Abstract
Psoriasis vulgaris (PV) not only affects patients' skin health but also increases the risk of coronary heart disease and diabetes, which brings both physical and mental harms. Its pathogenesis is complex, and the multitarget effect of traditional Chinese medicine (TCM) is especially advantageous. Because a considerable number of randomized controlled trials related to TCM exhibit design defects, small sample size, or inadequate intervention time, so the status of TCM in the treatment of PV cannot be fully clarified. We reviewed the controlled clinical trials published over the past decade and selected 17 high-quality articles from over 2000 papers. The results suggest that TCM might be beneficial for decrease in PASI scores, thus, TCM might be an effective alternative therapy for PV management. The safety of TCM on PV was also assessed in our analysis. The more strictly designed and long-term observations of TCM for PV are supposed to be conducted in the future.
Introduction
Psoriasis is a common chronic inflammatory, relapsing and refractory skin disease with a global incidence of 2–3% (Citation1). Psoriasis vulgaris (PV), also called plaque-type psoriasis, is presented in around 90% of the total cases (Citation2). Typical lesions are erythematous, sharply demarcated, pruritic plaques covered in silvery scales and the plaques can coalesce and cover large areas of skin. Although, the pathogenesis of psoriasis is complex and not understood yet, the sustained inflammation that leads to uncontrolled keratinocyte (KC) proliferation and dysfunctional differentiation is considered to be the hallmark of psoriasis (Citation3). A vast array of studies has shown that psoriasis can increase the risk of obesity, diabetes, and cardiovascular disease (Citation4). Furthermore, about 57% of patients are plagued by anxiety and depression (Citation5). Conventional therapies of western medicine with psoriasis are systematically using of retinoic acid, immunosuppressant, biological agents and topical glucocorticoids or vitamin D3 derivatives (Citation1,Citation2), which may add patients’ economic burden or present unstable curative efficacy along with adverse reactions like intolerability (Citation6). Therefore, it is urgent to establish an effective strategy for PV management.
Traditional Chinese medicine (TCM) has a long history in the treatment of PV. Compared with other treatments, TCM shows a competitive advantage owing to its holism concept and multitarget effect. TCM pays attention to individualized treatments that are based on syndrome differentiation such as blood-dryness, blood-stasis, and blood-heat. A large number of clinical trials have confirmed that TCM can alleviate psoriatic symptoms (Citation7–23). Moreover, the applications of advanced analysis and detection technology reveal some of the therapeutic mechanisms behind TCM's potency such as anti-angiogenesis, inducing KCs apoptosis and anti-inflammation (Citation24–26).
Evaluation of clinical evidence from RCTs is recognized as the gold standard of assessing an intervention. Although, some former reviews have been performed on oral TCM efficacy, the screening criteria of them lack enough sample size, sufficient intervention period and specific methodological quality assessment of the enrolled studies (Citation27–29). Thus, it might be difficult to precisely evaluate the efficacy and safety of TCM. Taking these limitations into consideration, we screened high-quality studies (according to the Jadad Scale) published over the past decade to evaluate the efficacy of TCM on PV via an updated systematic review and meta-analysis. Furthermore, we reviewed the effective underlying mechanisms of TCM to provide potential treatment targets of psoriasis.
Materials and method
Search strategy and data sources
This review was stated according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. Eligible studies were identified by searching the relevant articles published from January 2010 to February 2020 in the following databases: PubMed, Medline, Embase, Web of Science, Cochrane Library, China National Knowledge Internet (CNKI), WanFang, Sinomed and VIP. Search terms included the following: (‘psoriasis’ or ‘psoriasiform’ or ‘psoriatic’ or ‘papulosquamous’ or ‘palmoplantar pustulosis’ or ‘pustulosis palmaris et plantaris’ or ‘acrodermatitis continua’ or ‘impetigo herpetiformis’) and (‘RCT’ or ‘randomized controlled trial’ or ‘random’ or ‘randomized’ or ‘randomly’ or ‘controlled clinical trial’) and (‘TCM’ or ‘traditional Chinese medicine’ or ‘formula’ or ‘decoction’ or ‘prescription’ or ‘Chinese herbal compound prescription’ or ‘Chinese herbal medicine’ or ‘Chinese patent medicine’ or ‘Chinese patent drug’).
