Abstract
Background
The topical corticosteroid halobetasol propionate (HP) and retinoid tazarotene (TAZ) are effective in psoriasis treatment. Fixed-combination HP 0.01%/TAZ 0.045% lotion has demonstrated efficacy and safety in moderate-to-severe plaque psoriasis.
Objective
To investigate the maintenance of therapeutic effects after cessation of once-daily HP/TAZ treatment.
Methods
In two phase 3 studies (NCT02462070; NCT02462122), adults with moderate-to-severe psoriasis received HP/TAZ for 8 weeks. Data at week 12 were analyzed post hoc to evaluate posttreatment maintenance of treatment success (clear/almost clear skin), improvements in signs of psoriasis (erythema, plaque elevation, scaling), and reductions in affected body surface area (BSA). In a 52-week open-label study (NCT02462083), participants stopped HP/TAZ treatment after achievement of treatment success; data were analyzed post hoc to assess time to retreatment.
Results
Across all studies, most participants who achieved treatment success maintained this effect for at least one month posttreatment. Treatment effects were similarly maintained for improvements in signs of psoriasis and reductions in BSA. Some participants continued to improve after cessation of treatment. Maintenance of treatment success and time to retreatment were greater for participants who achieved clear skin.
Conclusion
HP/TAZ lotion provides therapeutic effects that persist after treatment cessation, supporting its use in long-term management of plaque psoriasis.
Keywords:
Introduction
Psoriasis is a lifelong inflammatory skin condition in which periods of remission are punctuated by relapse or disease flare. For approximately 80% of patients with psoriasis, topical therapies are the first line of treatment (Citation1). Prolonged use of many topical therapies is associated with safety concerns, however, posing a major challenge to the long-term management of this chronic disease. Thus, there is an ongoing need for psoriasis treatments providing therapeutic benefits that persist long after treatment cessation, thereby increasing the duration of disease remission.
Topical corticosteroids (TCS) are a mainstay of psoriasis treatment, providing rapid, anti-inflammatory actions (Citation2). However, despite the chronic nature of psoriasis, safety concerns limit continuous use of most TCS to 2–4 weeks (Citation3) and only one TCS – halobetasol propionate (HP) 0.01% lotion – is approved for 8 weeks of continuous use (Citation4). Moreover, discontinuation of TCS use is associated with relapse or rebound effects (Citation3). The retinoid tazarotene (TAZ) is as efficacious as topical corticosteroids, with therapeutic benefits persisting for up to 12 weeks after treatment cessation (Citation5). However, TAZ monotherapy may be underutilized in the clinic due to irritation concerns (Citation6). The combination of TAZ with a potent-to-superpotent TCS has been shown to be more efficacious and to provide greater duration of therapeutic effects after treatment cessation than either TAZ or TCS monotherapy, and is associated with fewer safety concerns (Citation2,Citation7,Citation8).
A fixed-combination HP 0.01%/TAZ 0.045% lotion (HP/TAZ; Duobrii®; Ortho Dermatologics, Bridgewater, NJ, USA) was developed to leverage the beneficial properties of both active ingredients, while mitigating safety concerns, in a single easy-to-use formulation (Citation6). HP/TAZ lotion provides greater permeation efficiency of the active ingredients compared with higher-dose HP or TAZ creams (alone or layered) (Citation6) with reduced potential for irritation and other adverse events (Citation9). Overall efficacy and safety results for HP/TAZ lotion from two phase 3 randomized clinical trials and a 52-week, long-term open-label study have been presented in detail (Citation10–13). The objective of the post hoc analyses presented here was to evaluate the maintenance of therapeutic effects with HP/TAZ lotion by assessing efficacy 4 weeks posttreatment in the phase 3 studies, and the time to retreatment after HP/TAZ cessation in the long-term open-label study.
Materials and methods
Overview of studies and participants
All studies were performed at multiple study centers in the United States in accordance with ethical principles from the Declaration of Helsinki, International Conference on Harmonization guidelines, Good Clinical Practice, and local regulatory requirements. Institutional review boards or ethics panels approved the protocols at all sites. All participants provided written informed consent prior to any study-related procedures, including permission for their photos to be used for non-promotional purposes.
