Abstract
Psoriasis is a common chronic and complex inflammatory skin disease that affects over 125 million people worldwide. Management of psoriasis in daily clinical practice in Saudi Arabia is variable. Local preferences for management differ, which may have a bearing on the treatment selection. Biologic therapy is now a well-established strategy for managing moderate-to-severe plaque psoriasis. There is a clear need for national consensus statements due to the extended role and high availability of literature on these agents. As a result of an initiative of the Ministry of Health, a multidisciplinary expert panel of dermatologists and pharmacists with practical experience in the clinical management of psoriasis were invited to be part of a work group to update the previous practical guidelines on the biologic treatment of psoriasis published in the Journal of Dermatological Treatment, 2014. The overall aim of this consensus document is to deliver evidence-based recommendations on the use, screening, and monitoring of biologic therapy in patients with moderate-to-severe plaque psoriasis. These recommendations also address the use of biologic therapy in special patient populations. They were developed after rigorous evaluation of existing international guidelines as well as the latest emerging evidence. Updates of the present consensus document will be provided as needed to incorporate new data or agents.
Introduction
Background
Psoriasis is a common, chronic, inflammatory skin disease. The worldwide prevalence is about 2%, but this differs between countries (Citation1). Its prevalence tends to be lower in Asian and some African populations, and higher (up to 11%) in Caucasian and Scandinavian populations (Citation2–6). At present, psoriasis is recognized as a genetically determined, inflammatory T-cell mediated systemic disease. It affects skin, nails, scalp, and joints with several comorbidities which include cardiovascular diseases, metabolic syndrome, inflammatory bowel disease, uveitis, and psychological changes. Psoriasis has an unpredictable course. It can arise at any age and is most common in the age group of 20–30 and 50–69 years (Citation5,Citation7,Citation8). In 80% of cases, psoriasis is mild or moderate and adequately treated with topical corticosteroids, vitamin D analogs, and phototherapy. One-fifth of patients suffer from severe psoriasis, requiring systemic drugs, such as acitretin, methotrexate, or biologic therapies (Citation9). Biologics are complex engineered molecules including monoclonal antibodies and receptor fusion proteins that are different from conventional systemic therapies in that they target specific inflammatory pathways. Currently available biologics for psoriasis target two pathways crucial in the development and chronicity of the psoriatic plaque: the IL-23/Th17 axis and TNF-α-signaling (Citation3). They are administered subcutaneously or intravenously on different schedules.
Purpose, aims, and scope
Many issues within the management of psoriasis in daily clinical practice are the subject of debate and not addressed by evidence-based medicine. Biologic therapy is now a well-established strategy for managing moderate-to-severe plaque psoriasis. There is a clear need for national consensus recommendations due to the extended role and high availability of literature on these agents. As a result of an initiative of the Ministry of Health of the Kingdom of Saudi Arabia, an expert board of dermatologists and pharmacists with recognized practical experience in the clinical management of psoriasis were called to be part of a work group to update the earlier practical guidelines for the biologic treatment of psoriasis published in 2014 (Citation10).
The overall aim of this consensus document is to deliver evidence-based recommendations on the use, screening, and monitoring of biologic therapy in patients with moderate-to-severe plaque psoriasis. These recommendations also aim to propose updated decision-making algorithms for practitioners involved in the treatment of these patients and consideration is given to special patient populations. They are not prescriptive, however, and are intended to support rather than replace physician’s clinical judgment (see Box 1).
Box 1 Disclaimer on the use of clinical practice guidelines
Clinical practice guidelines are evidence-based decision-making tools for managing health conditions. They are based on the best information available at the time of writing and are to be updated regularly. The present guidelines are not meant to serve as strict treatment guidelines. They are also not intended to replace the clinical judgment of practicing physicians but are only tools to help manage patients who require biologic psoriasis treatment. Decisions concerning treatment must always be taken on a case-by-case basis and the prescribing physicians need to personalize care and tailor the treatment regimen to patients’ personal circumstances and medical history. Physicians should also consult the approved product monographs within their institution’s formulary for each drug for dosage, special warnings and precautions for use, contraindications, and monitoring of side effects and potential harms. Institution formulary restrictions may also need to be considered when selecting treatment options. Prescribing physicians should refer to their institution’s formularies during the decision-making process for choosing specific agents within a recommended specific class.
