Abstract
Background
Nail psoriasis (NP) is common and of high importance in patients with psoriasis. Complete resolution of NP at week 24‒26 is an unambiguous nail outcome accessible for indirect treatment comparison of biologics.
Objective
To evaluate the comparative efficacy of approved biologics in achieving complete resolution of NP at week 24‒26.
Methods
A network meta-analysis (NMA) was conducted to indirectly compare the efficacy of six biologics in achieving complete resolution of NP at week 24‒26 in patients with moderate-to-severe psoriasis and concomitant NP. Complete resolution of NP was defined as a score of zero on the Nail Psoriasis Severity Index (NAPSI), modified NAPSI (mNAPSI) or Physician’s Global Assessment of Fingernails (PGA-F).
Results
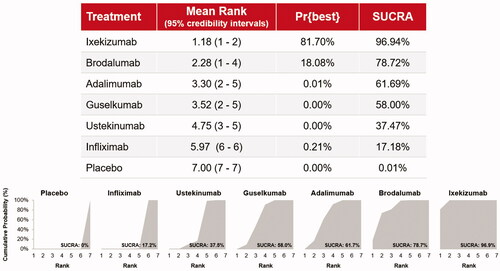
The probability of achieving complete resolution of NP was highest for ixekizumab (46.5%; 95% credibility interval [CrI] 35.1‒58.0; Surface Under the Cumulative RAnking curve [SUCRA] 97%), followed by brodalumab (37.0%; 17.0‒61.0; 79%), adalimumab (28.3%; 24.4‒32.4; 62%), guselkumab (27.7%; 21.1‒35.1; 58%), ustekinumab (20.8%; 10.2‒35.2; 37%), and infliximab (0.8%; 0.0‒8.9; 17%).
Conclusion
In patients with moderate-to-severe psoriasis and concomitant NP, ixekizumab has the greatest likelihood among approved biologics of achieving complete resolution of NP at week 24‒26. Findings should be interpreted carefully because of inherent study limitations.
Introduction
Nail psoriasis (NP) affects up to 50% of patients with psoriasis and up to 80% of patients with psoriatic arthritis (Citation1,Citation2), with an estimated lifetime risk of up to 90% (Citation1,Citation3,Citation4). NP is characterized by the presence of various morphological changes resulting from inflammation at the nail matrix or nail bed, leading to functional impairment and adverse effects on health-related quality of life (HR-QoL) (Citation1‒Citation5). Patients with psoriasis who have nail involvement have greater impairment of HR-QoL than those without NP (Citation6). NP may be underrecognized because of challenges in making a differential diagnosis, as NP may appear similar to onychomycosis or other nail disorders (Citation3,Citation5). It is also undertreated and is widely acknowledged to be a therapeutic challenge. NP is more difficult to treat than skin lesions of psoriasis, and limited penetration of topical agents through the nail plate plus poor treatment adherence renders them usually ineffective (Citation1,Citation3). Systemic therapy is often considered by dermatologists to be inappropriate for patients without or with limited cutaneous involvement, especially if the number of psoriatic nails is low (Citation1). Nonetheless, many systemic medications, particularly biologics, are efficacious in the treatment of NP, although often with a delayed and less pronounced effect relative to the cutaneous improvement. Even though pivotal studies for biologics evaluating psoriasis nowadays include active comparators, short-term use in these trials usually precludes meaningful analyses of the nails, as efficacy can usually not be evaluated before 3‒6 months (Citation3). Recent trials suggest that the maximum effect is not reached for up to 1 year, or even beyond (Citation7‒Citation11).
Recently, various head-to-head and placebo-controlled trials evaluating biologics in patients with psoriasis with a study duration of at least 24 weeks have been completed that also included nail assessments (Citation7,Citation9‒Citation16). With few exceptions (Citation10,Citation14,Citation16), nail outcomes were reported separately as post hoc analyses from studies primarily investigating drug efficacy on psoriasis. In combination, these trials allow an assessment of the relative efficacy of the involved biologics on NP. However, assessment is further complicated by the lack of consensus on which of the many scoring systems for NP is the most appropriate, owing to a lack of reproducibility and differences in selection and type of evaluated features (Citation17,Citation18).