Study selection and eligibility criteria
Chunyan He and Xinmiao Wang screened the literature and extracted data for eligibility independently. Haoran Wu settled the disagreements. The clinical trials enrolled in the meta-analysis should fulfill the following criteria: (a) participants in the studies had a definite diagnosis of PV (plaque) and were randomly assigned to receive TCM, western medicine, the combination of TCM and western medicine, placebo or nonintervention orally; (b) studies with a sample size ≥60; (c) studies with treatment duration ≥8 weeks; (d) studies designed to focus on the comparison of TCM and placebo/nonintervention, TCM and western medicine or TCM combined with western medicine and western medicine; (e) studies that adopted Psoriasis Area and Severity Index (PASI) as efficacy evaluation index; (f) designed as RCTs; (g) studies achieving a Jadad score from 4 to 7 (strongest) (Citation30). We excluded clinical trials with the following characteristics: (a) non-randomized studies; (b) studies’ participants were not required to receive TCM, TCM combined with western medicine, western medicine, placebo or nonintervention orally; (c) studies’ patients recruited without a definite diagnosis; (d) studies that lacked objective measures only with reports of symptomatic variation in patients; (e) studies with a Jadad score from 0 to 3.
Statistical analysis
The RevMan5.3 software provided by the Cochrane Collaboration was used to analyze all the data. Accompanied by 95% confidence intervals (CIs), continuous outcomes were pooled for the calculation of weighted mean differences, while categorical outcomes were pooled for the calculation of relative risks (RRs). Study heterogeneity was assessed with the I2 statistics. If I2 < 50%, a fixed-effect (FE) model was utilized; otherwise, the random-effect (RE) model was utilized. A funnel-plot analysis was used to assess publication bias.
Results
A total of 2031 papers were identified during database searching (492 from WanFang, 468 from CNKI, 424 from Sinomed, 338 from VIP, 81 from Embase, 78 from Cochrane, 77 from Web of Science, 42 from PubMed and 31 from Medline). Of these, 1061 records were excluded as duplicates, 714 were excluded according to their titles or abstracts, 239 articles were excluded in the full-text screening round and 17 remained. In the end, the remaining 17 articles were assessed for eligibility, all of which published in Chinese and conducted in China. A flow chart showed the process of literature screening, study selection, and reasons for exclusion, which could be found as Supporting Information Figure S1. In total, 1871 PV patients from 17 clinical trials were enrolled. All basic characteristics of the enrolled RCTs are presented in . Besides, the methodological quality evaluation and the pharmacological effects of TCM ingredients are summarized in Supporting Information Tables S1 and S2.
Table 1. Summary of the included studies.
TCM versus placebo/nonintervention
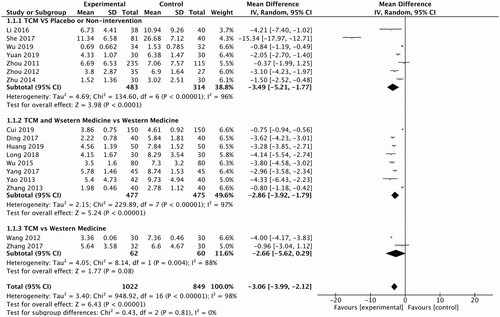
Seven RCTs that observed TCM therapeutic efficacy on PV were taken into analysis. Intervention duration was from 8 to 12 weeks, and the sample sizes ranged from 60 to 350. The Jadad scale was utilized to assess the methodological quality of the studies from 4 to 7; 3 of them were 7, and the rest were 4. Four trials used empirical decoctions as TCM interventions compared with the administration of placebo (Citation10,Citation12,Citation21,Citation22), the other three compared with nonintervention (Citation14,Citation18,Citation23).
Results of outcome measure evaluation suggested that TCM is more effective than placebo in reducing PASI score (n = 797; RR-3.49; 95% CI −5.21, −1.77; ). These four studies were all designed to be randomized, double-blind and placebo-controlled clinical trials except Li et al. 2016. Patients in the experimental group were treated with different prescriptions according to syndrome differentiation in two trials (She 2017; Zhou 2011). Zhou (2012) aimed to investigate the efficacy of Liangxue Huoxue (cooling and activating blood) complex prescription in 62 PV patients. After a mean follow-up of 8 weeks, PASI scores reduced more in the treatment group than the placebo group (p < .05) (Citation22). She (2017) enrolled 121 patients with PV. The patients of the experimental group were divided into two subgroups and received different recipes according to the syndrome differentiation (blood-dryness subgroup receiving NO.1 recipe, n = 44; blood-stasis subgroup receiving NO.2 recipe, n = 37). In the experimental group, the decrease of the PASI score was faster that of the placebo-control group after the 12-week treatment (p < .01) (Citation12). In a randomized, double-blind, multicenter, parallel-controlled trial, Zhou (2011) found that there was a trend toward a large decrease of PASI score in the TCM group compared with the placebo-control, which showed treating psoriasis with TCM blood differentiation was better than placebo (p > .05) (Citation21).