For all studies, key inclusion criteria were an age of ≥18 years and diagnosis of moderate-to-severe plaque psoriasis, defined as an Investigator’s Global Assessment (IGA) score of 3 or 4 (5-point scale; 0 = clear, 1 = almost clear; 2 = mild; 3 = moderate; 4 = severe) and an affected body surface area (BSA) of 3–12%, excluding involvement of the face, scalp, palms, soles, axillae, and intertriginous areas. CeraVe® hydrating cleanser and CeraVe® moisturizing lotion (L’Oreal, NY) were provided for optional use as needed for moisturization/cleaning of the skin.
Pooled phase 3 studies
Data from 2 multicenter, randomized, double-blind, vehicle-controlled phase 3 studies (NCT02462070; NCT02462122) were pooled for analysis. Study designs have been described previously (Citation10,Citation11); briefly, participants were randomized (2:1) to receive HP/TAZ or vehicle lotion once daily for 8 weeks with a 4-week posttreatment follow-up visit at week 12.
The primary efficacy endpoint in these studies was treatment success, defined as a ≥2-grade improvement from baseline IGA score and a score of 0 (clear) or 1 (almost clear) at week 8. Secondary efficacy endpoints included change from baseline in affected BSA and treatment success at the target lesion for individual signs of psoriasis (erythema, plaque elevation, and scaling), defined as a ≥2-grade improvement from baseline in severity (5-point scale; 0 = none, 4 = severe).
Multiple shift analyses were performed to determine participants who maintained or gained treatment effects at week 12 (4 weeks posttreatment). For treatment success (overall and individual signs of psoriasis), maintenance of treatment effect was defined as the percentage of participants achieving success at week 8 who also achieved success at posttreatment follow-up (week 12); gain of treatment effect was defined as the percentage of participants who had not achieved success at week 8 but who achieved success posttreatment. Shifts in IGA score from week 8 to week 12 were also measured to gauge posttreatment maintenance or improvement of psoriasis severity. Maintenance of affected BSA was assessed as the percentage of participants whose BSA remained the same or decreased from week 8 to week 12. All analyses were descriptive in nature; as such, statistical comparisons were not performed.
Long-term open-label phase 3 study
The details of this 1-year, multicenter, open-label study (NCT02462083) have been described previously (Citation12). Briefly, participants were treated with HP/TAZ lotion once daily for 8 weeks; for the remainder of the 52-week study, treatment was used intermittently, as needed, in 4-week intervals. At week 8 and all subsequent study visits (every 4 weeks), participants were evaluated for treatment success, defined as an IGA score of 0 (clear) or 1 (almost clear). Those who achieved treatment success stopped treatment for 4 weeks; those who did not achieve treatment success continued treatment for 4 weeks. At week 12, participants who had not achieved ≥1-grade improvement from baseline IGA were discontinued from the study. Maximum continuous exposure during the study was 24 weeks; if 24 weeks of continuous treatment had been received at any point in the study, the participant needed an IGA score of 0 or 1 to continue the study.
Participants enrolled for at least 8 weeks in this study and who stopped HP/TAZ therapy after achieving treatment success were assessed for maintenance of treatment success, defined as the time to retreatment with HP/TAZ. This was evaluated separately for participants who achieved an IGA score of 0 (clear) and for those who achieved an IGA score of 1 (almost clear). Maintenance of effect was measured as the cumulative percentage of participants who remained treatment successes for more than 4, 8, 12, and 16 weeks, or until end of study (i.e. no relapse) after cessation of HP/TAZ treatment. As with the pooled phase 3 studies, all analyses were descriptive in nature and statistical comparisons were not performed.