The least subjective and ambiguous outcome – and arguably the most important treatment goal from both a patient and clinician perspective – is complete resolution of NP (Citation15), which can be assessed using instruments such as the Nail Psoriasis Severity Index (NAPSI), modified NAPSI (mNAPSI), and the Physician’s Global Assessment of Fingernails (PGA-F). Even though not reported consistently (e.g. Transfigure (Citation8)), complete resolution of fingernail psoriasis (NAPSI = 0, mNAPSI = 0, PGA-F = 0) currently represents the only nail outcome accessible for indirect treatment comparisons at a time point that first allows meaningful analysis (week 24‒26). Complete nail resolution is common to multiple trials and is similar regardless of scale/scoring system used (Citation15). Therefore, we conducted a comprehensive network meta-analysis (NMA) that indirectly assessed the attainment of complete resolution of fingernail psoriasis at 24‒26 weeks for six approved biologics as well as placebo for patients with NP and moderate-to-severe psoriasis.
Methods
Literature review
A targeted literature review was performed to identify randomized controlled trials of biologics licensed by the European Medicines Agency and the US Food and Drug Administration for the treatment of moderate-to-severe psoriasis reporting NAPSI (or mNAPSI) or PGA-F. The population of focus for this NMA was adults with moderate-to-severe chronic plaque psoriasis and concomitant fingernail psoriasis defined as either NAPSI ≥1, mNAPSI ≥1, or PGA-F ≥ 1. Patient populations with psoriatic arthritis meeting these criteria were not excluded. A comprehensive database search was undertaken (with searches last updated on 28 April 2020), using MEDLINE and the Cochrane Central Register of Controlled Trials and was limited to English-language studies of human subjects. Additionally, two authors (C.S. and C.S.) performed a manual check of the reference lists of recently published reviews on this topic (Citation1‒Citation3,Citation5).
To be included in the analyses the identified studies were required to have a suitable comparator (another biologic, placebo, or both) for the entire follow-up period and to report at least one efficacy outcome of interest (NAPSI = 0, mNAPSI = 0, PGA-F = 0) at week 24‒26. Only data from trials’ treatment arms in which the treatment assignment was maintained during the maintenance period were used. For example, trials such as Transfigure (Citation8) could not be included in the analysis because it did not have a suitable comparator at week 24‒26 (patients in the placebo group crossed over to secukinumab at week 16). summarizes the key study design features of the identified eligible trials.
Table 1. Design features of clinical trials included in the network meta-analysis.
Comparators
All comparators in this analysis used on-label dosing (Citation19‒Citation24). Treatments included the anti-tumor necrosis factor (TNF) agent adalimumab (initial dose of adalimumab 80 mg, followed by 40 mg every second week starting 1 week later), the interleukin (IL)-17 receptor A (RA) inhibitor brodalumab (210 mg, at weeks 0, 1, 2, and every 2 weeks thereafter), the IL-23p19 inhibitor guselkumab (100 mg, at weeks 0, 4, and every 8 weeks thereafter), the anti-TNF agent infliximab (5 mg/kg at weeks 0, 2, 6, and every 8 weeks thereafter), the IL-17A inhibitor ixekizumab (160 mg starting dose at week 0, followed by 80 mg every 2 weeks from week 2–12 and every 4 weeks thereafter), and the IL-12/23p40 antibody ustekinumab (45/90 mg weight-based dosing at weeks 0, 4, and every 12 weeks thereafter) ().
Outcomes
In this analysis, the outcomes of interest were the proportion of patients with moderate-to-severe psoriasis and concomitant fingernail psoriasis defined as NAPSI ≥1, mNAPSI ≥1, or PGA-F ≥ 1 that achieved complete resolution of NP (NAPSI = 0, mNAPSI = 0, PGA-F = 0) at week 24‒26. Although not formally proven, one assumption for this analysis was that the outcomes of interest reflect attainment of complete resolution of NP and therefore should be comparable within and across clinical trials. This assumption is supported by data from a randomized controlled trial in which complete clearance of fingernail psoriasis occurred in a similar proportion of patients whether assessed by PGA-F = 0 or NAPSI = 0 (Citation15).