The efficacy of TCM in lowering the PASI score is better than nonintervention. Wu (2019) recruited 72 PV patients treated with Peiyuan Qingxie (strengthening primordial energy and dispelling the pathogenic factors) prescription or nonintervention. The results showed the TCM intervention has a significant curative effect on lowering the PASI score (p < .001) (Citation14).
TCM versus western medicine
Two RCTs showed that treatment with Silver Soup combined with Seedlings Medicine Away Tinea Pill and the tripterygium hypoglaucum (levl.) hutch mixture was equally effective as acitretin in reducing PASI score on PV patients (Citation13,Citation19). The Jadad score of them were both 4. Wang (2012) randomly assigned 60 patients to receive Silver Soup combined with Seedlings Medicine Away Tinea Pill or acitretin, the level of PASI score decreased obviously in both groups (p < .05) after a 12-week follow-up, and there was no statistical difference between the experimental and control groups (Citation13). In the other study, 62 participants with PV were assigned to receive tripterygium hypoglaucum (levl.) hutch mixture or acitretin randomly for 8 weeks. The mean changes in PASI score from baseline were significantly decreased in both groups, while the difference between groups was not notable (p > .05) (Citation19).
TCM and western medicine versus western medicine
Eight RCTs that compared TCM and western medicine therapeutic efficacy on PV were taken into analysis. Intervention duration was from 8 to 12 weeks, and the sample sizes ranged from 60 to 300 participants. The Jadad score of the studies was from 4 to 5. Most of them were 4 except Zhang (2013) with 5. In this comparison, seven studies used empirical decoctions as TCM interventions, whereas the other one used Chinese patent drugs (Citation20). Five RCTs used single oral retinoic acid drugs as the control group (Citation7,Citation15–17,Citation20), two used topical vitamin D3 analog (Citation9,Citation11) and one RCT used a combination of oral retinoic acid drugs, oral vitamin E and topical retinoic acid drugs (Citation8).
Analysis of outcome showed that the combination of TCM and western medicine was better than single western medicine in decreasing PASI score (n = 952; RR −2.86; 95% CI −3.92, −1.79; ). In a double-blind, randomized clinical trial, a total of 80 PV patients were assigned to receive an oxymatrine capsule combined with acitretin or only acitretin. After an 8-week follow-up, the patients' PASI scores decreased significantly in the experimental group (p < .01); however, the control group not. Oxymatrine capsule combined with acitretin had advantages in treating psoriasis over single acitretin (Citation20). Long et al. (2018; n = 60) and Huang and Lin (2019; n = 100), respectively, compared the same self-made decoction combined with topical calcipotriol with only topical calcipotriol for a 12-week treatment. In the experimental group, the decrease of the PASI score was more notable than the control group (p < .05; p < .01) (Citation9,Citation11). As for another trial, a total of 80 patients with plaque-type psoriasis were randomly assigned to receive Ziyin Huoxue Runzao(nourishing yin fluid and blood, moistening dryness) decoction combined with basic treatment including oral acitretin, oral vitamin E and topical tazarotene gel or only the basic treatment. The results showed the PASI score decreased obviously in both groups (p < .01) and the experimental group was better at improving plaque-type psoriasis than the control (p < .01) (Citation8).
Potential pharmacological mechanisms of action of TCM ingredients on PV
Some high-quality clinical studies have demonstrated the therapeutic effects of TCM on PV, and we systematically summarized their findings in .
Table 2. Therapeutic effects of TCM.
Among our enrolled TCM formulae, Huoxue Jiedu decoction (HXJDD) (Citation10) exerts potential antipsoriatic pharmaceutical effects such as cell cycle arrest and apoptosis in KCs, anti-inflammation, anti-angiogenesis by inhibiting VEGF-A, antioxidation, interfering T-lymphocyte trafficking. Additionally, the Spleen-strengthening and Detoxification decoction (Citation17) was reported to prevent the development of PV by inhibiting the inflammation via the PPARγ pathway, maintaining the balance of Th1/Th2 and Th17/Treg, inducing apoptosis via JAK2/STAT3 pathway, antioxidation, inhibiting platelet aggregation. Oxymatrine capsules (Citation20) could induce cell cycle arrest and autophagy induction in KCs by PI3K/Akt/mTOR pathway.
