Abstract
Background
Cyclosporine A (CsA) is one of the systemic therapeutic options for moderate-to-severe psoriasis, based on its efficacy and rapidity of action. The current study investigated the response to CsA in patients with moderate-to-severe plaque psoriasis.
Materials and Methods
TRANSITION was an observational, cross-sectional, multicentre study which evaluated the proportion of partial- and suboptimal-responders among patients with moderate-to-severe plaque psoriasis treated with continuous CsA for ≥12 weeks. Patients demonstrating a Psoriasis Area and Severity Index (PASI) response of ≥90, ≥75 and <90, ≥50 and <75 and <50 were defined as responders, suboptimal-responders, partial-responders, and non-responders, respectively.
Results
A total of 196 patients (mean age, 46.6 years; 62.8% males) from 14 sites in Italy were evaluated. At the study visit, the mean (SD) PASI score was 4.2(5.5) compared with 15.3(7.1) prior to the last CsA cycle. For response categories, 39.8%, 22.4%, 16.8%, and 20.9% of patients were responders, suboptimal-responders, partial-responders, and non-responders to CsA treatment. Overall, 28.6% of patients permanently discontinued treatment with CsA (lack of efficacy [10.2%], poor tolerability and voluntary discontinuation [3.6% each], and other [11.7%]).
Conclusion
Patients were only partially satisfied with CsA treatment, reporting measurable impact on quality of life. Only 40% patients showed a satisfactory response to CsA.
Introduction
Psoriasis is one of the most common human skin diseases, affecting 2–3% of the general population (Citation1). It is a complex disorder, characterized by inflammation, increased keratinocyte hyperproliferation, and altered epidermal differentiation (Citation2). Approximately 80–90% of psoriatic patients have chronic plaque psoriasis, characterized by recurrent exacerbations and remissions of thickened, erythematous, scaly patches of skin, which can occur anywhere on the body (Citation3–5). Psoriasis is associated with a high degree of morbidity; patients often experience embarrassment and social discrimination for the appearance of their skin, reduction in employment and income, and an overall decrease in quality of life (QoL) (Citation6,Citation7).
Patients with moderate to severe psoriasis represent approximately 15–25% of the psoriasis population and generally require systemic therapy (Citation4). Following improvements in therapeutics to manage psoriasis, such as the introduction of biologic agents, patients and physicians have raised their expectations in terms of treatment/response, with the goal of therapy now being clear or almost clear skin. Under the Psoriasis Area and Severity Index (PASI), a 90% reduction in PASI score (PASI 90) is considered the new threshold for desirable efficacy, as opposed to the previous PASI 75 standard for regulatory approvals (Citation8,Citation9). A PASI 90 response, cited as a state of ‘nearly complete clearance’, has been shown to correlate with improved health-related QoL outcomes compared with PASI 75 response (Citation10,Citation11).
Cyclosporine A (CsA) has been considered one of the systemic therapeutic options for moderate to severe psoriasis based on its effectiveness and rapidity of action. To our knowledge, there are no data available to date on disease activity for patients during the relatively long (up to 6 months) ‘off’ period between treatment cycles. The aim of this observational study was to capture the proportion of patients that, irrespective of the cycle of systemic therapy with CsA, could be defined as partial-responders or suboptimal-responders. The study also addressed treatment satisfaction perceived by the patients comparing efficacy assessment, performed by clinicians with pre-defined patient-reported outcomes.
Materials and methods
Study design
TRANSITION was an observational, cross-sectional, multicentre study () that aimed to evaluate the percentage of partial and suboptimal-responders among patients with moderate to severe plaque type psoriasis that were prescribed a continuous CsA treatment of at least 12 weeks, in the 6-month period prior to the study visit, regardless of whether they were in an ‘off’ or ‘on’ period of the treatment cycle.
