Abstract
Background
Methotrexate (MTX) is frequently used in the treatment of moderate-to-severe psoriasis, however, there is limited data on health-related quality-of-life (HRQoL), psoriasis clinical outcomes and hepatic fibrosis in MTX-treated patients in routine clinical practice.
Objectives
To investigate the impact of moderate-to-severe psoriasis in MTX-treated patients in Spain regarding to HRQoL, psoriasis clinical data and risk of hepatic fibrosis.
Methods
Observational, non-interventional, cross-sectional, retrospective, multicentre study, performed in Spain in moderate-to-severe plaque psoriasis patients treated with MTX > 16 weeks prior to inclusion.
Results
Despite ongoing treatment, 17.1% of 457 evaluable patients reported moderate-to-extreme impact on HRQoL (DLQI > 5); 21.4% BSA > 5 and 35.2% moderate-to-severe pruritus (VAS ≥ 4). Persistent severe psoriasis (PASI ≥ 10 and/or DLQI ≥ 10) was observed in 10.7%. Hepatic steatosis was identified in 64.1% of patients (HSI ≥ 36) and 37.2% of the patients were at-risk of advanced fibrosis which was associated to the MTX treatment duration.
Conclusions
The study identified unmet needs in moderate-to-severe plaque psoriasis patients treated with MTX, revealing a significant proportion of sub-optimally controlled patients in terms of HRQoL and different domains of the disease. This study also found patients at-risk of advanced fibrosis, with evidence suggesting a correlation between longer exposures to MTX and higher risk of advanced fibrosis.
Introduction
Psoriasis is a chronic inflammatory immune-mediated disease affecting the skin, joints and other organs. Prevalence ranges between 1.5% and 5% of the general population (Citation1). The disease has a major impact in patients’ health-related quality of life (HRQoL), affecting both employment and social activities (Citation2,Citation3).
The impact on HRQoL is commonly measured through the Dermatology Life Quality Index (DLQI) (Citation4), which has been considered as one of the parameters determining treatment goals, together with the Psoriasis Area Severity Index (PASI), the Body Surface Area (BSA) and the Physician’s Global Assessment (PGA), either individually or collectively (Citation5). Despite the significant number of currently available drugs for moderate-to-severe psoriasis, published clinical practice studies indicate that patients are frequently unable to achieve clear skin or a clinically significant improvement in their HRQoL (Citation6–8), with patients on conventional systemic therapeutics reaching worst results than the ones on biologics (Citation8–10).
Psoriasis is associated with multiple comorbidities, especially amongst moderate-to-severe cases (Citation11), some of which potentially increase cardiovascular risk, such as metabolic disorders including obesity, diabetes, dyslipidaemia, metabolic syndrome, and nonalcoholic fatty liver disease (NAFLD). NAFLD spectrum ranges from simple steatosis (an excess of fat in the liver), progressing, in some cases, to nonalcoholic steatohepatitis (NASH), advanced fibrosis and cirrhosis (Citation12–14). The risk of liver-related morbidity and mortality is high in people with NAFLD, resulting in significant health-care costs (Citation15). Psoriasis and NAFLD share a common proinflammatory background (Citation14,Citation16,Citation17), and studies indicate that NAFLD affects up to 50% of psoriatic patients, whom are also more likely to develop severe forms of NAFLD (Citation16).
As psoriasis requires long-term treatment, and the accumulated toxicity is generally higher with conventional systemic treatments than with other therapies (Citation5,Citation14,Citation16,Citation18), the potential for drug-induced liver injury should be considered when choosing medication (Citation16,Citation19). Caution is particularly recommended in administering methotrexate (MTX) in patients with obesity, type 2 diabetes, or NAFLD (Citation16). The incidence of liver disease associated to the use of MTX is higher in psoriasis than in other inflammatory diseases (Citation20), likely because of the distinct metabolic profile of patients (Citation16), and studies have suggested that treatment with MTX could contribute to the development of hepatic fibrosis (Citation15).
In Spain, as in other European countries, MTX is one of the most frequently used conventional systemic dugs, as it is considered first-line treatment (Citation21). Despite being widely used, there is limited evidence on the clinical results and impact on HRQoL achieved by Spanish patients with moderate-to-severe psoriasis treated with MTX in clinical practice (Citation22), as well as to the risk for advanced hepatic fibrosis.