Characteristics of included studies
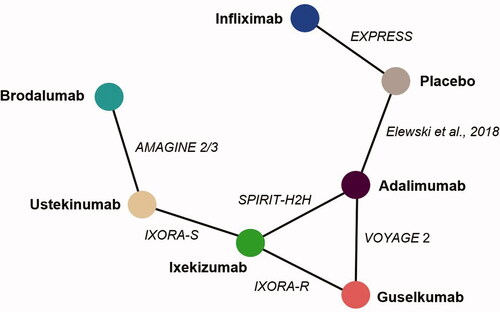
A total of seven phase III randomized controlled trials met our inclusion criteria and reported maintenance data on nail clearance at week 24‒26. Individual patient-level data were available for IXORA-S (Citation11), IXORA-R (Citation12,Citation13), and SPIRIT-H2H (Citation10,Citation16). All studies were performed in patients with moderate-to-severe psoriasis with a varying degree of concomitant psoriatic arthritis, except for the SPIRIT-H2H trial, in which patients had to have psoriatic arthritis and only the subgroup of patients with moderate-to-severe psoriasis and NP was included (Citation25). Of note, two studies performed in patients with moderate-to-severe psoriasis and NP could not be included as no suitable comparator was available and/or the frequency of complete nail resolution was not reported (Citation8,Citation26). provides the baseline patient characteristics from the included studies, and presents the network diagram highlighting the available evidence for achieving complete nail resolution at week 24‒26.
Table 2. Baseline patient characteristics from clinical trials included in the network meta-analysis.
Statistical analysis
Bayesian NMAs were performed using JAGS and R (R2JAGS package). With respect to statistical methods, a binomial model with a logit link was used for nail clearance data. Models were fitted using three chains and non-informative priors, and convergence diagnostics were checked. Both fixed- and random-effect models were run, and the deviance information criterion (DIC) was used to assess which model fit the data best.
Results from the Bayesian analysis are presented as a point estimate (posterior probability of nail clearance) and associated 95% credibility interval (CrI). A 95% CrI can be interpreted as a 95% probability that the true treatment effect lies within the interval. As for any Bayesian analysis, the probability of being ranked best treatment and Surface Under the Cumulative RAnking curve (SUCRA) were calculated and reported to reflect the extent to which each intervention is among the best therapeutic options.
Results
Nail resolution NMA – 24‒26 weeks
Seven studies included comparative data (two placebo-controlled trials (Citation9,Citation14) and five head-to-head active-comparator trials (Citation7,Citation11,Citation13,Citation15,Citation16)) on week 24‒26 outcomes that connected to form an evidence network comparing seven interventions (). Examination of DIC indicated that the fixed-effect model was more suitable to fit the data, although results for the random-effect model were similar. The probabilities of achieving complete resolution of NP at week 24‒26 were 46.5% (95% CrI 35.1‒58.0) for ixekizumab, 37.0% (95% CrI 17.0‒61.0) for brodalumab, and 28.3% (95% CrI 24.4‒32.4), 27.7% (95% CrI 21.1‒35.1), 20.8% (95% CrI 10.2‒35.2), and 0.8% (95% CrI 0.0‒8.9) for adalimumab, guselkumab, ustekinumab, and infliximab, respectively (). All drugs except infliximab were significantly better at achieving nail clearance than placebo. The percentage of patients achieving complete nail resolution was numerically higher with ixekizumab than with all comparators, and the difference was statistically significant vs adalimumab and infliximab. Based on mean effect values, ixekizumab had the greatest effect followed by brodalumab; treatment with brodalumab is expected to result in more responders (i.e. patients with complete nail resolution) than treatment with adalimumab, guselkumab, ustekinumab, or infliximab, although the differences were not statistically significant, except vs infliximab.
Figure 2. Forest plot of treatment differences (and 95% credibility intervals) for complete resolution of nail psoriasis (Nail Psoriasis Severity Index [NAPSI] = 0, modified NAPSI [mNAPSI] = 0, or Physician’s Global Assessment of Fingernail Psoriasis [PGA-F] = 0) at weeks 24‒26.