Patient population
This study was conducted in 14 study centers across Italy. The key inclusion criteria included male or female patients, aged ≥18 years, with moderate to severe plaque type psoriasis diagnosed for at least 6 months prior to inclusion who were treated with a continual CsA cycle of ≥12 weeks in the last 6 months prior to the single study visit. Drug-induced psoriasis, or use of any other systemic therapy for psoriasis, including psoralen and ultraviolet A, during the 6 months prior to enrollment were the key exclusion criteria.
Study objectives
The primary objective of the study was to estimate the percentage of partial- and suboptimal-responder patients affected by moderate to severe plaque type psoriasis that were prescribed with a continuous CsA treatment of at least 12 weeks, regardless of whether they were in an ‘off’ or ‘on’ period of the treatment cycle. The key secondary objectives included:
evaluation of the percentage of responders, non-responders, partial-responders, and suboptimal-responders assessed by PASI response (Citation12)
evaluation of the percentage of patients who permanently discontinued CsA
to describe the patients treatment satisfaction assessed by Treatment Satisfaction Questionnaire of Medication (TSQM) v1.4 questionnaire (Citation13)
to describe the QoL assessed by the Dermatology Life Quality Index (DLQI), overall and stratified by PASI response (Citation14)
Assessments
PASI was used for the evaluation of the primary objective. The PASI assessment combines the assessment of the severity of lesions and the area affected into a single score with a range of 0 (no disease) to 72 (maximal disease). The PASI score before the last CsA cycle (±15 days) was collected at the study visit. The following definitions of response were used:
Non-responders: patients achieving <50% improvement (reduction) in PASI score compared with the PASI assessed before the start of the last CsA cycle
Partial-responders: patients achieving an improvement (reduction) between 50% and <75% in PASI score compared with PASI assessed before the start of the last CsA cycle
Suboptimal-responders: patients achieving an improvement (reduction) between 75% and <90% in PASI score compared with PASI assessed before the start of the last CsA cycle
Responders: patients achieving ≥ 90% improvement (reduction) in PASI score compared with PASI assessed before start of last CsA cycle
Patient-reported outcomes were used to get information on patients’ QoL and satisfaction related to the actual treatment. The patient completed questionnaires at the study visit. The DLQI is widely used in clinical practice to assess the impact of skin diseases on patients’ QoL (Citation15–18). Treatment satisfaction was assessed by means of the TSQM version 1.4 (Citation13). Correlation between clinician and patient satisfaction related to treatment (through comparison among PASI and TSQM v.1.4) were evaluated.
Statistical analysis
All statistical analyses were performed using SAS® Version 9.4, according to the data analysis section of the study protocol. Quantitative variables were described by means, standard deviation (SD), and qualitative variables by absolute and relative frequency. Spearman coefficients were provided for analysis correlation.
Results
Demographics and patient characteristics
A total of 204 patients who had moderate to severe plaque psoriasis provided informed consent. Of these, 196 patients from 14 sites had evaluable data without major protocol deviations and were included in the analyses. The mean (SD) age was 46.6 (15.4) years, ranging from 18 to 89 years. The study visit occurred after a mean (SD) of 139 (89.8) days from CsA first intake. The mean (SD) PASI score was 4.15 (5.53). Almost two-thirds of the study population was male. Of the 73 female patients, 50.7% were of childbearing potential. Most patients in the study were Caucasian (189 patients, 96.4%; ). Collectively, patients were marginally overweight with a body mass index (BMI) ranging from 16 to 41 kg/m2. The majority of patients were nonsmokers; the mean (SD) estimated tobacco consumption was 59.5 (91.6) pack-years for current smokers and 20.6 (15.1) pack-years for former smokers. At least one concomitant disease was reported by 89 patients (45.4%) ().
Table 1. Demographics and baseline characteristics.
Table 2. Medical history: current medical condition of patients.
Permanent discontinuation of CsA
In total, 56 patients (28.6%) permanently discontinued treatment with CsA, mostly due to ‘other’ reasons (11.7%) besides lack of efficacy (10.2%), poor tolerability, or voluntary discontinuation (3.6% each) ().
