ or ‘mild’ disease in CAFÉ and CHRONOS and ‘clear,’ ‘mild’ or ‘moderate’ disease in SOLO by the end of treatment.
Figure 4. Percentage of patients in each total SCORAD score severity category over time, by study: (a) SOLO 1 and 2 (pooled data), 16-week monotherapy; (b) CAFÉ, 16 weeks with concomitant TCS; (c) CHRONOS, 52 weeks with concomitant TCS. Values were set to missing after rescue treatment; missing total SCORAD scores were set to the highest category (severe). q2w: every 2 weeks; SCORAD: SCORing Atopic Dermatitis; TCS: topical corticosteroids.
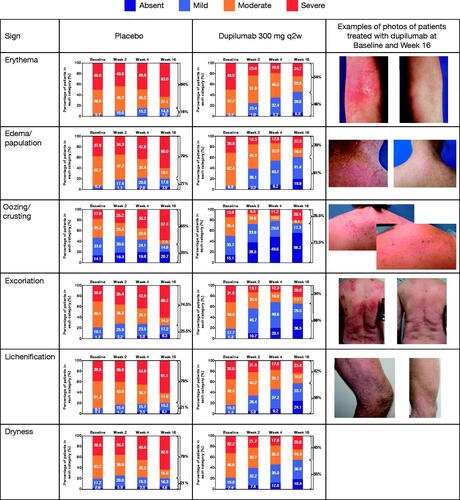
Categorical changes in signs severity score at each timepoint through Week 16 in patients treated with dupilumab monotherapy or placebo in SOLO 1 and 2 are presented in and in Figure S1 for CAFÉ and CHRONOS. LS mean percent change in sign intensity score over time is shown in Figure S2. The distribution across severity groups in all individual signs shifted over time. Dupilumab showed significant improvement in most signs vs. placebo as early as Week 1 with a greater proportion of patients with absent or mild signs by the end of treatment, whereas a greater proportion of patients had severe signs in the placebo group. Table S4 shows by which week the improvement in signs was significant vs. control. By Week 16, the majority of patients had mild or absent signs (except for erythema with monotherapy) in dupilumab vs. the majority of patients having moderate or severe signs in the control arm (, Figure S1).
Figure 5. Distribution of signs severity over time in patients treated with placebo and dupilumab 300 mg q2w: SOLO 1 and 2, 16-week monotherapy. Missing SCORAD scores set to the highest value (severe). Severity strata for severity grading are absent (0), mild (1), moderate (2), and severe (3). q2w: every 2 weeks; SCORAD: SCORing Atopic Dermatitis.
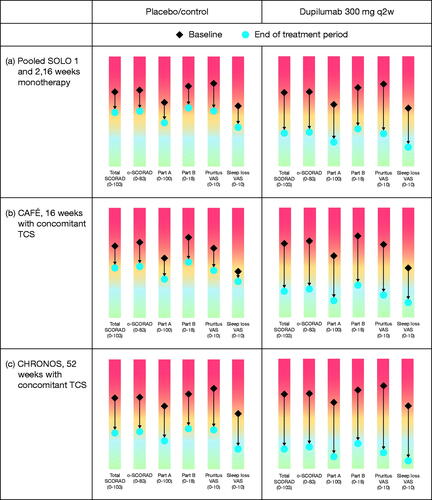
At the end of treatment (Week 16 in SOLO and CAFÉ and Week 52 in CHRONOS), dupilumab-treated patients showed consistent improvements assessed as LS mean percent reduction in SCORAD Part A (area), Part B (signs) and Part C (symptoms) (). Changes in absolute values from baseline (diamond) to Week 16/52 (dot) for SCORAD components are shown in the rainbow plots in for the control and q2w regimens across all studies. In all three studies, dupilumab vs. control led to greater improvements in total SCORAD, o-SCORAD, the extent of disease and individual signs and symptoms (VAS pruritus and VAS sleep loss), with ≥1 grade of improvement in severity for those outcomes based on established severity strata.