![Figure 2. Forest plot of treatment differences (and 95% credibility intervals) for complete resolution of nail psoriasis (Nail Psoriasis Severity Index [NAPSI] = 0, modified NAPSI [mNAPSI] = 0, or Physician’s Global Assessment of Fingernail Psoriasis [PGA-F] = 0) at weeks 24‒26.](/cms/asset/55f37d81-be43-4902-8651-b0c3bfe7a013/ijdt_a_1892024_f0002_c.jpg)
Based on the Bayesian NMA, ixekizumab was associated with the highest probability to be ranked best (81.7%), followed by brodalumab (18.1%; ). The SUCRA value also indicates that ixekizumab is the most efficacious therapy in 96.9% of Bayesian iterations with a mean rank of 1.2 (standard deviation [SD] ± 0.4), followed by brodalumab with a SUCRA value of 78.7% and a mean rank of 2.3 (SD ± 1.0). The probability that the other drugs ranked best to achieve complete resolution of NP was <1%, and the SUCRA values for adalimumab (61.7%), guselkumab (58.0%), ustekinumab (37.5%), and infliximab (17.2%) were markedly lower.
In terms of number needed to treat for complete nail clearance at weeks 24‒26 vs placebo, ixekizumab had the lowest value (2.2; 95% CrI 1.8‒2.9), followed by brodalumab (2.7; 95% CrI 1.7‒5.9), adalimumab (3.6; 95% CrI 3.2‒4.1), guselkumab (3.6; 95% CrI 2.9‒4.8), ustekinumab (4.8; 95% CrI 2.9‒9.8), and infliximab (147.2; 95% CrI 12.9 to >1000; ).
Figure 4. Number needed to treat for complete resolution of nail psoriasis (Nail Psoriasis Severity Index [NAPSI] = 0, modified NAPSI [mNAPSI] = 0, or Physician’s Global Assessment of Fingernail Psoriasis [PGA-F] = 0) at weeks 24‒26 versus placebo.
![Figure 4. Number needed to treat for complete resolution of nail psoriasis (Nail Psoriasis Severity Index [NAPSI] = 0, modified NAPSI [mNAPSI] = 0, or Physician’s Global Assessment of Fingernail Psoriasis [PGA-F] = 0) at weeks 24‒26 versus placebo.](/cms/asset/a11e84c6-bac7-4070-9c87-7d9d7aa3ca1a/ijdt_a_1892024_f0004_c.jpg)
Taken together, these data highlight the efficacy of the investigated inhibitors of IL-17 cytokines, ixekizumab and brodalumab, in achieving complete nail clearance as early as weeks 24‒26.
Discussion
This study compares the relative efficacy of biologics in achieving complete clearance of NP in patients with moderate-to-severe psoriasis at weeks 24‒26, and the IL-17A inhibitor ixekizumab has the highest probability of being ranked best. Ixekizumab also had the highest ranking in a recent NMA of biologics and small molecules in treating NP (Citation27). However, our NMA evaluated results at 24‒26 weeks, whereas this analysis compared short-term efficacy at week 10‒16, which was generally too short a time period to adequately assess nail outcomes (Citation3), and treatment efficacy was based on NAPSI improvement rather than complete nail resolution (Citation27). Results from the current study confirm and extend that biologics approved for the treatment of psoriasis are efficacious in achieving complete resolution of NP after 24‒26 weeks in comparison with placebo and that ixekizumab had significantly higher response rates (i.e. complete nail resolution) than adalimumab and infliximab and numerically higher response rates than all other comparators. Ranking analysis performed with SUCRA strongly suggests that ixekizumab is the best treatment when aiming at complete resolution of NP at week 24‒26, followed by brodalumab, adalimumab, guselkumab, ustekinumab, and infliximab.
Despite the efficacy of biologics in improving cutaneous signs of psoriasis (Citation28,Citation29), it cannot be automatically assumed that efficacy in skin also translates to NP improvement. NP can be regarded as an intermediate stage of the natural progression from cutaneous psoriasis to psoriatic arthritis and, in turn, as an indicator for the development of psoriatic arthritis (Citation30,Citation31). A recently published statistical model suggests that improvement in NP correlates with both improvement in skin and longer duration of treatment in patients with psoriasis (Citation32). As a corollary, one could assume that the relative efficacy provided by NMAs can be used as a surrogate to determine the best treatment for a patient with NP. Yet, it is possible that drug- and class-specific differences exist, given the micro-anatomical link between NP and psoriatic arthritis (Citation30,Citation31) in combination with the differential treatment effects of the various drug classes on psoriatic arthritis (Citation1‒Citation5,Citation18). This is, for instance, exemplified by the comparable effect of ixekizumab and guselkumab on skin clearance but not on nail clearance at week 24 in the IXORA-R trial (Citation13). Even though for many biologics improvement in NP continues until 52 weeks of continuous treatment (Citation7‒Citation11), it remains to be seen whether this discrepancy in the clearance rates of NP between these two drugs remains.