FirST study has uncovered unmet needs in moderate-to-severe psoriasis patients treated with the most widely used conventional systemic drugs for this disease: MTX. The main goals were to describe the HRQoL impact of moderate-to-severe plaque psoriasis in patients treated with MTX for at least 16 weeks, and the clinical response and risk of developing hepatic fibrosis in these patients.
Materials and methods
Study design
FirST is an observational, non-interventional, cross-sectional, retrospective, multicentre study, conducted in 49 Spanish hospitals and/or centers with dermatology consultations, distributed across the territory, according to routine clinical practice.
Patients had to be ≥18 years, diagnosed with moderate-to-severe plaque psoriasis and treated with MTX for at least 16 weeks prior to inclusion (timing of response to MTX treatment) (Citation21). Patients who had been previously or simultaneously treated with immunomodulatory biologics were excluded from the study, as well as those with severe concomitant diseases that could affect the evaluation of psoriasis impact in HRQoL (e.g. cancer, psychiatric diseases, other skin diseases), those participating in a clinical trial at the time of the study, or who presented other types of psoriasis.
Moderate-to-severe psoriasis was defined using the following criteria (Citation5): PASI > 10 or BSA > 10 or DLQI > 10 or PGA > 5; psoriasis that requires or has required systemic treatment at some point; psoriasis involving exposed areas (e.g. face), palms, soles, genitals, scalp, nails and recalcitrant plaques; psoriasis associated with psoriatic arthritis; or when there is a functional and/or psychosocial impact on the patient.
Clinical data related to psoriasis parameters were obtained from a single routine visit between June 2018–March 2019; other parameters were collected retrospectively from patients’ medical records. The scores to measure hepatic steatosis and fibrosis were calculated using retrospective data from the study visit up to 16 weeks prior to inclusion.
Study endpoints
The main objective of FirST was to describe the HRQoL impact of moderate-to-severe plaque psoriasis in patients treated with MTX for at least 16 weeks, being the primary endpoint the percentage of patients achieving DLQI 0/1. Secondary objectives included the: clinical response by PASI (Citation5,Citation23), BSA (Citation23), PGA (Citation5,Citation23,Citation24) and DLQI (Citation25); proportion of patients with an adequate response to treatment (Citation5); level of pruritus assessed through the Visual Analogue Scale (VAS) (Citation26); and risk of hepatic fibrosis.
Response to treatment was measured according to the criteria recommended by a consensus document on the evaluation and treatment of moderate-to-severe psoriasis (Citation5) (criteria 1: PASI < 5 or PGA = 0/1 or DLQI < 5). Furthermore, as the study was focused on HRQoL, an alternative criteria was added (criteria 2: (PASI < 5 or PGA ≤ 1) and DLQI < 5).
The risk of advanced fibrosis was studied according to the NAFLD fibrosis score (NAFLD-FS) (Citation27,Citation28) and Fibrosis-4 score (FIB-4) (Citation29), the risk of hepatic steatosis was predicted through the Hepatic Steatosis Index (HSI) (Citation30), and patients were stratified according to cutoffs described in Table S1. The subgroups of intermediate and high-risk were clustered into an at-risk of advanced fibrosis group, according to recently published algorithms for the risk stratification when using noninvasive tests (Citation31,Citation32).
Statistical analysis
Categorical variables were presented as numbers with percentages, while continuous variables as mean with standard deviation (SD).
The relation between the indexes of hepatic fibrosis and steatosis and sociodemographic and clinical characteristics of the study population were tested for significance using t-test and analysis of variance for continuous variables, and Chi-square test for categorical variables. Three multivariate analyses were performed to evaluate the factors related to DLQI, NAFLD-FS and FIB-4. Independent factors for DLQI models were age, weight, localization of psoriasis lesions, psoriatic arthritis, treatments, history of anxiety and depression and VAS of pruritus score. NAFLD-FS and FIB-4 models factors also included other variables as MTX treatment duration, hypertension and diabetes diagnosis, increased waist circumference and DLQI. Only variables with a level of significance ≤0.2 in the univariate analysis were included in each multiple regression model. The variables used to compute FIB-4 or NAFLD-FS were excluded. R2 coefficients of determination were calculated for each model. In the case of DLQI and FIB-4 models, a logarithmic transformation was applied to the dependent variable.