Figure 6. LS mean percent reduction in Part A (area), Part B (signs) and Part C (symptoms): (a) SOLO 1 and 2 (pooled data), 16-week monotherapy; (b) CAFÉ, 16-week with concomitant TCS; (c) CHRONOS, 52-week with concomitant TCS. Lines closer to the outer edge of the spider plot represent greater improvement from baseline. LS: least squares; qw: weekly; q2w: every 2 weeks; SCORAD: SCORing Atopic Dermatitis; TCS: topical corticosteroids.

Figure 7. Change in LS mean absolute values from baseline (diamond) to Week 16/52 (dot) for SCORAD components: (a) SOLO 1 and 2 (pooled data), 16-week monotherapy; (b) CAFÉ, 16 weeks with concomitant TCS; (c) CHRONOS, 52 weeks with concomitant TCS. BSA: body surface area; LS: least squares; q2w: every 2 weeks; SCORAD: SCORing Atopic Dermatitis; o-SCORAD: objective SCORAD; TCS: topical corticosteroids; VAS: visual analog scale.
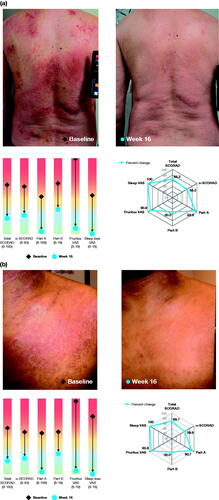
shows examples of patients treated with dupilumab monotherapy and their improvement in all SCORAD assessments from baseline to Week 16.
Figure 8. Examples of patients treated with dupilumab monotherapy and their individual improvement in SCORAD outcomes. Photos correspond to the target lesion assessed for SCORAD in SOLO studies (dupilumab monotherapy) in q2w group (approved dose). (a) 64-year-old patient, AD for 19 years; (b) 25-year-old patient, AD for 18 years. The spider graphics show LS mean percent reduction in each outcome; lines closer to the outer edge of the spider plot represent greater improvement from baseline. The rainbow graphics display change in LS mean absolute values from baseline (diamond) to Week 16 (dot) for each outcome. BSA: body surface area; LS; least squares; q2w: every 2 weeks; SCORAD: SCORing Atopic Dermatitis; o-SCORAD: objective SCORAD; VAS: visual analog scale.
Safety
Detailed safety results have been previously reported (Citation33–35). Overall, dupilumab had a favorable safety profile across all studies. Treatment-emergent adverse events occurred at similar rates across treatment groups. Conjunctivitis and injection-site reaction, mostly of mild-to-moderate severity, were more frequent in the dupilumab-treated groups in all studies, whereas AD exacerbations and skin infections occurred more frequently in patients in the control groups. Conjunctivitis and infections in dupilumab clinical studies have been described elsewhere (Citation38,Citation39). Because no clinically significant laboratory abnormalities have been identified in the dupilumab clinical program, no laboratory monitoring was required (Citation40).
Discussion
These analyses demonstrate that dupilumab 300 mg q2w, as monotherapy and in combination with TCS, significantly improves SCORAD outcomes in adults with moderate-to-severe AD compared with placebo with or without TCS. These improvements were observed throughout treatment in four randomized controlled trials, which included 2,444 patients in total. Furthermore, statistically significant improvements with dupilumab were observed in all components of the SCORAD score, including o-SCORAD (extent of disease [Part A] and intensity of signs [Part B]) and subjective SCORAD (symptoms: pruritus and sleep loss [Part C]).
In dupilumab-treated adults, rapid improvements in the extent of affected area, signs and symptoms were observed in the first weeks of treatment, and improvements were sustained over the 16- and 52-week treatment periods. Most outcomes (SCORAD, o-SCORAD, the extent of disease, pruritus, sleep loss and the majority of individual signs) significantly improved after a single dose of dupilumab; these improvements were progressive over 16–28 weeks and sustained over 52 weeks.