Table 3. Reasons for permanent discontinuation from the last CsA cycle.
Percentage of partial-, suboptimal- and non-responders by PASI
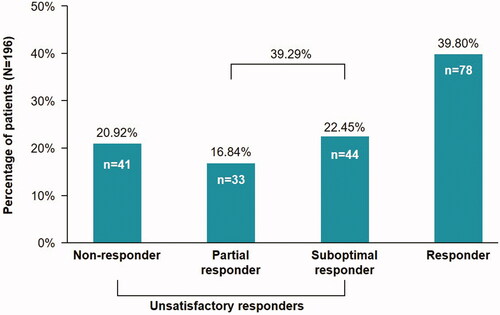
A total of 77 patients (39.3%) were partial- or suboptimal-responders and 41 patients (20.9%) were non-responders (), suggesting that CsA was not the optimal treatment option for this patient group.
Figure 2. Percentage of partial- and suboptimal-responder patients. Non-responder: PASI response <50%; Partial Responder: PASI response is between 50% and <75%; Suboptimal Responder: PASI response is between 75% and <90%; Responder: PASI response ≥90%; N: number of evaluable patients; n: number of patients who satisfied the criteria; PASI: Psoriasis Area and Severity Index.
Overall PASI response of the evaluable patient population
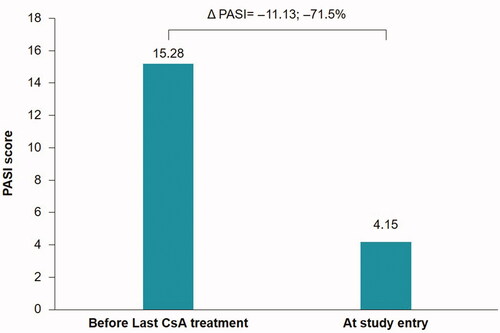
The mean (SD) PASI score prior to the last CsA cycle was 15.28 (7.07), whereas at the study visit the mean (SD) PASI score was 4.15 (5.53). The mean absolute and percentage change in the PASI score between assessment time points was –11.13 (8.04) and –71.15%, respectively ().
Quality of life by DLQI
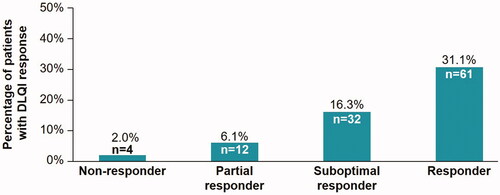
A total of 109 patients (55.6%) reported a DLQI response of 0 or 1. The mean (SD) overall DLQI score was 3.76 (5.49). The number of patients with a DLQI response of 0 or 1 correlated with the observed PASI responses. Here the highest number of patients that reported a DLQI response of 0 or 1 were responders ().
Patient satisfaction by TSQM
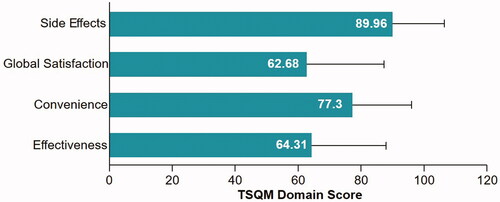
The TSQM domain of effectiveness had a mean (SD) score of 64.31(23.31) and the convenience domain had a mean (SD) score of 77.30 (18.45), while global satisfaction had the lowest mean (SD) score of 62.68 (24.27) and side effects had the highest mean (SD) score of 89.96 (16.28) ().
Correlation between clinicians’ and patients’ satisfaction related to treatment
The TSQM scores increased with improved patient satisfaction, whereas PASI scores decreased with improved disease control defined through decrease in psoriasis severity. There was a negative monotonic correlation between the PASI score and each TSQM domain (). The correlations between PASI and the domains of effectiveness, convenience, and global satisfaction were statistically significant, while the domain of side effects did not demonstrate a statistically significant correlation to the PASI score. In particular, the effectiveness and global satisfaction domains were moderately negatively correlated with the PASI score, whereas the correlation between PASI and convenience was weak.