Data were analyzed with Statistical Analysis System (SAS) Enterprise Guide 7.15, considering a statistical significance (p) of .05 for all statistic tests performed.
Ethical considerations
The study was conducted according to guidelines on observational post-authorization studies for medicinal products for human use specified in Order SAS/3470/2009 of the Spanish Agency of Medicines and Medical Devices (AEMPS) and following the ethical principles of the Declaration of Helsinki and the local regulation, including privacy laws. The study was classified by AEMPS and received a favorable opinion from the Ethic Committee for Research with medicinal products of Hospital 12 de Octubre (Madrid), with the ethics approval number 18/244. All patients signed a written informed consent before being included in the study.
Results
Patients profile
A total of 476 patients were recruited and 457 (96.0%) of them were evaluable patients, as they fulfilled all the inclusion criteria, had signed an informed consent and had completed the DLQI.
Mean age (standard deviation, SD) of evaluable patients was 53.3 (14.0) years, 60.2% were over 50 years and 56.5% were male. Mean (SD) Body Mass Index (BMI) was 28.0 (5.1) Kg/m2, with 42.5% being overweight (BMI 25–29.99) and 29.3% obese (BMI ≥30). Around 19.3% were smokers (>10 daily cigarettes), and 2.8% were alcohol consumers (>20 g daily alcohol consumption on female and >40 g in male). Comorbidities were found in 60.0% of patients. Patients had been treated with MTX for a mean (SD) of 130.7 (141.6) weeks, measured as the time elapsed between the start of MTX treatment and the study visit ().
Table 1. Sociodemographic and clinical characteristics of the study population.
Impact of psoriasis on HRQoL in MTX-treated patients
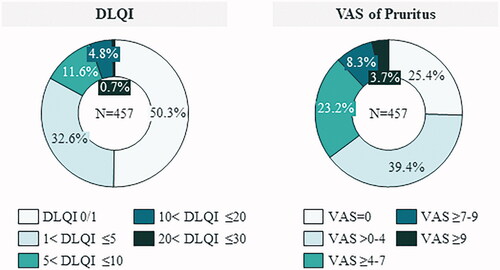
Mean (SD) DLQI score was 3.0 (4.0). Half of patients (50.3%) achieved DLQI 0/1 (no impact on HRQoL), however 17.1% had a moderate, high or extreme impact of the disease on their HRQoL (DLQI > 5), despite their MTX treatment ().
Figure 1. Description of the impact of psoriasis on HRQoL in MTX-treated patients. DLQI: dermatology quality-of-life index; VAS: visual analogue scale.
The relation between the impact of psoriasis on HRQoL and patients’ sociodemographic and clinical variables was assessed through a multiple linear regression model. Younger age, the presence of depression, the presence of scalp psoriasis and psoriasis in visible areas, and the severity of pruritus were all associated with higher DLQI classification, i.e. a worse HRQoL ().
Table 2. Multivariate analysis of the sociodemographic and clinical characteristics related to the DLQI score.
Clinical response
Mean scores for PASI, BSA and PGA are shown in . Considering PASI, 82.6% of patients scored PASI < 5, 12.7% 5 ≤ PASI ≤ 10 and 4.6% PASI > 10. Considering BSA, 52.5% of patients scored BSA < 3, 37.2% 3 ≤ BSA ≤ 10 and 10.3% BSA >10. PGA 0/1 was achieved by 58.2% of patients ().
Figure 2. Description of the patients’ clinical response. BSA: body surface area; PASI: psoriasis area severity index; PGA: physician’s global assessment.
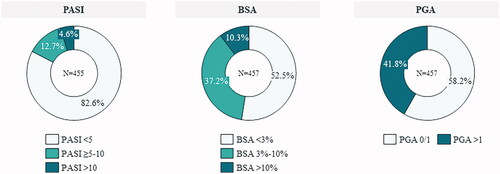
Table 3. Clinical parameters of the study population.