The use of scores to document disease severity and assess the effect of treatment is increasing with the availability of new treatments for AD. Although EASI score is recommended by the Harmonizing Outcome Measures for Eczema (HOME) initiative as the core outcome instrument for measuring AD signs in clinical trials (Citation21), both EASI and o-SCORAD were identified as extensively validated among 16 instruments, and SCORAD is used in both clinical trials and clinical practice by dermatologists globally. Furthermore, o-SCORAD was endorsed by HOME as a secondary scoring tool to evaluate objective AD signs in clinical trials in addition to EASI, whenever possible. Although both EASI and o-SCORAD evaluate affected area and intensity of signs, EASI places a higher weighting on the area and o-SCORAD places a higher weighting on signs. Vakharia et al (Citation41) found that SCORAD had a numerically stronger correlation with patient-reported severity than all other measures examined, including EASI. SCORAD is also more sensitive in the evaluation of localized severe lesions and lesions in sensitive areas whose function may be compromised, assigning additional points for lesions located on hands, feet, and genitals (Citation23).
Moreover, both SCORAD and its patient-oriented variant (PO-SCORAD) are available as free apps in multiple languages, enabling patients to perform a self-assessment of AD severity. The correlation of SCORAD and PO-SCORAD has been demonstrated (Citation42), and recently the strata for PO-SCORAD was defined in adults with AD: mild (1–27), moderate (28–56), and severe (57–103) (Citation43).
Overall, these data show that most adults respond to dupilumab, as shown by improvements in signs, symptoms and disease severity measured by SCORAD. Furthermore, the data add to prior evidence demonstrating the benefit of dupilumab with regard to improvements in signs and symptoms of AD, as well as QoL and psychological health, as reported with other key treatment outcome measurements, including EASI, the Peak Pruritus Numerical Rating Scale (NRS), POEM, the Dermatology Life Quality Index and the Hospital Anxiety and Depression Scale (Citation33–35,Citation44).
Of note, observed improvements in pruritus VAS in response to dupilumab differ from those measured by the Peak Pruritus NRS (Citation45), despite both being 10-point scales of pruritus severity. These differences may be because they assess pruritus differently. In the SCORAD VAS, patients are asked: ‘What was the average itch in the last 3 days?’ whereas the Peak Pruritus NRS asked, ‘On a scale of 0–10, what was the worst level of itch during the last 24 hours?’ (Citation46). Interpreting results from various studies must consider these different questions. Our analyses show that the vast majority of patients achieved a clinically significant benefit in pruritus and sleep loss with a score corresponding to mild or absent by the treatment end, a considerable positive impact given that the majority of patients had severe pruritus at baseline.
A clear improvement in lichenification was observed even in the 16-week studies, with most patients moving from moderate/severe to none/mild (). Lichenification is considered the AD sign most resistant to treatment, with improvement likely taking longer than with other signs. Lichenification in AD involves epidermal acanthosis and dermal fibrosis (Citation47). A possible explanation for early improvement with dupilumab may be that IL-4 and IL-13 are not only associated with a defective barrier with increased permeability to allergens but also increase collagen synthesis through ERK pathway activation (Citation48). By blocking the IL-4 receptor, dupilumab may induce tissue remodeling in both the epidermis and dermis.
Strengths of this analysis include an examination of four large, multinational randomized controlled trials, assessment of dupilumab as monotherapy and in combination with TCS, and multiple sensitivity analyses. Additionally, all efficacy measures have strengths and limitations, and SCORAD adds another means to confirm dupilumab efficacy across both physician- and patient-assessed signs and symptoms. Limitations include the post hoc nature of some analyses.
In conclusion, in all our large phase 3 trials, dupilumab administered as monotherapy or with concomitant TCS resulted in rapid, sustained and statistically and clinically significant improvements in total SCORAD and all component scores for the extent of disease and intensity of signs and symptoms compared with control groups of adults with uncontrolled AD.