As most studies included in this indirect comparison did not require a minimum degree of NP severity, some patients might have had relatively mild disease at baseline, and their improvement may not have been clinically relevant. Even though the definition of clinically meaningful has not been validated in NP (Citation14), it appears likely – analogous to the situation in cutaneous psoriasis (Citation33) – that reaching completely cleared NP translates into a significant improvement in HR-QoL (Citation34,Citation35). Notwithstanding the definition, at weeks 24 and 52 of continuous ixekizumab treatment, the proportion of patients with complete nail clearance is comparable irrespective of having significant NP (defined as NAPSI ≥16 and ≥4 fingernails involved) or not (Citation11). This effect is less obvious for ustekinumab and has not been reported for (most) other treatments.
The results of this study should be interpreted within the context of certain limitations. First, a targeted literature review rather than a systematic literature review was used, meaning it was not conducted according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) requirements (Citation36). However, the authors have considerable experience in this area and are confident that the targeted search strategy captured all relevant clinical trial data. The majority of identified studies were post hoc analyses of randomized trials conducted in patients with moderate-to-severe psoriasis, which could also be considered a limitation since only one study was performed in patients with moderate-to-severe fingernail psoriasis (i.e. as an inclusion criterion (Citation14) ()). Also, as in all NMAs, the presence of cross-trial differences, including the design, patient characteristics and outcome selection, may influence the treatment effect and introduce bias in the comparison (Citation37,Citation38). For example, there was some heterogeneity across trials for the minimum required NP score (e.g. NAPSI ≥ 1 vs NAPSI ≥ 6; ), and the datasets originated primarily from dermatologist-led trials (Citation7,Citation9,Citation11‒Citation15), but in some cases from rheumatologist-led studies (Citation10,Citation16). Most trials evaluated the efficacy of treatment on fingernail psoriasis (Citation9‒Citation16), although one study focused on the most affected nail and did not specify fingernail psoriasis (Citation7). In addition, missing data were handled differently in the individual studies (), potentially resulting in an overestimation of the treatment effect in studies using multiple imputation (Citation14) or as observed (Citation7,Citation9) instead of the most conservative non-responder imputation (Citation10‒Citation13,Citation15,Citation16). These differences altogether may affect both the reliability of the results of the NMA and the generalizability to patients with psoriasis treated in the real-world clinical setting. As most of the studies included in this analysis did not require a minimum severity of nail disease as a study entry criterion, the sample size for this analysis was smaller than in the respective clinical trial programs, in turn explaining the low degree of certainty evidenced by large CrIs.
The NMA was also limited to some extent by the scarcity of available clinical trial data (e.g. only one to three randomized trials for each intervention), and this also contributed to the large CrIs. In particular, the treatment effect of infliximab was probably underestimated in the network because of the connection through placebo (from the EXPRESS trial (Citation9)) and a relatively high placebo response for complete nail resolution at week 24 in EXPRESS compared with, for example, the placebo-controlled trial with adalimumab (Citation14). A likely explanation is that patients entering the infliximab trial could have any degree of NP, whereas those entering the adalimumab trial were required to have more severe NP (). The main consequence of the treatment effect being informed only through the placebo-controlled EXPRESS trial is that the treatment effect of infliximab was underestimated (0.8% of patients achieving complete nail clearance) and the 95% CrI was wide (0.0‒8.9). It is noteworthy that patients treated with infliximab in EXPRESS had a 57.2% mean improvement in NAPSI score from baseline to week 24, which was a statistically significant improvement compared with placebo (−4.1%; p < .001) (Supplementary Table S1). In addition, infliximab was superior to placebo in achieving complete nail clearance at week 24 (26.2% vs 5.1%; p < .001). We also conducted an exploratory analysis of 52-week results for nail clearance; however, the line-shaped nature of the network did not allow for the statistical model to converge, and additional long-term study data are needed. Various clinical trials suggest that the maximum effect may not be reached for up to or beyond 52 weeks (Citation7‒Citation11), and further assessment of a maintenance effect would be interesting; however, indirect treatment comparison is restricted by the limited data from long-term randomized trials. Finally, given the absence of a universally accepted and used NP scale (Citation5,Citation17), harmonization on reporting NP outcome measures is urgently needed to increase the comparability of clinical trials and to facilitate the conduct of NMAs. Important strengths of the NMA include the application of robust statistical approaches and the addition of the subgroup of patients with NP, moderate-to-severe psoriasis, and psoriatic arthritis from the SPIRIT-H2H trial (Citation10,Citation16).