Interestingly, 10.7% of patients showed a persistent moderate-to-severe psoriasis according to criteria PASI ≥ 10 and/or DLQI ≥ 10, despite ongoing treatment with MTX. Adequate response to treatment was not reached by 8.3% of patients according to criteria 1 (PASI < 5 or PGA = 0/1 or DLQI < 5) or by 30.0%, according to criteria 2 ((PASI < 5 or PGA ≤ 1) and DLQI < 5).
The mean (SD) score obtained in VAS scale of pruritus was 2.9 (2.6). Only 25.4% of patients showed no pruritus while 35.2% of patients showed moderate-to-extreme pruritus (VAS ≥4) ().
Risk of hepatic fibrosis and steatosis
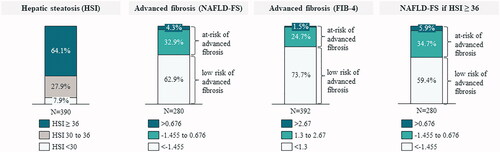
The variables required to calculate hepatic scores were available in 390 patients for the HSI; 280 for the NAFLD-FS; and 392 for the FIB-4. Hepatic steatosis was identified in 64.1% of patients, ruled-out in 7.9%, and 27.9% of the cases were inconclusive. Applying the NAFLD-FS, 37.2% of the patients were classified as at-risk of advanced fibrosis and 62.9% at low-risk. Using FIB-4, 26.2% of the patients were classified at-risk and 73.7% at low-risk of advanced fibrosis. Risk of advanced fibrosis was observed in 40.6% of patients with hepatic steatosis and in 16.0% of those without hepatic steatosis, according to NAFLD-FS ().
Figure 3. Description of the risk of hepatic steatosis and risk of advanced fibrosis. his: hepatic steatosis index; NAFLD-FS: nonalcoholic fatty liver disease fibrosis score; FIB-4: fibrosis-4.
The risk of hepatic fibrosis increased with age (p < .0001, mean age for patients at-risk of advanced fibrosis was 64.2 years vs. 46.6 from those at low-risk). Gender, on the other hand, was not significantly related to the risk of hepatic fibrosis or steatosis (). As expected, patients with metabolic syndrome had also greater risk of hepatic fibrosis (p < .0001, 74.4% of at-risk patients amongst those with metabolic syndrome vs. 37.2% in total cohort). A statistically significant relation was also found regarding the presence of other comorbidities, while no significant relation was identified to psoriatic arthritis.
Table 4. Relation between the indexes of hepatic fibrosis and steatosis and sociodemographic and clinical characteristics of the study population.
Other variables, such as BMI, weight and diabetes, were significantly related to the risk of hepatic fibrosis measured by NAFLD-FS (as expected, being part of its formula), but not when measured by FIB-4 ().
Although no statistically significant relation was observed between the MTX treatment duration and the HSI (), patients at-risk of advanced fibrosis experienced longer MTX treatments than those in the low-risk group (158.1 vs. 118.0 weeks for NAFLD-FS and 181.7 vs. 108.6 weeks for FIB-4, respectively) ().
Figure 4. Relationship between HSI, NAFLD-FS and FIB-4 scores and time on treatment with methotrexate. (a) Mean time on MTX according to HSI, NAFLD-FS and FIB-4 risk groups. (b) Share of patients with low-risk and at-risk of advanced fibrosis according to time on MTX, measured by FIB-4. FIB-4: fibrosis-4; HSI: hepatic steatosis index; NAFLD-FS: nonalcoholic fatty liver disease fibrosis score; W: weeks.
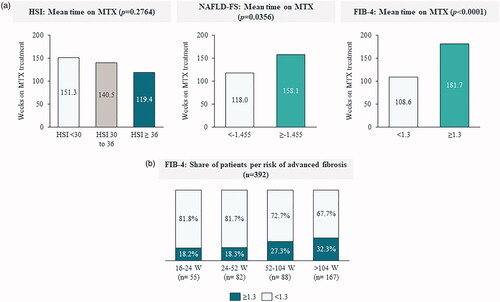
Multivariate analyses, using either the NAFLD-FS or the FIB-4 as dependent variables, found a positive relation between the MTX treatment duration and the risk of advanced fibrosis, both measured by NAFLD-FS (p = .0002) or FIB-4 (p = .0070), relation which was independent from other model variables (). As shown in , the proportion of patients at-risk of advanced fibrosis (FIB4 > 1.3) increased from 18.2%, for MTX treatment between 16–24 weeks, to 27.3% and 32.3%, for treatments that lasted between 52-104 weeks and >104 weeks, respectively.