Author contribution statements
BS, LE, and AG contributed to study concept and design. SB, AW, JIS, and MD acquired data. ABR conceived the idea of the post hoc analyses described in this manuscript. ZC conducted the statistical analyses on the data. ABR drafted the manuscript with the medical writer and created figures. SB, AW, JIS, MD, GP, JCA-H, ZC, BS, LE, AG, YL, and ABR interpreted the data, provided critical feedback on the manuscript, approved the final manuscript for submission, and were accountable for the accuracy and integrity of the manuscript.
Supplemental Material
Download PDF (1.2 MB)Acknowledgments
We thank the patients, their families, and the health care providers who participated in these studies. Marthe Vuillet and Ana B. Rossi of Sanofi Genzyme developed the spider gram and rainbow graphics shown in . We thank Linda Williams of Regeneron Pharmaceuticals, Inc., and El-Bdaoui Haddad of Sanofi Genzyme for their contributions.
Disclosure statement
SB has received research grants from Pierre Fabre Dermo-Cosmétique; personal fees from Bioderma, Ferring, La Roche-Posay, Novalac, and Sanofi Genzyme; and nonfinancial support from AbbVie, Janssen, and Novartis. AW is an investigator for Eli Lilly, Galderma, LEO Pharma, MedImmune, Novartis, Pfizer, Regeneron Pharmaceuticals, Inc., and Sanofi Genzyme; consultant for Almirall, Anacor, Eli Lilly, Galderma, LEO Pharma, MedImmune, Novartis, Pfizer, Regeneron Pharmaceuticals, Inc., and Sanofi Genzyme; and has received research grants from Beiersdorf, LEO Pharma, and Pierre Fabre. JIS is an investigator for AbbVie, Celgene, Eli Lilly, GlaxoSmithKline, Incyte, LEO Pharma, Realm Therapeutics, Regeneron Pharmaceuticals, Inc., and Roche; a consultant for AbbVie, Anacor Pharmaceuticals, Eli Lilly, Galderma, GlaxoSmithKline, Incyte, Kiniksa Pharmaceuticals, LEO Pharma, MedImmune, Menlo Therapeutics, Pfizer, Procter & Gamble, Realm Therapeutics, and Regeneron Pharmaceuticals, Inc.; and a speaker for Regeneron Pharmaceuticals, Inc. and Sanofi Genzyme. MD has received research support, consulting/advisory board agreements, and/or honoraria for lecturing from AbbVie, Eli Lilly, Galápagos, LEO Pharma, MEDA Pharma, Pfizer, Pierre Fabre, Regeneron Pharmaceuticals, Inc., and Sanofi Genzyme. GP is an investigator for AbbVie, Eli Lilly, Galderma, IFC Cantabria, LEO Pharma, Novartis, Pfizer, Pierre Fabre, and Sanofi Genzyme. JCA-H is a consultant for AbbVie, Celgene, Eli Lilly, Galderma, Janssen, LEO Pharma, Novartis, Pfizer, and Sanofi Genzyme. ZC, BS, and YL are employees and shareholders of Regeneron Pharmaceuticals, Inc. LE is an employee of Sanofi and may hold stock and/or stock options in the company. AG was an employee and shareholder of Regeneron Pharmaceuticals, Inc., at the time the study was completed. ABR is an employee of Sanofi Genzyme and may hold stock and/or stock options in the company.
Additional information
Funding
References
- Bieber T. Atopic dermatitis. Ann Dermatol. 2010;22(2):125–137.
- Brunner PM, Silverberg JI, Guttman-Yassky E, et al. Increasing comorbidities suggest that atopic dermatitis is a systemic disorder. J Invest Dermatol. 2017;137(1):18–25.
- Gittler JK, Shemer A, Suárez-Fariñas M, et al. Progressive activitation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. 2012;130(6):1344–1354.
- Goleva E, Berdyshev E, Leung DY. Epithelial barrier repair and prevention of allergy. J Clin Invest. 2019;129(4):1463–1474.