Table 4. Correlation between PASI score and each domain of TSQM, using the Spearman correlation coefficient.
Discussion
With the availability of improved therapeutic options, such as biologics, to manage psoriasis, patients and physicians have heightened expectations in terms of treatment/response, with the goal of therapy now being clear or almost clear skin. A PASI 90 response, the new threshold for desirable efficacy, has been shown to correlate with improved health-related quality of life (HRQoL) outcomes compared with PASI 75, and a larger percentage of patients (60%) who reach PASI 90 report no HRQoL impairment compared with those who reach PASI 75 (40%) (Citation19–21). These data support the importance of a patient’s QoL as a treatment goal for psoriasis, considering that 88% of patients believe that psoriasis affects their emotional well-being and 82% report that it interferes with their enjoyment of life (Citation22).
The aim of TRANSITION was to capture the proportion of patients who, regardless of the cycle of systemic therapy with CsA, could be defined as partial-responders as judged by PASI responses from 50 to 75, or as suboptimal-responders as judged by PASI responses from 75 to 90. Conversely, a patient was defined as a non-responder if the PASI response was less than 50% of the original PASI score, suggesting that CsA was not the optimal treatment option for these patients. As stated by Gisondi et al., the basis for the Italian guidelines for psoriasis treatment, PASI 75 was the benchmark of primary endpoints for most clinical trials of psoriasis (Citation23,Citation24). However, the ultimate goal of therapy is complete clearance of skin lesions, and an improvement of 90% or greater (PASI 90 response) is currently considered as the most relevant treatment outcome (Citation25), especially in patients with severe disease. In the present study, 77 patients (39.3%) showed either a partial or suboptimal response to CsA.
The mean (SD) reduction in PASI score between assessments was 11.13 (8.04). The mean percentage change in the PASI score was 71.15%, falling in the partial-responder range. This value corresponded well with the literature reported value of 71.5% for a CsA dose of 5 mg/kg/day (Citation26). The responder class (PASI 90) comprised of 78 patients (39.8%), which was the largest proportion of patients. The suboptimal-responder class (PASI score between 75 and 90) comprised 44 patients (22.4%); the partial responder class (PASI between 50 and 75), which had the lowest proportion of patients, comprised 33 patients (16.8%); and the non-responder class comprised 41 patients (20.9%). The percentage of patients attaining PASI 75 or above (i.e. responders and suboptimal-responders) was 62.2%, which corresponded well with the reported range in the literature of 50–70% (Citation27–30).
The TSQM domain of effectiveness had a mean (SD) score of 64.3 (23.3), with a minimum of 0 and a maximum of 100. The relatively low scores for effectiveness and global satisfaction indicated only partial overall satisfaction with CsA treatment.
Overall, a mean (SD) DLQI of 3.76 (5.49) indicated that psoriasis had a small impact on QoL for the evaluable population. The dimension of symptoms and feelings had the highest mean DLQI score, indicating that symptoms and feelings had the greatest negative impact on QoL. The number and proportion of patients with a DLQI score of 0 or 1, indicating that the disease had no impact on QoL, was greater for the higher PASI response categories. Correlation analysis demonstrated a negative correlation between the PASI score and each TSQM domain using the Spearman correlation coefficient. Patients gave higher scores to the effectiveness, convenience, and global satisfaction domains as the severity of psoriasis decreased, whereas disease severity did not show a correlation with satisfaction based on side effects. Patients also reported better QoL (by DLQI) with reduced psoriasis severity (decreased PASI score).
In total, 56 patients (28.6%) permanently discontinued treatment with CsA. The most common reason for discontinuation was ‘other’ (23 patients, 11.7%), followed by lack of efficacy (20 patients, 10.2%), and either poor tolerability or voluntary discontinuation (7 patients each, 3.6%). This was a lower overall rate of discontinuation than previously reported for CsA, where discontinuation due to ‘other’ reasons matched well with the literature value of 15.8%, while discontinuation due to lack of efficacy and adverse events (intolerance) were reported to be much higher (26.3% and 57.9%, respectively) in the literature (Citation31).