Table 5. Multivariate analysis of the sociodemographic and clinical characteristics related to the NAFLD-FS and FIB-4 scores.
Discussion
To our knowledge, this is the first study addressing the relation between HRQoL, clinical response and risk of hepatic steatosis and/or fibrosis in patients with moderate-to-severe plaque psoriasis treated with MTX. As a result, several unmet needs have been identified.
Despite ongoing treatment, a substantial proportion of patients was sub-optimally controlled in different domains of the disease: persistent severe psoriasis was observed in 10.7% of patients (PASI ≥ 10 and/or DLQI ≥ 10); 17.1% reported moderate-to-extreme impact on HRQoL (DLQI > 5); 17.4% to 21.4% had PASI > 5 or BSA > 5, respectively; and 35.2% moderate-to-severe pruritus (VAS ≥ 4). Results are consistent with previous studies reporting a significant proportion of patients with high disease severity and poor HRQoL among psoriasis patients treated with conventional systemic drugs in clinical practice (Citation8). A Swedish study revealed that 18% of patients had persisting moderate-to-severe psoriasis (PASI ≥ 10 and/or DLQI ≥ 10), despite ongoing systemic treatment (Citation8). Of these, 69% were using conventional systemic treatments and 54% of patients were using MTX as monotherapy. Mean (SD) PASI was 4.1 (4.6), and DLQI was 4.1 (5.2). Treatments had been used, on average, for over 3 years, with a minimum of 12 weeks of treatment, which is similar to our study (average of 2.5 years, with a minimum of 16 weeks of treatment with MTX). A U.S. study has also revealed sub-optimal clinical results on patients treated with MTX, with a median (IQR) PASI of 3.8 (1.8–6.6), BSA of 3.0 (1.0–6.0), PGA of 1.7 (1.3–2.0), and DLQI of 3 (1–5) (Citation10). Patients on MTX had a median (IQR) duration of treatment of 10.5 (4.0–24.0) months, which is inferior to the one from the FirST study, but still meeting the minimum 16 weeks for inclusion. The study has also revealed better PASI and DLQI outcomes in patients treated with biologics than in those treated with MTX (Citation8,Citation10), even after adjusting for confounding factors. This conclusion is supported by a Swiss registry-based study, which found that patients with biologic treatment reached a lower DLQI than the ones with conventional systemic therapeutics (Citation9). It is worth noting that the presented results might be positively biased toward MTX given that patients were biologic naive and likely those with a good initial response and tolerance to MTX (treatment for at least 16 weeks).
Notwithstanding the recommended caution regarding treating patients with NAFLD with potentially hepatotoxic drugs, such as MTX (Citation3,Citation14,Citation33), the FirST study has identified a significant proportion of patients at-risk of hepatic steatosis and fibrosis amongst MTX-treated patients, as measured by HSI, NAFLD-FS and FIB-4, thus contributing to existing evidence that the risk of advanced fibrosis is common in moderate-to-severe psoriasis (Citation13,Citation20,Citation34). In a recent review of the current state of noninvasive tools for the assessment of liver disease in NAFLD, experts stated that those patients with intermediate and high risk of fibrosis (up to one-third of patients in FirST study) should be sent to a referral center for further assessment (Citation31).
A statistically significant relation was found between the duration of MTX treatment and the fibrosis biomarkers NAFLD-FS (p = .0002) and FIB-4 (p = .0070), suggesting a direct relation between the time of MTX treatment and the risk of hepatic fibrosis. Specifically, the longer the time of MTX treatment, the higher the risk of fibrosis appearance. Other variables were also independent contributors to the risk of hepatic fibrosis (for both NALFD-FS and FIB4), such as alcohol consumption and hypertension.