In conclusion, the results of this NMA in patients with moderate-to-severe psoriasis and concomitant NP strongly suggest that, among biologic treatment options, the IL-17A inhibitor ixekizumab has the greatest likelihood of achieving complete resolution of NP at week 24‒26, followed in ranking by the IL-17RA inhibitor brodalumab. Findings should be interpreted with a degree of caution in view of the inherent study limitations, including the need for long-term efficacy data. Nevertheless, this analysis serves as a foundation for further studies once more information regarding NP becomes available.
Acknowledgments
The authors would like to acknowledge Greg Plosker and Karen Goa (Rx Communications, Mold, UK) for medical writing assistance with the presentation of this manuscript.
Disclosure statement
Professor Reich has served as advisor and/or paid speaker for and/or participated in clinical trials sponsored by Abbvie, Affibody, Almirall, Amgen, Avillion, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Centocor, Covagen, Dermira, Forward Pharma, Fresenius Medical Care, Galapagos, GlaxoSmithKline, Janssen-Cilag, Kyowa Kirin, Leo, Eli Lilly and Company, Medac, Merck Sharp & Dohme, Novartis, Miltenyi Biotec, Ocean Pharma, Pfizer, Regeneron, Samsung Bioepis, Sanofi, Sun Pharma, Takeda, UCB, Valeant, Xenoport. Professor Conrad has served as scientific adviser and/or clinical study investigator and/or paid speaker for AbbVie, Actelion, Amgen, BMS, Celgene, Galderma, Incyte, Janssen, LEO Pharma, Eli Lilly and Company, MSD, Novartis, Pfizer, Samsung, and UCB. Professor L. E. Kristensen has received grant/research support from UCB, Biogen, Janssen pharmaceuticals, and Novartis; Speakers bureau from Pfizer, AbbVie, Amgen, UCB, Bristol-Myers Squibb, Biogen, MSD, Novartis, Eli Lilly and Company, and Janssen pharmaceuticals. Dr S. D. Smith has received honorariums for serving on industry advisory boards and/or consultancy, teaching, and education activities for Janssen Cilag, Eli Lilly and Company, Novartis, MSD, Abbvie, Sanofi Genzyme, Pfizer, Leo Pharma, Amgen, Biogen, UCB, and BMS. Dr S. D. Dr Smith has also been an investigator of clinical trials sponsored by Eli Lilly and Company, BMS, UCB, Novartis, Abbvie, Sanofi Genzyme, and Regeneron, and is co-editor of the journal Opinions and Progress in Cosmetic Dermatology. Pr L. Puig has perceived consultancy/speaker’s honoraria from and/or participated in clinical trials sponsored by Abbvie, Almirall, Amgen, Baxalta, Biogen, Boehringer Ingelheim, Celgene, Gebro, Janssen, JS BIOCAD, Leo-Pharma, Eli Lilly and Company, Merck-Serono, MSD, Mylan, Novartis, Pfizer, Regeneron, Roche, Sandoz, Samsung-Bioepis, Sanofi, and UCB. Dr P. Rich has acted as principal investigator of clinical trials sponsored by Abbvie, Arcutis, Bristol-Myers Squibb, Dermavant, Eli Lilly and Company, Novartis, Sun Pharma, and UCB, and has participated on a Leo advisory board. C. Sapin, T. Holzkaemper, U. Koppelhus, and C. Schuster are employees of Eli Lilly and Company, Indianapolis, IN, USA.
Additional information
Funding
References
- Rigopoulos D, Stathopoulou A, Gregoriou S. Small molecules and biologics in the treatment of nail psoriasis. Skin Appendage Disord. 2020;6(3):134–141.