The frequency of Chinese herbs used in the trials was ranked, and the mechanism of herbs with the top nine frequency was summarized and analyzed in . The most frequently used herb for PV is Radix Rehmanniae Recens. Catalpol is the main component of Radix Rehmanniae Recens, and it could improve the lesion of psoriasis by alleviating oxidative stress, activation of AMPK-mediated mitochondrial biogenesis, up-regulating expressions of cleaved Caspase-3 and PARP (Citation31–33). The main component of Radix Salviae Miltiorrhizae, Tanshinone IIA has been confirmed its ability of anti-psoriasis. Besides antioxidation, it might inhibit the growth of KCs via enhancing activation of cleaved caspase-3 and PARP and improve depression via activating the ERK-CREB-BDNF signaling pathway (Citation34–36). Angelica Sinensis polysaccharide could attenuate inflammation by repression of NF-κB and JAK2/STAT3 pathways (Citation24,Citation37). Astilbin was found to improve differentiation in HaCaT KCs with suppression of KRT5 and KRT14 and induction of KRT1 and KRT10 (Citation25,Citation26,Citation38). In addition to anti-inflammation, Shikonin might promote apoptosis and suppresses growth in KCs (Citation39).
Table 3. Mechanism of Chinese herbs.
Evaluation of adverse events
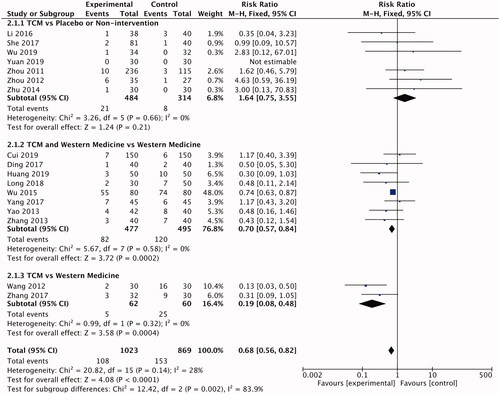
The incidence of adverse events (AEs) was reported in all the trials, which were used to assess the safety of TCM on PV. Treatment of psoriasis with TCM was safe as placebos or nonintervention (RR 1.64; 95% CI: 0.75–3.55; ). AEs in the TCM occurred less frequently than the control group with an intervention of western medicine (RR 0.68; 95% CI: 0.56–0.82; ), suggesting that TCM was safer than western medicine. And it made no statistical differences to the TCM combined with western medicine group and western medicine group in AEs (RR 0.70; 95% CI: 0.57–0.84; ). Details about the types of AEs are provided in Supporting Information Table S2. The most common AEs that occurred in the experimental group was diarrhea, which was possibly caused by gastroenteric intolerance. The duration of this symptom was not long and biochemical examination of liver and kidney functions did not show any injury. Thus, none of the participants in the experimental group dropped out due to diarrhea. The TCM was considered to be much safer than western medicine in treating PV.
Publication bias
The potential publication bias was expressed by a funnel plot, which was shown in the Supporting Information Figure S2. According to the symmetric dispersion points, it suggested that there was no indication of publication bias in the comparison of TCM and the control groups.
Discussion
Overall, the findings we described above indicated that TCM had a good application prospect in the remedy of psoriasis. We chose the most widely and frequently used parameter, PASI, as the only outcome measure, which was a more specific means of quantifying the extent and severity of psoriasis than the total body surface area (BSA) (Citation47). TCM decreased the PASI score and arrested the progression of psoriasis, with similar efficacy to western medicine. The incidence of AEs during the intervention period suggested that TCM was safer than western medicine for PV therapy, which could avoid clinicians’ suspicion of its toxicology. And the integrated TCM and western medicine behaved better than the single-use of western medicine in lowering the PASI score without increasing the risk of AEs. Compared with placebo or nonintervention, TCM exerted an obvious effect on decreasing PASI scores and the incidences of AEs making no significant difference with each other. Additionally, Wu and Li (2015), Zhou (2012) and Li et al. (2016) evaluated the quality of life with Dermatology Life Quality Index (DLQI) both in the experimental and control groups, and the results of which indicated that TCM could improve patients’ daily life quality dramatically upon controls (p < .05).