Finally, the presence of steatosis in 64.1% of patients indicates a higher presence of NAFLD than the 25.8% estimated in the adult Spanish population (Citation15,Citation35) or the 47% NAFLD prevalence observed among patients with plaque psoriasis who were not treated with MTX or other potentially hepato-toxic drug (Citation16,Citation34). Similarly, the proportion of at-risk of advanced fibrosis in the MTX treated psoriatic patients in our study, 26.2% (FIB-4) and 37.2% (NAFLD-FS), is higher than reported in general population: 11.4% (FIB-4) or 8.6% (NAFLD-FS) (Citation36) in Sweden; 2.8% in France (FibroTest) (Citation37) and 3.6% in Spain (measured with transient elastography) (Citation38). These differences are aligned with a previous study reporting higher steatosis and NAFLD-FS in patients with psoriasis than in controls (Citation34,Citation39–42). Although the study excluded MTX treated patients, population with a greater alcohol intake was included, which, as in FirST, was a specific cause of liver damage (Citation39). Despite not including a control group, our results suggest a negative impact of MTX on the risk of hepatic fibrosis in moderate-to-severe psoriasis and support previous findings showing MTX as a statistically significant contributor to the risk of NAFLD (Citation43).
Pairing the risk of advanced fibrosis with the sub-optimal clinical results achieved by a set of patients, the results from FirST not only support the recommendation that MTX requires a careful hepatic evaluation in patients with moderate-to-severe psoriasis, but further advise a reflection on whether other treatments with lower toxicity could result in better outcomes and at lower hepatic risk for these sub-optimally controlled patients (Citation8).
Limitations
This is a cross-sectional retrospective study that lacks prospective follow-up, thus not detecting changes over time. The study population was limited to biologic naive patients meeting the inclusion criteria of >16 weeks on MTX treatment, thus possibly excluding some MTX nonresponders. Laboratory data for the calculation of hepatic scores were only available for some patients and hepatic steatosis or fibrosis were not evaluated through techniques such as elastography or liver biopsy. Some of the risk factors for liver disease are used to compute FIB-4 or NAFLD-FS scores, thus limiting analyses on their specific contribution to the risk of hepatic fibrosis. Finally, only MTX dose at time of visit was obtained, and not accumulated MTX dose throughout the MTX treatment period, thus not enabling potential correlations between MTX dose and liver fibrosis development to be evaluated.
Conclusions
Using clinical practice outcomes from 49 Spanish dermatology consultations, FirST study has identified substantial unmet needs related to HRQoL, clinical parameters and risk of advanced hepatic fibrosis in patients with moderate-to-severe psoriasis treated with MTX during at least 16 weeks. A significant percentage of patients was found to be sub-optimally controlled, suggesting a holistic management approach might be needed, focusing on patients' quality-of-life and pruritus, psoriasis severity, body surface area involvement and risk for hepatic fibrosis. Results suggest a correlation between the duration of MTX treatment and the risk of liver fibrosis, as patients with longer exposures to MTX exhibited a higher risk of advanced fibrosis, advising that this risk should be thoroughly assessed by clinicians when managing patients with moderate-to-severe psoriasis. These findings should be considered when selecting treatment options and patients should receive support in improving lifestyle factors and access to effective therapies, allowing better treatment outcomes and not contributing to hepatotoxicity.
Supplemental Material
Download PDF (282.7 KB)Acknowledgments
The authors would like to thank all investigators who participated in the FirST study (Table S2), Mafalda Carmo (IQVIA, Spain) and Carmen Barrull (IQVIA, Spain) for medical writing support and editorial assistance.
Disclosure statement
Dr Rivera received personal fees from AbbVie, Celgene, Janssen, LEO Pharma, Lilly, Novartis, UCB, and Almirall, not related to the submitted work. Dr Vilarrasa received personal fees from AbbVie, Almirall, Amgen, Boehringer Ingelheim, Celgene, Janssen-Cilag, LEO Pharma, Lilly, MSD-Schering-Plough, Novartis, Pfizer and UCB, not related to the submitted work. Dr Ribera received personal fees from AbbVie, Almirall, Celgene, Gebro Pharma, Janssen, LEO Pharma, Lilly, Novartis, Pfizer, Sandoz, not related to the submitted work. Dr Roe received grants from Novartis, during the conduct of the study. Dr Kueder-Pajares received support from Novartis, during the conduct of the study; as well as personal fees from Novartis, LEO Pharma, and UCB not related to the submitted work. Dr Usero-Bárcena received personal fees from Novartis, during the conduct of the study; as well as non-financial support from Novartis, AbbVie, Janssen, and Almirall, not related to the submitted work. Dr García-Donoso received grants from Fundación para la Investigación Biomédica del Hospital Universitario 12 de Octubre, during the conduct of the study. Dr Olveira received personal fees and non-financial support from Novartis, during the conduct of the study; as well as grants, personal fees and non-financial support from Gilead, and grants, personal fees and non-financial support from Intercept, not related to the submitted work. Dr Ferran received personal fees from AbbVie, Almirall, Celgene, Janssen, MSD, Lilly, Novartis, and Pfizer, not related to the submitted work. Dr Guinea and Dr Martín are employees of Novartis. Dr Zayas, Dr Martínez-Molina, Dr Mataix Díaz, Dr Rodríguez-Nevado, and Dr de la Mano have no conflict of interest to declare.