- Sobolewski P, Walecka I, Dopytalska K. Nail involvement in psoriatic arthritis. Reumatologia. 2017;55(3):131–135.
- Haneke E. Nail psoriasis: clinical features, pathogenesis, differential diagnoses, and management. Psoriasis. 2017;7:51–63.
- Jiaravuthisan MM, Sasseville D, Vender RB, et al. Psoriasis of the nail: anatomy, pathology, clinical presentation, and a review of the literature on therapy. J Am Acad Dermatol. 2007;57(1):1–27.
- Bardazzi F, Starace M, Bruni F, et al. Nail psoriasis: an updated review and expert opinion on available treatments, including biologics. Acta Derm Venereol. 2019;99(6):516–523.
- Augustin M, Reich K, Blome C, et al. Nail psoriasis in Germany: epidemiology and burden of disease. Br J Dermatol. 2010;163(3):580–585.
- Elewski B, Rich P, Lain E, et al. Efficacy of brodalumab in the treatment of scalp and nail psoriasis: results from three phase 3 trials. J Dermatolog Treat. 2020:1–5.DOI:10.1080/09546634.2020.1749546
- Reich K, Sullivan J, Arenberger P, et al. Secukinumab shows high and sustained efficacy in nail psoriasis: 2.5-year results from the randomized placebo-controlled TRANSFIGURE study. Br J Dermatol. 2020.DOI:10.1111/bjd.19262
- Rich P, Griffiths CE, Reich K, et al. Baseline nail disease in patients with moderate to severe psoriasis and response to treatment with infliximab during 1 year. J Am Acad Dermatol. 2008;58(2):224–231.
- Smolen JS, Mease P, Tahir H, et al. Multicentre, randomised, open-label, parallel-group study evaluating the efficacy and safety of ixekizumab versus adalimumab in patients with psoriatic arthritis naïve to biological disease-modifying antirheumatic drug: final results by week 52. Ann Rheum Dis. 2020;79(10):1310–1319.
- Wasel N, Thaçi D, French LE, et al. Ixekizumab and ustekinumab efficacy in nail psoriasis in patients with moderate-to-severe psoriasis: 52-week results from a phase 3, head-to-head study (IXORA-S). Dermatol Ther. 2020;10(4):663–670.
- Blauvelt A, Papp K, Gottlieb A, et al. IXORA-R study group. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 12-week efficacy, safety and speed of response from a randomized, double-blinded trial. Br J Dermatol. 2020;182(6):1348–1358.
- Blauvelt A, Leonardi C, Elewski B, et al. A head-to-head comparison of ixekizumab versus guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomised, double-blinded trial. Br J Dermatol. 2020.DOI:10.1111/bjd.19509
- Elewski BE, Okun MM, Papp K, et al. Adalimumab for nail psoriasis: efficacy and safety from the first 26 weeks of a phase 3, randomized, placebo-controlled trial. J Am Acad Dermatol. 2018;78(1):90–99.
- Foley P, Gordon K, Griffiths CEM, et al. Efficacy of guselkumab compared with adalimumab and placebo for psoriasis in specific body regions: a secondary analysis of 2 randomized clinical trials. JAMA Dermatol. 2018;154(6):676–683.
- Mease PJ, Smolen JS, Behrens F, et al. A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naïve patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann Rheum Dis. 2020;79(1):123–131.
- Busard CI, Nolte JYC, Pasch MC, et al. Reporting of outcomes in randomized controlled trials on nail psoriasis: a systematic review. Br J Dermatol. 2018;178(3):640–649.
- Kyriakou A, Patsatsi A, Sotiriadis D. Biologic agents in nail psoriasis: efficacy data and considerations. Expert Opin Biol Ther. 2013;13(12):1707–1714.