Limitations/bias
The selection of sites and patients participating in the study did not follow a random selection procedure and this could have led to a risk of selection bias. There are well-described associations between psoriasis disease activity and weather conditions. In order to control this bias, the enrollment period lasted 1 year, covered all weather conditions, and the centers involved were located in cities all over Italy. Another possible bias was the variability of duration of the CsA cycle with respect to inclusion in the study that led to a PASI evaluation that is different between patients. However, this was the main objective of the study, which aimed to observe the effect of CsA in the ‘on’ or ‘off’ period.
Conclusion
In the current study about 60% of patients showed an unsatisfactory response to CsA (39% either a partial or a suboptimal response, and 21% a non-response). Patients who did not achieve optimal response with CsA treatment, with a mean duration of 4.5 months showed a moderate impact on QoL as per DLQI. Overall treatment with CsA seems to be associated with limited clinical efficacy. Hence, optimization of therapy should be considered in the majority of these patients.
Acknowledgements
The authors would like to thank Avishek Anant and Mohammad Fahad Haroon (Novartis Healthcare Pvt. Ltd., Hyderabad, India) for editorial and medical writing support, which was funded by Novartis Farma SpA, Origgio, Italy, in accordance with the Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Disclosure statement
S. P. Cannavò has been a consultant, adviser for AbbVie, Celgene, Eli- Lilly, Janssen, LEO-Pharma, Novartis, and Sanofi. A. Cuccia has been an advisor board member and/or consultant and/or received speakers’ honoraria and/or received grants and/or received research support and/or participated in clinical trials with the following pharmaceutical companies: Almirall, Celgene, Eli Lilly, Janssen-Cilag, Novartis, Pierre Fabre. G. Stinco has been a consultant, adviser for Novartis Farma Spa, UCB Pharma Spa, and Ely Lilly Italia Spa. F. Prignano has been a consultant, adviser and clinical study investigator for Eli -Lilly, Abbvie, Novartis, Leo-Pharma, Abiogen-Pharma, Celgene, and Janssen-Cilag. F. Marsili, M. Travaglini, R. Manzoni, R. Tiberio, A. Mazzotta, M. Germino, M. R. Bongiorno, S. Persechino, T. Florio, M. Pettinato, M. Tabanelli have no disclosures to make. R. Sarkar, E. Aloisi, M. Bartezaghi, R. Orsenigo are employees of Novartis.
Additional information
Funding
References
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Investigat Dermatol. 2013;133(2):377–385.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361(5):496–509.
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet (London, England). 2007;370(9583):263–271.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58(5):826–850.
- Raut AS, Prabhu RH, Patravale VB. Psoriasis clinical implications and treatment: a review. Crit Rev Ther Drug Carrier Syst. 2013;30(3):183–216.
- Gelfand JM, Feldman SR, Stern RS, et al. Determinants of quality of life in patients with psoriasis: a study from the US population. J Am Acad Dermatol. 2004;51(5):704–708.
- Horn EJ, Fox KM, Patel V, et al. Association of patient-reported psoriasis severity with income and employment. J Am Acad Dermatol. 2007;57(6):963–971.
- Menter A. Psoriasis. 1st ed.: London: Manson Publishing; 2010.
- Agency EM. Guideline on clinical investigation of medicinal products indicated for the treatment of psoriasis; 2004; [cited 2019 Dec 27]. Available from: https://www.ema.europa.eu/documents/scientific-guideline/guideline-clinical-investigation-medicinal-products-indicated-treatment-psoriasis_en.pdf
- Excellence NIfHaC. Psoriasis: assessment and management; 2012; [cited 2019 Dec 27]; Clinical guideline [CG153]]. Available from: https://www.nice.org.uk/guidance/cg153.