Results
Posttreatment maintenance or gain of treatment effect with HP/TAZ lotion (pooled phase 3 studies)
A total of 276 participants were randomized to HP/TAZ lotion treatment and included in the pooled intent-to-treat (ITT) population. Demographics, baseline characteristics, and disposition of these participants have been described previously (Citation10,Citation11). Briefly, mean age was 50.0 years, 36.6% were female, 84.1% were White, and 71.7% were not Hispanic or Latino; at baseline, 85.9% had an IGA score of 3 (moderate), and mean BSA was 6.0%.
In the overall study population, 40.6% of HP/TAZ-treated participants achieved treatment success at week 8 and 33.3% achieved treatment success at week 12 (using multiple imputation of missing values) (Citation11). Maintenance and gain of treatment effect were assessed for 234 participants for whom week 8 and week 12 efficacy results were available (). Of those who had achieved treatment success at week 8 (n = 101), 62.4% maintained treatment success at week 12 (4 weeks posttreatment); of those who had not achieved treatment success at week 8 (n = 133), 18 participants (13.5%) gained treatment success at week 12. Representative images of participants who experienced posttreatment maintenance or gain of treatment success are shown in and , respectively.
Figure 1. Maintenance or gain of treatment success 4 weeks posttreatment with HP/TAZ lotion (pooled phase 3 studies). Treatment success was defined as ≥2-grade improvement from baseline IGA and a score of 0 (clear) or 1 (almost clear). Data shown for Week 12 treatment success, stratified by participants with or without week 8 treatment success. HP/TAZ: halobetasol propionate 0.01% and tazarotene 0.045%; IGA: Investigator’s Global Assessment.
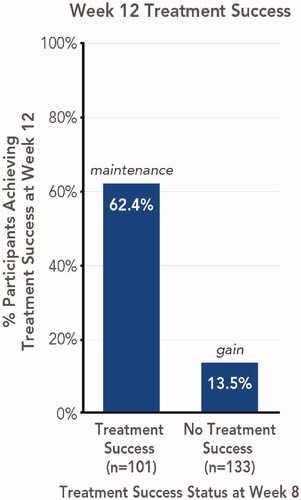
Figure 2. Posttreatment maintenance of treatment success with HP/TAZ lotion. Treatment success was defined as ≥2-grade improvement from baseline IGA and a score of 0 (clear) or 1 (almost clear). Representative images from a participant with target lesion on the lower back. Participant IGA: baseline, 3; weeks 8 and 12, 1. Participant BSA: baseline, 11%; week 8, 11%; week 12, 3%. BSA: body surface area; HP/TAZ: halobetasol propionate 0.01% and tazarotene 0.045%; IGA: Investigator’s Global Assessment.
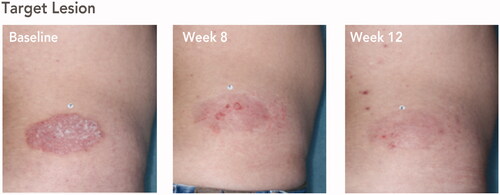
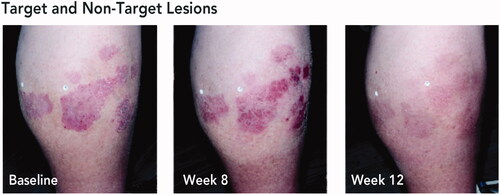
Figure 3. Posttreatment gain of treatment success with HP/TAZ lotion. Treatment success was defined as ≥2-grade improvement from baseline IGA and a score of 0 (clear) or 1 (almost clear). Representative images from a participant with target and non-target lesions on the lower leg. aLesions indicated by white stickers on participant’s leg; target lesion indicated by sticker with letter ‘T’. Participant IGA: baseline, 3; week 8, 2; week 12, 0. Participant BSA: baseline, 6%; week 8, 4%; week 12, 0%. BSA: body surface area; HP/TAZ: halobetasol propionate 0.01% and tazarotene 0.045%; IGA: Investigator’s Global Assessment.