Methods
The first (2014) version of the Saudi practical guidelines on the biologic treatment of psoriasis (Citation10) provided the methodological basis for developing the current consensus document guidelines.
A literature search was conducted in October 2019 using PubMed, using the search terms ‘psoriasis’, ‘guidelines’, and ‘biologic’ with a filter of publication dates within the last 5 years (2015–2019) to identify published international guidelines concerning the biologic treatment of psoriasis. The reference lists from the identified guidelines then were evaluated to locate additional articles.
An expert multidisciplinary work group including eight dermatologists and five pharmacists was then invited to four workshops held between October 2019 and February 2020 to discuss the need for national consensus recommendations for biologic treatment of psoriasis, review current available international guidelines, and develop recommendations applicable to the Saudi setting.
In the first workshop, a total of six guidelines were found to meet the criteria for use in the generation of the current guidelines: The European S3 Guidelines (2017) (Citation11), the British Association of Dermatologists Guidelines for Biologic Therapy for Psoriasis (2017) (Citation12), American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) guidelines (2019) (biologics and comorbidities) (Citation13,Citation14), the UK National Institute for Health and Care Excellence (NICE) Guidelines (2019) (Citation15), and the French guidelines on the use of systemic treatments for moderate-to-severe psoriasis in adults (2019) (Citation16). Other articles with updated emerging evidence were also identified and evaluated. Tasks were then distributed amongst the work group to formulate recommendations for different topics related to the biologic treatment of psoriasis based on the evidence.
Before the second and third workshops, the draft of the distributed tasks was circulated in the work group. During the workshops, the formal consensus methodology of the nominal group technique was used to agree upon the recommendations (Citation17). All work group members were entitled to vote on the recommendations. A statement was regarded as consented when the agreement was achieved by at least 75% of the voting experts. The strength of recommendation was not expressed.
The draft document was then compiled to include all the consensus recommendations and shared with the expert work group and MOH experts for further review and input during a 30-day feedback/comment period. Comments received were then discussed for further agreement by work group in a final workshop held in February 2020.
Key considerations before application of biologic therapy
Appropriate prescribers of biologic therapy
Biologic therapies should be prescribed by dermatologists with extensive clinical experience in the treatment of psoriasis with systemic agents, considering that the use of these therapies requires the appropriate patient selection and follow-up.
Eligibility criteria for biologic treatment
Biologic drugs are generally indicated in moderate-to-severe psoriasis with particular consideration for patients who have failed, could not tolerate, or cannot use at least one systemic treatment, preferably methotrexate (MTX) (Citation12).
In general, any of the following criteria is currently widely accepted as appropriate criteria for initiating biologic therapy:
Moderate-to-severe disease, which is defined as (Citation10):
≥10% body surface area (BSA) involvement
≥10 Psoriasis Area and Severity Index (PASI)
≥10 Dermatology Life Quality Index (DLQI)
Limited disease but with significant functional impairment and/or high levels of distress after the failure of topical therapy. These special circumstances include (Citation10):
Involvement of large areas of the scalp
Involvement of visible areas, such as the hands and face,
Involvement of intertriginous areas and genitalia
Involvement of palms and/or soles
The presence of treatment-resistant areas
Presence of psoriatic arthritis
Treatment goals
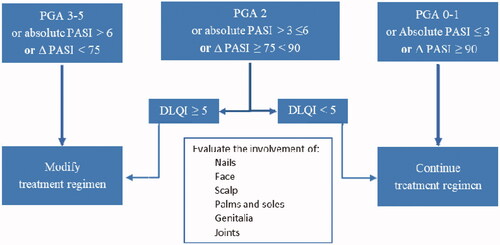
It is preferable to define treatment goals for each patient before starting biologic therapy. Assessment tools, such as PASI, physician global assessment (PGA), BSA, and DLQI are recommended for use in daily practice to establish and monitor the achievement of treatment goals and guide therapy (). The newly available biologic therapies for psoriasis are highly effective; and thus, we recommend that one of the following criteria should be achieved for the treatment goal of psoriasis (Citation16,Citation18):
Absolute PASI ≤3.