- European Medicines Agency: adalimumab (Humira®) summary of product characteristics [Internet]. Amsterdam (The Netherlands): EMA; 2020 [cited 2020 October 15]. Available from: https://www.ema.europa.eu/en/documents/product-information/humira-epar-product-information_en.pdf
- European Medicines Agency: brodalumab (Kyntheum®) summary of product characteristics [Internet]. Amsterdam (The Netherlands): EMA; 2020 [cited 2020 October 15]. Available from: https://www.ema.europa.eu/en/documents/product-information/kyntheum-epar-product-information_en.pdf
- European Medicines Agency: guselkumab (Tremfya®) summary of product characteristics [Internet]. Amsterdam (The Netherlands): EMA; 2020 [cited 2020 October 15]. Available from: https://www.ema.europa.eu/en/documents/product-information/tremfya-epar-product-information_en.pdf
- European Medicines Agency: infliximab (Remicade®) summary of product characteristics [Internet]. Amsterdam (The Netherlands): EMA; 2020 [cited 2020 October 15]. Available from: https://www.ema.europa.eu/en/documents/product-information/remicade-epar-product-information_en.pdf
- European Medicines Agency: ixekizumab (Taltz®) summary of product characteristics [Internet]. Amsterdam (The Netherlands): EMA; 2020 [cited 2020 October 15]. Available from: https://www.ema.europa.eu/en/documents/product-information/taltz-epar-product-information_en.pdf
- European Medicines Agency: ustekinumab (Stelara®) summary of product characteristics [Internet]. Amsterdam (The Netherlands): EMA; 2020 [cited 2020 October 15]. Available from: https://www.ema.europa.eu/en/documents/product-information/stelara-epar-product-information_en.pdf
- Reich K, Kristensen LE, Smith SD, et al. Ixekizumab shows early and sustained resolution of nail psoriasis in patients with psoriatic arthritis and moderate-to-severe psoriasis: 52-week results from a multicentre, randomised, open-label, rater-blinded study (SPIRIT-H2H). Poster FC02.06-846 presented at: 29th European Academy of Dermatology and Venereology (EADV) Congress; 2020 Oct 28‒November 1; Vienna, Austria.
- Ortonne JP, Paul C, Berardesca E, et al. A 24-week randomized clinical trial investigating the efficacy and safety of two doses of etanercept in nail psoriasis. Br J Dermatol. 2013;168(5):1080–1087.
- Szebényi J, Gede N, Hegyi P, et al. Efficacy of biologics targeting tumour necrosis factor-alpha, interleukin-17 -12/23, -23 and small molecules targeting JAK and PDE4 in the treatment of nail psoriasis: a network meta-analysis. Acta Derm Venereol. 2020;100(18):adv00318.
- Armstrong AW, Puig L, Joshi A, et al. Comparison of biologics and oral treatments for plaque psoriasis: a meta-analysis. JAMA Dermatol. 2020;156(3):258–269.
- Sbidian E, Chaimani A, Afach S, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2020;1:CD011535.
- McGonagle D, Ash Z, Dickie L, et al. The early phase of psoriatic arthritis. Ann Rheum Dis. 2011;70(1):i71–i76.
- Scher JU, Ogdie A, Merola JF, et al. Preventing psoriatic arthritis: focusing on patients with psoriasis at increased risk of transition. Nat Rev Rheumatol. 2019;15(3):153–166.
- Rusk AM, Fleischer AB. Jr. In psoriasis treatment, greater improvement in skin severity predicts greater improvement in nail severity. J Dermatolog Treat. 2020:1–4.DOI:10.1080/09546634.2020.1720578
- Griffiths CE, Reich K, Lebwohl M, et al. UNCOVER-2 and UNCOVER-3 investigators. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386(9993):541–551.
- Reich K. Approach to managing patients with nail psoriasis. J Eur Acad Dermatol Venereol. 2009;23(1):15–21.
- Rigopoulos D, Gregoriou S, Stratigos A, et al. Evaluation of the efficacy and safety of infliximab on psoriatic nails: an unblinded, nonrandomized, open-label study. Br J Dermatol. 2008;159(2):453–456.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Kawalec P, Holko P, Moćko P, et al. Comparative effectiveness of abatacept, apremilast, secukinumab and ustekinumab treatment of psoriatic arthritis: a systematic review and network meta-analysis. Rheumatol Int. 2018;38(2):189–201.
- McInnes IB, Nash P, Ritchlin C, et al. Secukinumab for psoriatic arthritis: comparative effectiveness versus licensed biologics/apremilast: a network meta-analysis. J Comp Eff Res. 2018;7(11):1107–1123.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318–1328.