The RE model and FE model were utilized for sensitivity analysis of the stability of the enrolled studies. The results showed that I2 did not change in the mutual conversion, suggesting that the findings were stable. As for the subgroup analysis, the various intervention might cause heterogeneity. In addition, the agents used as control were various in different studies, which could also cause heterogeneity among these studies. as Zhang (2013) used acitretin (Citation20), Huang and Lin (2019) used topical calcipotriol (Citation9), and Ding (2017) used a combination of acitretin, vitamin E and topical tazarotene gel (Citation8). Even though some studies chose the same agents for the control group, the daily dose varied (Citation15–17,Citation20). Moreover, the patients in some studies were recruited according to TCM syndrome differentiation (Citation7,Citation10,Citation12,Citation13,Citation19). The modification of compound prescriptions or Chinese patent drugs based on the syndrome differentiation could also cause heterogeneity (Citation12,Citation21).
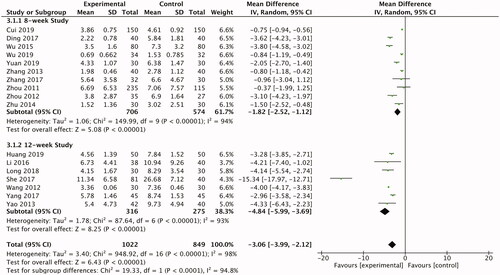
In detail, we divided the enrolled studies into two groups based on intervention duration. Patients received an 8-week follow up (n = 10) or 12-week (n = 7). The results of 8-week studies showed that the ability of TCM or TCM integrated with western medicine in lowering the PASI score outflanked the control groups (n = 1280; RR −1.82; 95% CI −2.52, −1.12; ). When the intervention duration extended to 12 weeks, TCM or TCM integrated with western medicine performed better as well (n = 591; RR −4.84; 95% CI −5.99, −3.69; ). The assessment of high-quality clinical trials with different intervention duration above gave convincing evidence that the therapeutic efficacy of TCM and TCM integrated with western medicine with PV were stable, which might make up the defects of the unstable efficacy of western medicine.
TCM theory, syndrome differentiation and treatment, might make a more detailed classification of PV, which was conducive to precision medicine. PV, also called Baibi in TCM, was classified by syndrome differentiation including the blood heat, blood stasis or blood dryness blood heat, or blood stasis based on the synthesis of physical characteristics and symptoms (Citation48). For example, patients with blood-heat syndrome often appeared in the early stage of psoriasis. Inflammation was dramatic in patients with this syndrome and herbs with an anti-inflammatory effect, such as Rhizoma Smilacis Glabrae, Fructus Gardeniae, Radix Isatidis etc. were selected to compose an efficient formula. Patients with blood-stasis syndrome often appeared in the middle stage of psoriasis, which was considered to be caused by blood stasis in TCM. Herbs with activating blood effects were selected such as Radix Salviae Miltiorrhizae, Radix Paeoniae Rubra, Caulis Spatholobi etc. for treatment. Patients with the blood-dryness syndrome often appeared in the late stage of psoriasis, and the skin lesions were mainly desquamation, gradually subsided. Herbs with nourishing yin fluid effects were selected such as Radix Rehmanniae Recens, Carapax et Plastrum Testudinis, Asparagus cochinchinensis (Lour.) Merr. etc.
The pathogenesis of psoriasis was incompletely understood but advances in the field had been revealing over the past 15 years, some of which were targeted by western medicine. For instance, methotrexate and acitretin could interfere with the JAK-STAT pathway and subsequently inhibit KC proliferation, expression of proinflammatory cytokines (Citation49–51). In addition, acitretin also reversed Th1 and Th17 preponderance in psoriasis patients to some degree (Citation52). But the blood lipid must be monitored during therapy with acitretin and the infection must be paid attention to when using methotrexate (Citation6). The therapeutic effect of topical calcipotriol was achieved by attenuating the T17 cell accumulation in both treated areas and draining lymph nodes. Biologic agents that targeted some cytokines such as TNF-α, IL-17, IL-23 were approved for the treatment of PV. This allowed for the specific targeting of parts of the immune system, rather than suppression of the entire immune system, leading to a favorable efficacy and safety profile (Citation53,Citation54). However, some studies revealed that they might cause adverse events like infection and idiopathic psoriasis (Citation6,Citation55). Moreover, a single drug was unable to meet the treatment of complex diseases such as psoriasis, while the combination might have an increasing risk of AEs. TCM compound prescription had a multitarget effect as shown in , which might improve the therapeutic efficacy and maintain a lower rate of AEs.