PASI 90 response.
PGA score (0–1).
DLQI (<5).
Treatment goals are generally evaluated between weeks 12 and 16.
Screening, precautions, and monitoring of biologics
Screening for tuberculosis (TB)
TB is common in Saudi Arabia (Citation19) as most cases are due to the reactivation of latent TB (LTBI). LTBI is defined by WHO as a state of persistent immune response to stimulation by Mycobacterium tuberculosis antigens without clinical evidence of active TB (Citation20).
The Saudi guidelines for testing and treatment of LTBI include statements by the Saudi Thoracic Society (STS), the Saudi Society of Medical Microbiology and Infectious Disease (SSMMID), the Saudi Association of Public Health (SAPH), and the Society of Family and Community Medicine, thereby providing national recommendations for targeted tuberculin testing and treatment regimens for patients with LTBI (Citation19). These guidelines recommend Tuberculin Skin Test (TST) with purified protein derivative (PPD) for the screening. TST should be done and assessed by trained professionals. The induration is measured and should be recorded in millimeters. The interpretation of TST is dependent on the size of induration and the risk status of the subjects (Citation19).
The interferon-gamma release assays (IGRA-QuantiFERON-TB Gold test) have been incorporated into TB screening (Citation10). The IGRA is highly specific and sensitive in detecting LTBI, and it is not affected by prior BCG vaccination (Citation19,Citation21). Either TST or IGRA can be used for LTBI testing before initiating a biologic therapy (Citation10,Citation13). The baseline chest radiograph is recommended for all patients (Citation12). It is also needed for any patient with a newly identified positive test (Citation13,Citation19).
During treatment with biologic therapy, annual screening is recommended, including medical history (Citation21). Either TST or IGRA -QuantiFERON test can be utilized (Citation10). Suspicion of TB infection should be maintained even after discontinuation of the biologic therapy for six months (Citation21).
Screening for hepatitis B and C
The patients who are eligible to receive immunosuppressive drugs, including biologics, must be screened for the following markers: antibody to hepatitis B core antigen (IgM/IgG), antibody to hepatitis B surface antigen (anti-HBsAg), HBsAg, and antibody to HCV (anti-HCV). In case HBV or HCV infection is diagnosed, a close collaboration with a hepatologist is required before and during immunosuppressive therapy.
Laboratory screening
summarize our proposed schedule of laboratory screening before prescribing a biologic therapy for psoriasis (Citation10,Citation12,Citation22–34).
Table 1. Laboratory screening prior to initiation of a biologic treatment (Citation10,Citation12,Citation22–34).
Table 2. Screening for comorbidities (Citation10,Citation12,Citation22–34).
Vaccinations
Vaccinations should be up to date and all recommended vaccines should be given before starting biologic therapy (Citation10,Citation12). Live vaccines (BCG, measles, mumps, rubella, yellow fever, oral polio, oral typhoid, varicella-zoster, and nasal influenza vaccine) can cause disseminated or fatal infections in immunocompromised individuals; therefore they are contraindicated in patients on biologic therapy and in infants (up to 6 months of age) whose mothers have received biologic therapy beyond 16 weeks gestation (Citation12).
Despite the data regarding the lack of placental transfer of certolizumab, there are no published data concerning the live vaccination in the newborns of mothers treated with this biologic therapy during pregnancy (Citation35). If a live vaccine is needed while on biologic therapy, discontinuation of the biologic agents is recommended. Some experts advise withdrawing the biologic therapy for 2–3 half-lives before and after vaccine administration, while others advise 4 weeks (or longer depending on the biologic’s half-life) before and until 1–2 weeks after vaccination (Citation13).