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ (Clinical Research ed). 2009;338:b1542–b1542.
- Langley RG, Feldman SR, Nyirady J, et al. The 5-point Investigator’s Global Assessment (IGA) Scale: a modified tool for evaluating plaque psoriasis severity in clinical trials. J Dermatol Treatment. 2015;26(1):23–31.
- Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2(1):12.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216.
- Badia X, Mascaro JM, Lozano R. Measuring health-related quality of life in patients with mild to moderate eczema and psoriasis: clinical validity, reliability and sensitivity to change of the DLQI. The Cavide Research Group. Br J Dermatol. 1999;141(4):698–702.
- Lewis V, Finlay AY. 10 years experience of the Dermatology Life Quality Index (DLQI). J Investigat Dermatol Symp Proc. 2004;9(2):169–180.
- Shikiar R, Bresnahan BW, Stone SP, et al. Validity and reliability of patient reported outcomes used in psoriasis: results from two randomized clinical trials. Health Qual Life Outcomes. 2003;1(1):53.
- Shikiar R, Willian MK, Okun MM, et al. The validity and responsiveness of three quality of life measures in the assessment of psoriasis patients: results of a phase II study. Health Qual Life Outcomes. 2006;4:71.
- Revicki DA, Willian MK, Menter A, et al. Relationship between clinical response to therapy and health-related quality of life outcomes in patients with moderate to severe plaque psoriasis. Dermatology (Basel, Switzerland). 2008;216(3):260–270.
- Torii H, Sato N, Yoshinari T, et al. Dramatic impact of a Psoriasis Area and Severity Index 90 response on the quality of life in patients with psoriasis: an analysis of Japanese clinical trials of infliximab. J Dermatol. 2012;39(3):253–259.
- Viswanathan HN, Chau D, Milmont CE, et al. Total skin clearance results in improvements in health-related quality of life and reduced symptom severity among patients with moderate to severe psoriasis. J Dermatol Treat. 2015;26(3):235–239.
- Armstrong AW, Schupp C, Wu J, et al. Quality of life and work productivity impairment among psoriasis patients: findings from the National Psoriasis Foundation survey data 2003–2011. PLOS One. 2012;7(12):e52935.
- Gisondi P, Giglio Girolomoni DM. Treatment approaches to moderate to severe psoriasis. Int J Mol Sci. 2017;18(11):E2427.
- Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303(1):1–10.
- Puig L. PASI90 response: the new standard in therapeutic efficacy for psoriasis. J Eur Acad Dermatol Venereol. 2015;29(4):645–648.
- Colombo MD, Cassano N, Bellia G, et al. Cyclosporine regimens in plaque psoriasis: an overview with special emphasis on dose, duration, and old and new treatment approaches. TheScientificWorldJournal. 2013;2013:1–11.
- Heydendael VM, Spuls PI, Opmeer BC, et al. Methotrexate versus cyclosporine in moderate-to-severe chronic plaque psoriasis. N Engl J Med. 2003;349(7):658–665.
- Maza A, Montaudie H, Sbidian E, et al. Oral cyclosporin in psoriasis: a systematic review on treatment modalities, risk of kidney toxicity and evidence for use in non-plaque psoriasis. J Eur Acad Dermatol Venereol. 2011;25(Suppl 2):19–27.
- Shintani Y, Kaneko N, Furuhashi T, et al. Safety and efficacy of a fixed-dose cyclosporin microemulsion (100 mg) for the treatment of psoriasis. J Dermatol. 2011;38(10):966–972.
- Yoon HS, Youn JI. A comparison of two cyclosporine dosage regimens for the treatment of severe psoriasis. J Dermatol Treat. 2007;18(5):286–290.
- Arnold T, Schaarschmidt ML, Herr R, et al. Drug survival rates and reasons for drug discontinuation in psoriasis. J German Soc Dermatol. 2016;14(11):1089–1099.