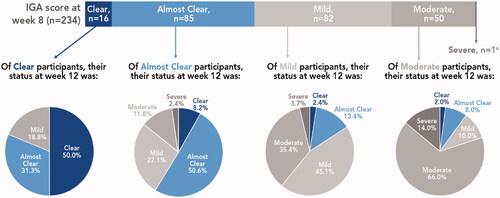
Regardless of treatment success outcomes at week 8, the majority of participants experienced posttreatment maintenance or improvement in psoriasis severity at 4 weeks posttreatment (week 12; ). Of the 16 participants who had an IGA score of clear at week 8, half maintained clear at week 12 and 31.3% were almost clear; overall, over 80% remained treatment successes. Among the 85 participants who were almost clear at week 8, half maintained almost clear and 8.2% improved to clear at week 12; overall, almost 60% of these participants remained treatment successes. Improvements in psoriasis severity were also seen in participants who were mild or moderate at week 8. Of 82 participants who were mild at week 8, 15.8% improved to clear or almost clear (45% maintained mild); of 50 participants who were moderate at week 8, 10% improved to clear or almost clear and 10% improved to mild (two-thirds maintained moderate). One participant was severe at week 8 and remained severe at week 12.
Figure 4. Posttreatment IGA severity by week 8 results (pooled phase 3 studies). aRemained severe at week 12. IGA assessed on a 5-point scale: 0 = clear, 1 = almost clear; 2 = mild; 3 = moderate; 4 = severe. Treatment success defined as ≥2-grade improvement from baseline IGA and a score of 0 (clear) or 1 (almost clear). IGA: Investigator’s Global Assessment.
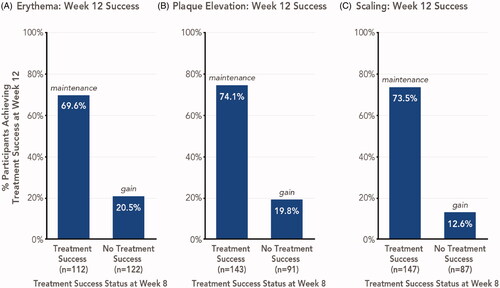
For individual signs of psoriasis (erythema, plaque elevation, and scaling) assessed in the overall study population, treatment success was achieved by 47.0–61.2% of participants at week 8 and by 43.4–51.4% of participants at week 12 (Citation11). Similar to overall treatment success, the majority (69.6–74.1%) of participants who achieved treatment success in signs of psoriasis at week 8 maintained this effect 4 weeks posttreatment; among participants who did not achieve success at week 8, 12.6–20.5% gained success at week 12 ().
Figure 5. Signs of psoriasis maintenance or gain of treatment success 4 weeks posttreatment with HP/TAZ lotion (pooled phase 3 studies). Treatment success was defined as ≥2-grade improvement from baseline in individual signs of psoriasis at the target lesion. Data shown for Week 12 success, stratified by participants with or without week 8 success. HP/TAZ: halobetasol propionate 0.01% and tazarotene 0.045%; IGA: Investigator’s Global Assessment.
Overall, there was a 37.6% reduction from baseline in mean BSA among HP/TAZ-treated participants at week 8, and a 34.1% reduction from baseline at week 12 (Citation11). BSA involvement was maintained or reduced (improved) from week 8 to week 12 in almost three-quarters (73.5%) of participants.
Time to retreatment after cessation of treatment with HP/TAZ lotion (long-term open-label phase 3 study)
In this study, 555 participants were treated with HP/TAZ lotion and 550 had post-baseline safety data. Detailed participant demographics, baseline characteristics, and disposition have been described previously (Citation12). Participants were generally similar to those in the pooled phase 3 studies. Mean age was 51.9 years, 34.4% were female, 86.0% were White, and 74.4% were not Hispanic or Latino; at baseline, 86.5% had an IGA score of 3 (moderate), and mean BSA was 5.6%.