Inactivated vaccines are safe to be given while on biologic therapy (Citation12). Yearly influenza (inactivated) and pneumococcal vaccines are recommended. Hepatitis A and B vaccines are recommended if not immunized (Citation36). About the Hajj season, patients who are planning to perform Hajj should be up to date on all routine vaccines, by MOH vaccine requirements and recommendations (Citation37).
In the era of COVID-19, patients with psoriatic disease, who do not have contraindications to vaccination, should be reassured and encouraged to receive any of the approved COVID-19 vaccines whenever available to them (mRNA-based or adenovirus vectored vaccine (Citation38). Biologic medications for psoriasis are not contraindicated to any currently available COVID-19 vaccines (Citation38).
Infections
Biological agents are associated with a minimal increase in the risk of infections (Citation10,Citation11). However, serious infections were reported albeit are very rare. Moreover, in case of serious or significant infections, biological agents should be suspended till complete recovery (Citation10,Citation11).
Therapeutic use of biologics for the management of psoriasis
Overview of biologic agents available for psoriasis in Saudi Arabia
lists all biologic therapies that are currently registered and approved by SFDA for the treatment of moderate-to-severe plaque psoriasis (Citation22–28,Citation30,Citation31,Citation39,Citation40). provides a summary of the evaluation of the efficacy of all biologic therapies that are currently registered and approved by SFDA for the treatment of moderate-to-severe plaque psoriasis, along with their dosing scheme, type of antibody, and half-life (Citation41–55). summarizes their tolerability and safety profiles from pivotal studies (Citation56–59).
Table 3. Biologic agents and biosimilars available for the treatment of psoriasis in Saudi Arabia.
Table 4. Dosing schemes, type of antibody, efficacy, and half-life of biologic agents/biosimilars available for the treatment of psoriasis in Saudi Arabia.
Table 5. Adverse event of biologic agents for the treatment of psoriasis.
Treatment algorithm
General considerations
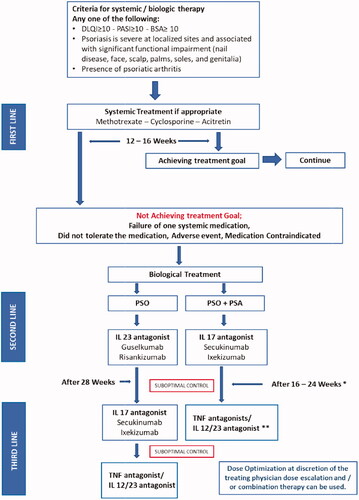
shows an overview of evaluating treatment options for the management of chronic psoriasis vulgaris. In current routine clinical practice, near-complete skin clearance with the least side effects should be the treatment goal for patients with moderate-to-severe psoriasis. In addition to defining treatment goals, and to improve psoriasis outcomes, it is important to implement strategies to promptly alter treatment regimens if goals are not met within about 12–16 weeks. When the clinical response of treatment with the chosen biologic therapy is unsatisfactory (suboptimal outcomes, primary or secondary treatment failure, or drug-related side effects), the possible courses of action that may be followed to improve the results are: (a) dose optimization, (b) combination strategies with non-biologic treatment, (c) switching to another biologic therapy.
Apremilast
Apremilast may be considered for a select group of patients. Apremilast is an oral phosphodiesterase inhibitor approved for Pso & PsA, and has a PASI 75 response of 33.1% at 16 weeks (Citation60). Subgroup analysis of pivotal studies data showed some response in palmoplantar, scalp, and nail psoriasis (Citation61,Citation62). There is no restriction to using it in patients with malignancies (Citation63). Additionally, institution formulary restrictions may need to be considered when selecting apremilast (i.e. in MOH, apremilast is non-formulary).
Transitioning
Benefits of transitioning
Switching psoriasis treatment is a common, accepted practice for improving patient outcomes (e.g. when patients are experiencing suboptimal efficacy and/or intolerability with a given therapy) (Citation64). There are no evidence-based studies on the duration of the interval between discontinuation of the previous medication and initiation of biologic therapy. This may depend on the treatment that is being discontinued, disease severity, and response to prior treatment, as well as on expert opinion, and it should be assessed on a case-by-case basis. Therefore, whereas some experts will start administration of a new biologic as soon as it is available for the patient, others may wait for a period of 3 or 4 half-lives of the previous therapy before the transition (Citation13).