The lack of RCTs with high-quality results was a major limitation in the present study. Although, we had enrolled studies with high Jadad scores, there was only one RCT with a Jadad score of 5 and 4 RCTs of 7. The quality of included RCTs could directly influence the veracity of the meta-analysis. In addition, all the RCTs were conducted in China, so we failed to access the efficacy of TCM in other countries. The intervention duration was not long enough to provide robust long-term efficacy evidence. Moreover, the efficacy of a single index was limited, so it cannot be compared and evaluated from many aspects. Six studies did not detail the full ingredients of their interventions so some herbs are missing from this review. Thus, the lack of other ethnic groups in the enrolled studies might limit the scope of their application. High-quality randomized controlled clinical trials with large samples enrolled more ethnic groups and long-term intervention could provide more convincing evidence in future meta-analysis.
Author contributions
Dan Dai and Haoran Wu drafted the manuscript. Ping Song revised the manuscript. Haoran Wu, Chunyan He and Xinmiao Wang searched the literature and extracted data. Dan Dai and Yiqi Luo made statistical analysis of all the data. All of the authors participated in the design and approved the final manuscript, approving the published version and agreeing to be accountable for the accuracy and integrity.
Supplemental Material
Download Zip (211.5 KB)Acknowledgment
The authors thank all the researchers of every trial mentioned above for their distinguished work.
Disclosure statement
The authors declare that they have no competing interests.
Data availability statement
The data that support the findings of this study are stored in Guang’anmen Hospital (Beijing, China), and available from the corresponding author on reasonable request.
Additional information
Funding
References
- Greb JE, Goldminz AM, Elder JT, et al. Psoriasis. Nat Rev Dis Primers. 2016;242:16082.
- Boehncke WH, Schon MP. Psoriasis. Lancet. 2015;386(9997):983–994.
- Rendon A, Schakel K. Psoriasis pathogenesis and treatment. Int J Mol Sci. 2019;20(6):1475.
- Jensen P, Skov L. Psoriasis and obesity. Dermatology (Basel). 2016;232(6):633–639.
- Liang SE, Cohen JM, Ho RS. Screening for depression and suicidality in psoriasis patients: a survey of US dermatologists. J Am Acad Dermatol. 2019;80(5):1460–1462.
- Dauden E, Carretero G, Rivera R, et al. Long term safety of nine systemic medications for psoriasis: a cohort study using the Biobadaderm Registry. J Am Acad Dermatol. 2020;83(1):139–150.
- Cui Y. Clinical curative effect of using Liangxue Tuyin decoction in the treatment of psoriasis with blood-heat syndrome and its effect on accelerating skin lesion. J Sichuan Tradit Chin Med. 2019;37(07):151–154.
- Ding R. Observation on the therapeutic effect of Ziyin Huoxue Runzao decoction combined with Western Medicine in the treatment of plaque Psoriasis. Mod J Integr Tradit Chin Western Med. 2017;26(18):2030–2032 + 2043.
- Huang QH, Lin JJ. Observation on the Therapeutic effect of combination of traditional Chinese and Western Medicine on psoriasis vulgaris. J Dermatol Venereol. 2019;41(06):820–822.
- Li TT, Wang P, Zhou DM, et al. Clinical observation of Huoxue Jiedu decoction for psoriasis vulgaris of Blood stasis syndrome. J Pract Dermatol. 2016;9(02):133–135 + 138.
- Long HQ, Chen L, Bi DD, et al. Observation on the efficacy and safety of integrated traditional Chinese and Western Medicine in the treatment of psoriasis vulgaris. J Dermatol Venereol. 2018;40(06):839–840.
- She YY. A Clinical Research on The Treatment of psoriasis vulgaris in stationary stage blood dryness syndrome, blood stasis syndrome with calm-the-mind-and-relieve-itching formula. China Academy of Chinese Medical Sciences; 2017.
- Wang WN. Observation of the curative effect of the silver soup combined with seedlings medicine away tinea pill treatment of psoriasis with wind-hot-and-blood-dryness syndrome. Guiyang University of Chinese Medicine; 2012.