Overall, 318 participants (57.8%) achieved treatment success (IGA score of 0 [clear] or 1 [almost clear]) at some point during this study (Citation12). Consistent with the results of the pooled phase 3 studies, the majority of participants experienced maintenance of treatment success after cessation of HP/TAZ treatment. Of 226 participants enrolled at least 8 weeks in the study and who stopped therapy after achieving treatment success, time to retreatment was more than 4 weeks for 55.3%, more than 8 weeks for 28.3%, more than 12 weeks for 19.5%, and more than 16 weeks for 12.4%; 6.6% did not relapse for the duration of the study and therefore did not require any retreatment (Citation12). Mean and median times to relapse were 61.0 and 30.0 days, respectively.
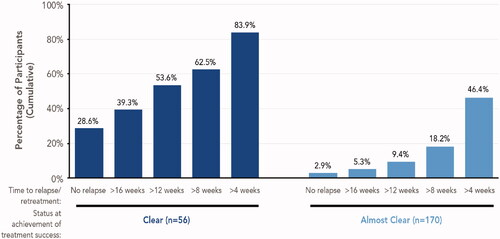
Per study protocol, participants stopped HP/TAZ treatment (until their next assessment) upon achievement of clear or almost clear skin (Citation14). Duration of remission may have been greater, however, if all participants had been allowed to continue HP/TAZ treatment until achieving clear skin, which better aligns with dosage and administration guidelines in the prescribing information (‘discontinue treatment when control is achieved’) (Citation14). To evaluate this possibility, we performed separate analyses of maintenance of treatment success for participants who achieved clear skin and participants who achieved almost clear skin (). Of the 56 (24.8%) participants who achieved clear skin, 83.9% did not require retreatment for more than 4 weeks, 62.5% for more than 8 weeks, 53.6% for more than 12 weeks, and 39.3% for more than 16 weeks; 28.6% did not relapse for the duration of the study and therefore did not require any retreatment. Mean and median times to relapse for these participants were 104.7 days and 85.0 days, respectively. Among the 170 (75.2%) participants who achieved treatment success with almost clear skin, the duration of posttreatment effect was shorter than for participants who achieved clear skin ().
Figure 6. Maintenance of treatment success (long-term open-label study). Data shown for participants enrolled at least 8 weeks in the study and who stopped therapy after achieving treatment success, defined as an IGA score of 0 (clear) or 1 (almost clear). Cumulative data shown; within each subgroup, participants included in each bar are not mutually exclusive. Number of weeks to retreatment based on a 7-day week: 4 weeks = 28 days; 8 weeks = 56 days; 12 weeks = 84 days; 16 weeks = 112 days. IGA: Investigator’s Global Assessment.
Discussion
In the treatment of psoriasis, the importance of providing sustained therapeutic effects and increasing time in remission cannot be understated, as it is a chronic disease that frequently recurs after discontinuation of treatment (Citation2). Lower rates of relapse and disease flare are associated with improved quality of life and lower levels of productivity loss (Citation15), underscoring the ongoing need for therapies that provide durable therapeutic benefits. Increasing posttreatment maintenance of effects would have the added benefit of reducing overall drug exposure, thus potentially reducing treatment-related safety and tolerability concerns. However, in a survey of patient satisfaction, only one-third of respondents currently using topical treatments rated their treatment positively (good/very good/excellent) for not losing effectiveness over time or for making them feel like their psoriasis was under control (Citation16). Indeed, patients are frequently dissatisfied with their psoriasis treatment, despite the expansion of treatment options available, because therapies too often fail to achieve or maintain skin clearance (Citation17).
Combining topical therapies can improve outcomes in the treatment of psoriasis. In a clinical trial, fixed-combination HP/TAZ lotion provided greater efficacy in the treatment of moderate-to-severe psoriasis than HP or TAZ alone (Citation18). The present analyses assessed the maintenance of treatment effects after cessation of HP/TAZ treatment. In two phase 3 studies, the majority of participants who achieved treatment success after 8 weeks of treatment continued to experience treatment success 4 weeks posttreatment. Additionally, some HP/TAZ-treated participants who did not achieve treatment success after 8 weeks continued to improve and achieved treatment success 4 weeks posttreatment. Similar findings were observed for psoriasis severity, signs of psoriasis, and BSA involvement. In a 52-week open-label study, the majority of participants experiencing treatment success did not require retreatment for over 4 weeks, with many experiencing much longer (>16 weeks) maintenance of treatment success.