Transitioning from conventional systemic therapy to biologic therapy
General considerations (Citation65): Recommendations for transitioning from conventional systemic therapy to biologic therapy will differ depending on the reason for transition. For example:
When transitioning due to safety reasons (development of medication-related side effects), a treatment-free interval may be necessary until the safety parameter has normalized or stabilized.
When transitioning due to lack of efficacy, which could be primary or secondary inefficacy, or due to suboptimal response; transitioning directly or with an overlap period can be considered.
Additionally, if a patient develops psoriatic arthritis, transitioning to a therapy that is efficacious in both psoriasis and psoriatic arthritis is required.
Irrespective of the reason for transitioning, approved induction dosages should be used for the new drug.
Transitioning from cyclosporine to biologic therapy (Citation65): Transitioning from cyclosporine to TNF antagonists and ustekinumab can be performed without a washout period. A short overlap period (e.g. 2–8 weeks) of cyclosporine with TNF antagonists or ustekinumab can be considered to reduce the risk of the rebound in partial responders but the overlap period should be minimized and the dose of cyclosporine tapered down as soon as possible.
Transitioning from MTX to biologic therapy (Citation64): Transitioning from MTX to TNF antagonists and ustekinumab can be performed without a washout period. MTX can be overlapped or used concurrently with TNF antagonists or ustekinumab.
Transitioning from one biologic therapy to another
General considerations before transitioning (Citation65): In the case of suboptimal response to a biologic therapy (etanercept, infliximab, adalimumab, and ustekinumab), dose optimization or combination strategies with non-biologic treatment is preferred before transitioning to another biologic therapy (Citation65). This decision should be made with consideration to patient situation/preference and the cost inflation by dose optimization. Recommendations for dose optimization include:
For adalimumab, an increase of dosage from 40 mg every other week to 40 mg/week (Citation65).
For etanercept, an increase of dosage from 50 mg/week to 50 mg twice weekly (Citation65).
For ustekinumab, with partial responders, an increase of dosage from 45 to 90 mg with 12-week dosing intervals. If this is unsuccessful, the interval can be shortened to 8 weeks (Citation65).
For infliximab, a reduction of the dosing intervals from every 8 weeks to every 6 weeks with 5 mg/kg can be considered in secondary non-responders, defined as the loss of at least 50% of the initial improvement. In special cases, an increase of the dosage >5 mg/kg can be considered (Citation64). The dose can be increased in some circumstances up to 10 mg/kg/dose and the interval can be shortened to 4 weeks (Citation66).
For secukinumab, dose optimization includes shortening the interval to every 2 weeks (Citation67).
For ixekizumab, a single report of administrating it every 2 weeks instead of 4 weeks (Citation68)
For IL-23 inhibitors, no data yet
General considerations after the decision to transition have been made (Citation65): Recommendations for transitioning from one biologic therapy to another will differ depending on the reason for transition. For example:
When transitioning due to lack of efficacy, no washout period is necessary; transition to the new biologic therapy at the time of the next scheduled dose of the original therapy, using the standard induction dose, followed by the maintenance dose.
When transitioning due to safety reasons (development of medication-related side effects), a treatment-free interval may be necessary until the safety issue has been resolved.
Adjusting biologic therapy
Dose reduction
During successful maintenance with biologics as monotherapy, a dose reduction can be considered to limit drug exposure. However, long-term efficacy and safety data have only been generated for the approved doses. Moreover, there is a risk of decreasing efficacy. In addition, there is some evidence to support that a longer interval might increase the risk of anti-drug antibody formation (Citation65). Decreasing the dose may be considered in patients on combination therapy (Citation65).
In clinical practice, dosing intervals have been increased with adalimumab and etanercept while maintaining clinical response (Citation65). With infliximab monotherapy, intervals should not be increased over the recommended intervals. The dose of infliximab may be reduced from 5 mg/kg body weight to a minimum of 3 mg/kg bodyweight particularly if combined with methotrexate (Citation65). With ustekinumab, the dose for a responding patient may be reduced from 90 to 45 mg. Moreover, few reports exist for the prolongation of intervals between injections (Citation69).