- Wollenberg A, Oranje A, Deleuran M, et al. ETFAD/EADV Eczema task force 2015 position paper on diagnosis and treatment of atopic dermatitis in adult and paediatric patients. J Eur Acad Dermatol Venereol. 2016;30(5):729–747.
- Sibbald C, Drucker AM. Patient burden of atopic dermatitis. Dermatol Clin. 2017;35(3):303–316.
- Simpson EL, Bieber T, Eckert L, et al. Patient burden of moderate to severe atopic dermatitis (AD): insights from a phase 2b clinical trial of dupilumab in adults. J Am Acad Dermatol. 2016;74(3):491–498.
- Simpson EL, Guttman-Yassky E, Margolis DJ, et al. Association of inadequately controlled disease and disease severity with patient-reported disease burden in adults with atopic dermatitis. JAMA Dermatol. 2018;154(8):903–912.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139(3):583–590.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70(2):338–351.
- Huet F, Faffa M-S, Poizeau F, et al. Characteristics of pruritus in relation to self-assessed severity of atopic dermatitis. Acta Derm Venerol. 2019;99(3):279–283.
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121(3):340–347.
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Mental health burden and comorbidity in atopic dermatitis in US adults. Paper presented at: Annual Meeting of the American Academy of Dermatology; 2019 Mar 1–5; Washington, DC.
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Symptoms and diagnosis of anxiety and depression in atopic dermatitis in U.S. adults. Br J Dermatol. 2019;181(3):554–565.
- Yew YW, Thyssen JP, Silverberg JI. A systematic review and meta-analysis of the regional and age-related differences in atopic dermatitis clinical characteristics. J Am Acad Dermatol. 2019;80(2):390–401.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135(1):56–66.
- Schmitt J, Langan S, Deckert S, et al. Assessment of clinical signs of atopic dermatitis: a systematic review and recommendation. J Allergy Clin Immunol. 2013;132(6):1337–1347.
- Charman CR, Venn AJ, Ravenscroft JC, et al. Translating Patient-Oriented Eczema Measure (POEM) scores into clinical practice by suggesting severity strata derived using anchor-based methods. Br J Dermatol. 2013;169(6):1326–1332.
- Chopra R, Silverberg JI. Assessing the severity of atopic dermatitis in clinical trials and practice. Clin Dermatol. 2018;36(5):606–615.
- Rehal B, Armstrong AW. Health outcome measures in atopic dermatitis: a systematic review of trends in disease severity and quality-of-life instruments 1985-2010. PLoS One. 2011;6(4):e17520.
- Schmitt J, Spuls PI, Thomas KS, et al. The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J Allergy Clin Immunol. 2014;134(4):800–807.
- European Task Force on Atopic Dermatitis. Severity scoring of atopic dermatitis: the SCORAD index. Consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1993;186:23–31.
- Kunz B, Oranje AP, Labrèze L, et al. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1997;195(1):10–19.
- Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32(5):657–682.
- Oranje AP, Glazenburg EJ, Wolkerstorfer A, et al. Practical issues on interpretation of scoring atopic dermatitis: the SCORAD index, objective SCORAD and the three-item severity score. Br J Dermatol. 2007;157(4):645–648.
- Chopra R, Vakharia PP, Sacotte R, et al. Severity strata for Eczema Area and Severity Index (EASI), modified EASI, Scoring Atopic Dermatitis (SCORAD), objective SCORAD, Atopic Dermatitis Severity Index and body surface area in adolescents and adults with atopic dermatitis. Br J Dermatol. 2017;177(5):1316–1321.
- Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32(6):850–878.
- Chopra R, Vakharia PP, Sacotte R, et al. Relationship between EASI and SCORAD severity assessments for atopic dermatitis. J Allergy Clin Immunol. 2017;140(6):1708–1710.