The posttreatment duration of disease remission can be increased by treating psoriasis until achievement of clear skin. In the pooled phase 3 clinical trials of HP/TAZ lotion, a greater percentage of participants who achieved clear skin maintained treatment success 4 weeks posttreatment compared with participants who achieved almost clear skin (81.3% versus 58.7%, respectively). In the long-term open-label study, the median time to relapse/retreatment was 55 days longer for participants who achieved clear skin versus those who achieved treatment success (clear or almost clear skin). These efficacy data align with patient-reported outcomes, in which individuals with clear skin are more likely to report no impact of psoriasis on quality of life than those with almost clear skin (Citation17). Moreover, drug efficacy can have a strong impact on patients’ preferences for and satisfaction with topical psoriasis therapies (Citation19), which in turn can profoundly affect adherence to treatment and overall treatment success. Thus, treating to clear skin and increasing the posttreatment duration of therapeutic effects may improve patient satisfaction and quality of life.
One challenge to the interpretation of these findings is that protocol-defined treatment regimens in the clinical trials do not represent real-world use of HP/TAZ lotion. Treatment until achievement of clear skin reflects the goals of both patients and clinicians in a real-world setting, and the prescribing information for HP/TAZ lotion indicates that daily treatment should continue until control is achieved, without providing any limitations on the duration of treatment (Citation14). In the long-term open-label study, however, participants were required to stop treatment if they achieved almost clear skin. Allowing treatment to continue until achievement of clear skin would potentially have yielded a longer maintenance of treatment effect. This is supported by the comparison of participants who achieved clear versus almost clear, in which a greater percentage of participants with clear skin did not require retreatment and were able to maintain treatment success for longer periods of time. In contrast to the flexible dosing of the long-term study, participants in the pooled phase 3 studies were required to stop treatment after a fixed 8-week treatment period, regardless of treatment success status; however, it is possible that more participants could have achieved treatment success with continued treatment past 8 weeks.
The fixed 8-week treatment regimen in the pooled phase 3 studies may also have led to inflated incidences of adverse events (AEs). Many participants in these studies achieved treatment success within the first 2–4 weeks of treatment but were required to continue treatment through 8 weeks (Citation10,Citation11); this continued treatment could have increased the likelihood of irritation and other adverse effects of prolonged TAZ or TCS exposure. In these studies, most AEs were of mild or moderate severity and the most common treatment-related AEs were contact dermatitis, pruritus, and application site pain (Citation10,Citation11). With longer-term treatment in the open-label study, rates of these AEs decreased to less than 1% after week 36 (Citation12,Citation13). Incidences of skin atrophy with HP/TAZ lotion were rare, even with up to 24 weeks of continuous treatment in the long-term open-label study (Citation10–13). HP/TAZ treatment was also associated with reductions in the severity of itching, dryness, and burning/stinging skin symptoms across the phase 3 and open-label studies (Citation10,Citation11). Although HP/TAZ lotion has demonstrated a favorable safety profile with up to 24 continuous weeks of use, treatment-related irritation remains a concern for clinical use (Citation9). Irritation can be mitigated by temporarily interrupting drug use (i.e. drug holiday) until irritation signs and symptoms have subsided. In addition, use of moisturizers concurrent with treatment and/or during periods of irritation-related treatment cessation can enhance epidermal barrier function and counteract adverse effects of prolonged TAZ and TCS exposure (Citation20,Citation21).