Additional information
Funding
References
- WHO. Global report on psoriasis. Geneva: World Health Organization; 2016.
- Gelfand JM, Feldman SR, Stern RS, et al. Determinants of quality of life in patients with psoriasis: a study from the US population. J Am Acad Dermatol. 2004;51(5):704–708.
- Daudén E, Castañeda S, Suarez C, et al. Abordaje integral de la comorbilidad del paciente con psoriasis. Actas Dermosiliogr. 2012;103(Supl 1):1–64.
- Puig L. PASI90 response: the new standard in therapeutic efficacy for psoriasis. J Eur Acad Dermatol Venereol. 2015;29(4):645–648.
- Dauden E, Puig L, Ferrandiz C, the Spanish Psoriasis Group of the Spanish Academy of Dermatology and Venereology, et al. Consensus document on the evaluation and treatment of moderate-to-severe psoriasis: Psoriasis Group of the Spanish Academy of Dermatology and Venereology. J Eur Acad Dermatol Venereol. 2016;30(Suppl 2):1–18.
- Armstrong AW, Robertson AD, Wu J, et al. Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: findings from the National Psoriasis Foundation surveys, 2003-2011. JAMA Dermatol. 2013; 149(10):1180–1185.
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70(5):871–881.e1-30.
- Norlin JM, Calara PS, Persson U, et al. Real-world outcomes in 2646 psoriasis patients: one in five has PASI >/=10 and/or DLQI >/=10 under ongoing systemic therapy. J Dermatol Treat. 2017;28(6):500–504.
- Jungo P, Maul JT, Djamei V, et al. Superiority in quality of life improvement of biologics over conventional systemic drugs in a Swiss Real-Life Psoriasis Registry. Dermatology (Basel, Switzerland). 2016;232(6):655–663.
- Gelfand JM, Wan J, Callis DK, et al. Comparative effectiveness of commonly used systemic treatments or phototherapy for moderate to severe plaque psoriasis in the clinical practice setting. Arch Dermatol. 2012;148(4):487–494.
- Singh S, Young P, Armstrong AW. Relationship between psoriasis and metabolic syndrome: a systematic review. Giornale Italiano di Dermatologia e Venereologia: organo Ufficiale, Societa Italiana di Dermatologia e Sifilografia. 2016;151(6):663–677.
- Glen J, Floros L, Day C, et al. Non-alcoholic fatty liver disease (NAFLD): summary of NICE guidance. BMJ (Clinical Research ed). 2016;354:i4428.
- Maybury CM, Porter HF, Kloczko E, et al. Prevalence of advanced liver fibrosis in patients with severe psoriasis. JAMA Dermatol. 2019;155(9):1028.
- Fiore M, Leone S, Maraolo AE, et al. Liver illness and psoriatic patients. Biomed Res Int. 2018;2018:3140983.
- Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20.
- Mantovani A, Gisondi P, Lonardo A, et al. Relationship between non-alcoholic fatty liver disease and psoriasis: a novel hepato-dermal axis? Int J Mol Sci. 2016;17(2):217.
- Ruiz de Morales JMG, Puig L, Daudén E, et al. Critical role of interleukin (IL)-17 in inflammatory and immune disorders: an updated review of the evidence focusing in controversies. Autoimmun Rev. 2020; 19(1):102429.