- Wu J. Clinical study on the treatment of retrograde psoriasis vulgaris by Peiyuan Yifu Purging. Yunnan University of Chinese Medicine; 2019.
- Wu Y-M, Li Q. Jianpi Jiedu Tang combined with avermectin A for training plaque psoriasis: a report of 80 cases. Liaoning J Tradit Chin Med. 2015;42(3):547–548.
- Yang F, Wang H, Qin T. Observation on the therapeutic effect of Qingre Liangxue Xiaoyin decoction combined with acitretin capsule in the treatment of psoriasis vulgaris. Shaanxi J Tradit Chin Med. 2017;38(11):1568–1569.
- Yao SP, Zhang CH, Guo F, et al. Clinical study on plaque psoriasis treated by spleen-strengthening and detoxification decoction combined with Avi A. China J Tradit Chin Med Pharmacy. 2013;28(09):2798–2800.
- Yuan LL. Effect of Qingre Huoxue Jiedu Prescription on Psoriasis NGF and CREB Signal Transduction Network. China Academy of Chinese Medical Sciences; 2019.
- Zhang CH. Efficacy of Tripterygium hypoglaucum (levl.) hutch mixture in blood-heat type of psoriasis vulgaris and its effect on IL-12 and IL-23. Yunnan University of Chinese Medicine; 2017.
- Zhang L. The clinical observation and immune adjustment of oxymatrine capsule and actretin in the treatment of psoriasis. J Chin Phys. 2013;15(3):331–333.
- Zhou DM. Wang Jusheng's academic thoughts and clinical experiences and the clinical study of treating psoriasis through blood differentiation. Beijing University of Chinese Medicine; 2011.
- Zhou MJ. Study of the Mechanisms of Liangxuehuoxue complex prescription on progressive psoriasis. Dalian Medical University; 2012.
- Zhu XY. Clinical observation in the treatment of psoriasis vulgaris by Yu Shi Xiao Bi Yin decoction. Zhejiang Chinese Medical University; 2014.
- Zhou Y, Guo X, Chen W, et al. Angelica polysaccharide mitigates lipopolysaccharide-evoked inflammatory injury by regulating microRNA-10a in neuronal cell line HT22. Artif Cells Nanomed Biotechnol. 2019;47(1):3194–3201.
- Wang W, Yuhai , Wang H, et al. Astilbin reduces ROS accumulation and VEGF expression through Nrf2 in psoriasis-like skin disease. Biol Res. 2019;52(1):49.
- Zhang C, Xu Q, Tan X, et al. Astilbin decreases proliferation and improves differentiation in HaCaT keratinocytes. Biomed Pharmacother. 2017;93:713–720.
- Parker S, Zhang CS, Yu JJ, et al. Oral Chinese herbal medicine versus placebo for psoriasis vulgaris: a systematic review. J Dermatolog Treat. 2017;28(1):21–31.
- Zhang CS, Yang L, Zhang AL, et al. Is oral Chinese herbal medicine beneficial for psoriasis vulgaris? A meta-analysis of comparisons with acitretin. J Altern Complement Med. 2016;22(3):174–188.
- Zhang CS, Yu JJ, Parker S, et al. Oral Chinese herbal medicine combined with pharmacotherapy for psoriasis vulgaris: a systematic review. Int J Dermatol. 2014;53(11):1305–1318.
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12.
- Yang L, Feng X, Li Y, et al. Therapeutic efficacy of catalpol against apoptosis in cardiomyocytes derived from human induced pluripotent stem cells. AMB Express. 2020;10(1):56.
- Xu DQ, Li CJ, Jiang ZZ, et al. The hypoglycemic mechanism of catalpol involves increased AMPK-mediated mitochondrial biogenesis. Acta Pharmacol Sin. 2020;41(6):791–799.
- Wang ZH, Zhan-Sheng H. Catalpol inhibits migration and induces apoptosis in gastric cancer cells and in athymic nude mice. Biomed Pharmacother. 2018;103:1708–1719.
- Li FL, Xu R, Zeng QC, et al. Tanshinone IIA inhibits growth of keratinocytes through cell cycle arrest and apoptosis: underlying treatment mechanism of psoriasis. Evid Based Complement Alternat Med. 2012;2012:927658.