A benefit of the 52-week open-label study is that it allowed for the evaluation of up to 24 weeks of continuous treatment with a superpotent TCS with long-term follow-up; this is especially noteworthy given the lack of long-term (>6 months) clinical trials evaluating posttreatment maintenance of efficacy for TCS and other topical psoriasis therapies (Citation22). In one other 52-week open-label study, a fixed-combination foam – containing the corticosteroid betamethasone dipropionate and the vitamin D analog calcipotriene (Cal/BD) – was assessed for time in remission after cessation of once-daily treatment (Citation23). Participants were treated to clear or almost clear skin and then either stopped treatment entirely until relapse or continued treatment with twice-weekly Cal/BD to prevent relapse. Among participants who stopped treatment entirely, the median time to first relapse was 30 days, similar to the overall median time to retreatment in the long-term open-label study of HP/TAZ lotion; prophylactic, twice-weekly Cal/BD treatment extended this time to first relapse to 56 days. In comparison, participants who stopped HP/TAZ treatment entirely after achievement of clear skin had a median time to relapse of 85 days. Thus, treatment to clear skin with HP/TAZ lotion was associated with a duration of remission that was longer than that seen with continued bi-weekly treatment with Cal/BD foam. This difference highlights both the importance of treatment to clear skin and the unique mechanism of action by which TAZ may contribute to the maintenance of treatment effects. TAZ addresses the pathophysiology of psoriasis by normalizing keratinocyte proliferation/differentiation, reducing immune cell infiltration, and decreasing the expression of proinflammatory markers, and is thus thought to return skin to a quiescent, ‘prelesional’ state (Citation24,Citation25).
Conclusion
In clinical trials, fixed-combination HP/TAZ lotion has consistently demonstrated greater efficacy over placebo and a favorable safety profile (Citation10,Citation11). The beneficial effects of HP/TAZ lotion were maintained for at least one month in the majority of participants who achieved clear or almost clear skin, and some participants gained treatment success after cessation of treatment. These results support the use of fixed-combination HP/TAZ lotion for the long-term treatment and management of plaque psoriasis.
Acknowledgements
Medical writing support was provided by Prescott Medical Communications Group (Chicago, IL) with financial support from Ortho Dermatologics. Ortho Dermatologics is a division of Bausch Health US, LLC.
Disclosure statement
M.G. Lebwohl is an employee of Mount Sinai and receives research funds from: AbbVie, Amgen, Arcutis, Boehringer Ingelheim, Dermavant, Eli Lilly, Incyte, Janssen Research & Development, LLC, LEO Pharma, Ortho Dermatologics, Pfizer, and UCB; is a consultant for Aditum Bio, Allergan, Almirall, Arcutis, Inc., Avotres Therapeutics, BirchBioMed Inc., BMD skincare, Boehringer-Ingelheim, Bristol-Myers Squibb, Cara Therapeutics, Castle Biosciences, Corrona, Dermavant Sciences, Evelo, Facilitate International Dermatologic Education, Foundation for Research and Education in Dermatology, Inozyme Pharma, Kyowa Kirin, LEO Pharma, Meiji Seika Pharma, Menlo, Mitsubishi, Neuroderm, Pfizer, Promius/Dr. Reddy’s Laboratories, Serono, Theravance, and Verrica.
L. Stein Gold has served as investigator/consultant or speaker for Ortho Dermatologics, LEO Pharma, Dermavant, Incyte, Novartis, AbbVie, Pfizer, Sun Pharma, UCB, Arcutis and Lilly.
J.Q. Del Rosso has served as a consultant, investigator, and/or speaker for Ortho Dermatologics, Abbvie, Amgen, Arcutis, Dermavant, EPI Heath, Galderma, Incyte, LEO Pharma, Lilly, MC2 Therapeutics, Pfizer, Sun Pharma, and UCB.
L. Green has served as consultant, speaker, and/or investigator for Arcutis, AbbVie, Amgen, Celgene, Dermavant, Janssen, Lilly, MC2, Novartis, Ortho Dermatologics, Sienna, Sun Pharma, and UCB.
A. Jacobson is an employee of Ortho Dermatologics and may hold stock and/or stock options in its parent company.
References
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643–659.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432–470.
- Bagel J, Thibodeaux QG, Han G. Halobetasol propionate for the management of psoriasis. Cutis. 2020;105:92–96. E94.
- Bryhali [package insert]. Bridgewater (NJ): Ortho Dermatologics; 2018.