- Nijsten T, Margolis DJ, Feldman SR, et al. Traditional systemic treatments have not fully met the needs of psoriasis patients: results from a national survey. J Am Acad Dermatol. 2005;52(3 Pt 1):434–444.
- Cheng HS, Rademaker M. Monitoring methotrexate-induced liver fibrosis in patients with psoriasis: utility of transient elastography. Psoriasis (Auckl). 2018;8:21–29.
- Taylor WJ, Korendowych E, Nash P, et al. Drug use and toxicity in psoriatic disease: focus on methotrexate. J Rheumatol. 2008;35(7):1454–1457.
- Carrascosa JM, de la Cueva P, Ara M, et al. Methotrexate in moderate to severe psoriasis: review of the literature and expert recommendations. Actas Dermosifiliogr. 2016;107(3):194–206.
- Fernández-Torres RM, Pita-Fernández S, Fonseca E. Quality of life and related factors in a cohort of plaque-type psoriasis patients in La Coruña, Spain. Int J Dermatol. 2014;53(11):e507–e511.
- AEDV. How to measure psoriasis severity?. Spanish Academy of Dermatology and Venereology. 2016.
- Langley RGB, Feldman SR, Nyirady J, et al. The 5-point Investigator's Global Assessment (IGA) Scale: a modified tool for evaluating plaque psoriasis severity in clinical trials. J Dermatolog Treat. 2015;26(1):23–31.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)-a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216.
- Reich A, Heisig M, Phan NQ, et al. Visual analogue scale: evaluation of the instrument for the assessment of pruritus. Acta Derm Venerol. 2012;92(5):497–501.
- Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45(4):846–854.
- EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia. 2016;59(6):1121–1140.
- Shah AG, Lydecker A, Murray K, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7(10):1104–1112.
- Lee JH, Kim D, Kim HJ, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42(7):503–508.
- Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(5):1264–1281.e4.
- EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–1402.
- Maybury CM, Jabbar-Lopez ZK, Wong T, et al. Methotrexate and liver fibrosis in people with psoriasis: a systematic review of observational studies. Br J Dermatol. 2014;171(1):17–29.
- Carrascosa JM, Bonanad C, Dauden E, en nombre del Grupo de Trabajo en Inflamación Sistémica en Psoriasis, et al. Psoriasis and nonalcoholic fatty liver disease. Actas Dermosifiliogr. 2017;108(6):506–514.
- Caballeria L, Pera G, Auladell MA, et al. Prevalence and factors associated with the presence of nonalcoholic fatty liver disease in an adult population in Spain. Eur J Gastroenterol Hepatol. 2010;22(1):24–32.
- Hagstrom H, Talback M, Andreasson A, et al. Ability of noninvasive scoring systems to identify individuals in the population at risk for severe liver disease. Gastroenterology. 2020;158(1):200–214.
- Poynard T, Lebray P, Ingiliz P, et al. Prevalence of liver fibrosis and risk factors in a general population using non-invasive biomarkers (FibroTest). BMC Gastroenterol. 2010;10(1):40.
- Caballeria L, Pera G, Arteaga I, et al. High prevalence of liver fibrosis among European adults with unknown liver disease: a population-based study. Clin Gastroenterol Hepatol. 2018;16(7):1138–1145.e5.
- Gisondi P, Barba E, Girolomoni G. Non-alcoholic fatty liver disease fibrosis score in patients with psoriasis. J Eur Acad Dermatol Venereol. 2016;30(2):282–287.
- Miele L, Vallone S, Cefalo C, et al. Prevalence, characteristics and severity of non-alcoholic fatty liver disease in patients with chronic plaque psoriasis. J Hepatol. 2009;51(4):778–786.
- Wenk K, Arrington K, Ehrlich A. Psoriasis and non-alcoholic fatty liver disease. J Eur Acad Dermatol Venereol. 2011;25(4):383–391.
- Candia R, Ruiz A, Torres-Robles R, et al. Risk of non-alcoholic fatty liver disease in patients with psoriasis: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2015;29(4):656–662.
- Rosenberg P, Urwitz H, Johannesson A, et al. Psoriasis patients with diabetes type 2 are at high risk of developing liver fibrosis during methotrexate treatment. J Hepatol. 2007;46(6):1111–1118.