- Lu J, Zhou H, Meng D, et al. Tanshinone IIA improves depression-like behavior in mice by activating the ERK-CREB-BDNF signaling pathway. Neuroscience. 2020;430:1–11.
- Yang G, Wang F, Wang Y, et al. Protective effect of tanshinone IIA on H2O2-induced oxidative stress injury in rat cardiomyocytes by activating Nrf2 pathway. J Recept Signal Transduct Res. 2020;40(3):1–9.
- Ren F, Li J, Wang Y, et al. The effects of angelica sinensis polysaccharide on tumor growth and iron metabolism by regulating hepcidin in tumor-bearing mice. Cell Physiol Biochem. 2018;47(3):1084–1094.
- Di TT, Ruan ZT, Zhao JX, et al. Astilbin inhibits Th17 cell differentiation and ameliorates imiquimod-induced psoriasis-like skin lesions in BALB/c mice via Jak3/Stat3 signaling pathway. Int Immunopharmacol. 2016;32:32–38.
- Yu YJ, Xu YY, Lan XO, et al Shikonin induces apoptosis and suppresses growth in keratinocytes via CEBP-δ upregulation. Int Immunopharmacol. 2019;72:511–521.
- Ma C, Wei Y, Liu Q, et al. Polysaccharides from Hedyotis diffusa enhance the antitumor activities of cytokine-induced killer cells. Biomed Pharmacother. 2019;117:109167.
- Lin L, Cheng K, Xie Z, et al. Purification and characterization a polysaccharide from Hedyotis diffusa and its apoptosis inducing activity toward human lung cancer cell line A549. Int. J. Biol. Macromol. 2019;122:64–71.
- Xu Y, Xu X, Gao X, et al. Shikonin suppresses IL-17-induced VEGF expression via blockage of JAK2/STAT3 pathway. Int. Immunopharmacol. 2014;19(2):327–333.
- Zhang X, Li J, Yu Y, et al Shikonin controls the differentiation of CD4 + CD25+ regulatory T cells by inhibiting AKT/mTOR pathway. Inflammation. 2019;42(4):1215–1227.
- Meng Y, Wang M, Xie X, et al. Paeonol ameliorates imiquimod-induced psoriasis-like skin lesions in BALB/c mice by inhibiting the maturation and activation of dendritic cells. International Journal of Molecular Medicine. 2017;39(5):1101–1110.
- Li B, He S, Liu R, et al. Total glucosides of paeony attenuates animal psoriasis induced inflammatory response through inhibiting STAT1 and STAT3 phosphorylation. J Ethnopharmacol. 2019;243:112121.
- Xiong H, Xu Y, Tan G, et al Glycyrrhizin ameliorates imiquimod-induced psoriasis-like skin lesions in BALB/c mice and inhibits TNF-α-induced ICAM-1 expression via NF-κB/MAPK in HaCaT cells. Cell Physiol Biochem. 2015;35(4):1335–1346.
- Oji V, Luger TA. The skin in psoriasis: assessment and challenges. Clin Exp Rheumatol. 2015;33(5 Suppl 93):S14–S19.
- Medicine. DBoCAoC, Medicine. DscoBAoC. Dermatology and venereology speciality committee BaotioTaWm. Evidence-based clinical practice guide of traditional Chinese medicine for psoriasis vulgaris. J Tradit Chin Med. 2014;55(01):76–82.
- Alqarni AM, Zeidler MP. How does methotrexate work? Biochem Soc Trans. 2020;48(2):559–567.
- Cronstein BN, Aune TM. Methotrexate and its mechanisms of action in inflammatory arthritis. Nat Rev Rheumatol. 2020;16(3):145–154.
- Qin X, Chen C, Zhang Y, et al. Acitretin modulates HaCaT cells proliferation through STAT1- and STAT3-dependent signaling. Saudi Pharm J. 2017;25(4):620–624.
- Niu X, Cao W, Ma H, et al. Acitretin exerted a greater influence on T-helper (Th)1 and Th17 than on Th2 cells in treatment of psoriasis vulgaris. J Dermatol. 2012;39(11):916–921.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–1072.
- Borren NZ, Ananthakrishnan AN. Safety of biologic therapy in older patients with immune-mediated diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2019;17(9):1736–1743.e4.
- Hu JZ, Billings SD, Yan D, et al. Histologic comparison of tumor necrosis factor-alpha inhibitor-induced psoriasis and psoriasis vulgaris. J Am Acad Dermatol. 2020;83(1):71–77.