- Macdonald LE, Karow M, Stevens S, et al. Precise and in situ genetic humanization of 6 Mb of mouse immunoglobulin genes. Proc Natl Acad Sci USA. 2014;111(14):5147–5152.
- Murphy AJ, Macdonald LE, Stevens S, et al. Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc Natl Acad Sci USA. 2014;111(14):5153–5158.
- US Food and Drug Administration [Internet]. Silver Spring (MD): FDA. DUPIXENT® (dupilumab). Highlights of prescribing information; 2017 [cited 2019 September 26]; Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761055s014lbl.pdf
- European Medicines Agency [internet]. London (UK): EMA. DUPIXENT® (dupilumab). Summary of product characteristics; [cited 2019 September 26]; Available from: https://ec.europa.eu/health/documents/community-register/2019/20190801145601/anx_145601_en.pdf
- Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–2348.
- de Bruin-Weller M, Thaçi D, Smith CH, et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFÉ. Br J Dermatol. 2018;178(5):1083–1101.
- Blauvelt A, de-Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389(10086):2287–2303.
- Schram ME, Spuls PI, Leeflang MM, et al. EASI, (objective) SCORAD and POEM for atopic eczema: responsiveness and minimal clinically important difference. Allergy. 2012;67(1):99–106.
- Reich A, Heisig M, Phan NQ, et al. Visual analogue scale: evaluation of the instrument for the assessment of pruritus. Acta Derm Venerol. 2012;92(5):497–501.
- Akinlade B, Guttman-Yassky E, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials. Br J Dermatol. 2019;181(3):459–473.
- Eichenfield LF, Bieber T, Beck LA, et al. Infections in dupilumab clinical trials in atopic dermatitis: a comprehensive pooled analysis. Am J Clin Dermatol. 2019;20(3):443–456.
- Wollenberg A, Beck LA, Blauvelt A, et al. Laboratory safety of dupilumab in moderate‐to‐severe atopic dermatitis: results from three phase III trials (LIBERTY AD SOLO 1, LIBERTY AD SOLO 2, LIBERTY AD CHRONOS). Br J Dermatol. 2019. [Epub ahead of print]. Accessed April 27, 2020.
- Vakharia PP, Chopra R, Sacotte R, et al. Validation of patient-reported global severity of atopic dermatitis in adults. Allergy. 2018;73(2):451–458.
- Stalder JF, Barbarot S, Wollenberg A, et al. Patient-Oriented SCORAD (PO-SCORAD): a new self-assessment scale in atopic dermatitis validated in Europe. Allergy. 2011;66(8):1114–1121.
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Severity strata for POEM, PO-SCORAD, and DLQI in US adults with atopic dermatitis. Ann Allergy Asthma Immunol. 2018;121(4):464–471.
- Cork MJ, Eckert L, Simpson EL, et al. Dupilumab improves patient-reported symptoms of atopic dermatitis, symptoms of anxiety and depression, and health-related quality of life in moderate-to-severe atopic dermatitis: analysis of pooled data from the randomized trials SOLO 1 and SOLO 2. J Dermatolog Treat. 2019. [Epub ahead of print]. Accessed April 27, 2020.
- Silverberg JI, Eckert L, Yosipovitch G, et al. Dupilumab treatment results in rapid and sustained improvements in itch in adults with moderate-to-severe atopic dermatitis. Paper presented at: 77th Annual Meeting of the Society for Investigative Dermatology; 2019. May 8–11; Chicago, IL.
- Yosipovitch G, Reaney M, Mastey V, et al. Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate‐to‐severe atopic dermatitis. Br J Dermatol. 2019;181(4):761–769.
- Brandt EB, Sivaprasad U. Th2 cytokines and atopic dermatitis. J Clin Cell Immunol. 2011;2:110.
- Bhogal RK, Bona CA. Regulatory effect of extracellular signal-regulated kinases (ERK) on type I collagen synthesis in human dermal fibroblasts stimulated by IL-4 and IL-13. Int Rev Immunol. 2008;27(6):472–496.