- Weinstein GD, Koo JY, Krueger GG, et al., Tazarotene Cream Clinical Study Group. Tazarotene cream in the treatment of psoriasis: two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48:760–767.
- Tanghetti EA, Stein Gold L, Del Rosso JQ, et al. Optimized formulation for topical application of a fixed combination halobetasol/tazarotene lotion using polymeric emulsion technology. J Dermatolog Treat. 2019.
- Lebwohl MG, Breneman DL, Goffe BS, et al. Tazarotene 0.1% gel plus corticosteroid cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;39:590–596.
- Koo JY, Martin D. Investigator-masked comparison of tazarotene gel q.d. plus mometasone furoate cream q.d. vs. mometasone furoate cream b.i.d. in the treatment of plaque psoriasis. Int J Dermatol. 2001;40:210–212.
- Del Rosso JQ, Kircik L, Lin T, et al. Halobetasol 0.01%/tazarotene 0.045% fixed-combination lotion in the treatment of plaque psoriasis: sensitization and irritation potential. J Clin Aesthet Dermatol. 2019;12:11–15.
- Stein Gold L, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287–293.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855–861.
- Lebwohl MG, Stein Gold L, Papp K, et al. Long-term safety and efficacy of a fixed-combination halobetasol propionate 0.01%/tazarotene 0.045% lotion in moderate-to-severe plaque psoriasis: phase 3 open-label study. J Eur Acad Dermatol Venereol. 2021.
- Lebwohl MG, Sugarman JL, Gold LS, et al. Long-term safety results from a phase 3 open-label study of a fixed combination halobetasol propionate 0.01% and tazarotene 0.045% lotion in moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2019;80:282–285.
- Duobrii [package insert]. Bridgewater (NJ): Ortho Dermatologics; 2020.
- Korman NJ, Zhao Y, Roberts J, et al. Impact of psoriasis flare and remission on quality of life and work productivity: a real-world study in the USA. Dermatol Online J. 2016;22:13030/qt4vb7q7rr.
- [Data on file]. Bridgewater (NJ): Ortho Dermatologics; 2021.
- Takeshita J, Callis Duffin K, Shin DB, et al. Patient-reported outcomes for psoriasis patients with clear versus almost clear skin in the clinical setting. J Am Acad Dermatol. 2014;71:633–641.
- Sugarman JL, Gold LS, Lebwohl MG, et al. A phase 2, multicenter, double-blind, randomized, vehicle controlled clinical study to assess the safety and efficacy of a halobetasol/tazarotene fixed combination in the treatment of plaque psoriasis. J Drugs Dermatol. 2017;16:197–204.
- Svendsen MT, Feldman SR, Tiedemann SN, et al. Psoriasis patient preferences for topical drugs: a systematic review. J Dermatolog Treat. 2019.
- Thiboutot D, Del Rosso JQ. Acne vulgaris and the epidermal barrier. J Clin Aesthet Dermatol. 2013;6:18–24.
- Del Rosso JQ, Cash K. Topical corticosteroid application and the structural and functional integrity of the epidermal barrier. J Clin Aesthet Dermatol. 2013;6:20–27.
- Segaert S, Calzavara-Pinton P, de la Cueva P, et al. Long-term topical management of psoriasis: the road ahead. J Dermatolog Treat. 2020.
- Lebwohl M, Kircik L, Lacour JP, et al. Twice-weekly topical calcipotriene/betamethasone dipropionate foam as proactive management of plaque psoriasis increases time in remission and is well tolerated over 52 weeks (PSO-LONG trial). J Am Acad Dermatol. 2020.
- Chandraratna RA. Tazarotene: the first receptor-selective topical retinoid for the treatment of psoriasis. J Am Acad Dermatol. 1997;37:S12–S17.
- Tanghetti E, Lebwohl M, Stein Gold L. Tazarotene revisited: safety and efficacy in plaque psoriasis and its emerging role in treatment strategy. J Drugs Dermatol. 2018;17:1280–1287.