Dose discontinuation/interruption
In cases of sustained response/clearance:
Discontinuation of biologic therapy is not generally recommended due to the risk of recurrence or failure to recapture the initial response (Citation65).
However, if agreed with the patient, and after achieving a clinical response of clear or almost clear with good QoL for a prolonged period (i.e. a minimum of 1 year), discontinuing biologic therapy can be considered with careful follow-up (Citation65).
There is little evidence to suggest which subgroups of patients can discontinue the biologic medication. These subgroups include (Citation65):
Patient preference
Patients with a history of disease-free intervals or previously stable plaque-type psoriasis
Absence of PsA
Low impact of disease on QoL
No worsening of the disease after previous dose reductions and treatment withdrawals
However, because biologic therapies are typically considered for patients with more severe disease, and as a second line after failing conventional systemic therapy; patients on biologics are less likely to fulfill these criteria. Furthermore, fewer treatment options are available in case of relapse after discontinuation.
Another consideration is that the risk of antibody formation increases with intermittent therapy. This is particularly important for the use of infliximab monotherapy where a higher risk of infusion reactions has been observed with intermittent therapy (Citation65).
Efficacy with biologic therapy following treatment discontinuation/interruption:
In patients receiving biologic therapy, there is a high likelihood of disease recurrence within several months of discontinuation of treatment, although some patients may maintain disease control for a prolonged period (Citation65). Generally, maintaining PASI-90 response for a longer duration is documented with IL-17 as compared to anti-TNF inhibitors, as well as a higher percentage of patients will recapture PASI-90 after restarting IL-17 in comparison to anti-TNF (Citation70).
Continuous biologic therapy generally results in greater improvements in efficacy over time compared with intermittent therapy (Citation65).
In clinical trials with primary responder patients, up to 20% fail, to regain a PASI 75 response after the re-initiation of the same biologic monotherapy. This decrease in efficacy may be greater with intermittent use of the drug (Citation65).
Where therapy has been withdrawn and restarted, an induction dosing schedule should be used for re-introduction of the biologic therapy, with the possible exception of infliximab (because of the increased risk of infusion reactions) (Citation65).
Use of biologics in special patient populations
Contents of this section
Psoriasis is a systemic inflammatory disease that is associated with an increased risk of comorbidities, which can have a significant impact on the decision to use one therapy over another. Choosing the right therapy in certain patient populations can also be challenging. Thus, it is important to tailor treatment regimens for psoriasis patients based on their individual special needs and characteristics.
This section of the guidelines covers the choice of biologic therapy in pregnancy and lactation, as well as in pediatrics and adolescents. In addition, recommendations of the choice of treatment are presented for patients with the following comorbidities: metabolic syndrome (including obesity), malignancy, demyelinating disease (multiple sclerosis), cardiovascular disease, congestive heart failure, inflammatory bowel disease, and lupus erythematosus. This section also provides insights for choosing appropriate biologic therapy for the treatment of moderate-to-severe psoriasis in the setting of chronic infections, such as hepatitis and tuberculosis.
Pregnancy and lactation
The treatment of psoriasis in pregnancy or patients planning a pregnancy can be challenging. It is reported that 55% of psoriasis patients improve during pregnancy, likely due to the immunomodulatory changes of pregnancy, while 23% experience worsening (Citation71). Patients who are pregnant or are lactating require special considerations to ensure treatment safety and efficacy. Generally, any medications in pregnancy should be avoided unless the benefits outweigh the risks. In women of childbearing potential or those who become pregnant, risks and benefits of continuing vs. stopping biologic therapy should be discussed (Citation12). It is preferred to discontinue all biologic agents before pregnancy or at 16–26 weeks of pregnancy (Citation72). In general, advise mothers who have received biologic therapy for psoriasis beyond 16 weeks’ gestation that their infants should not receive any live vaccinations until they have reached 6 months of age (e.g. rotavirus and BCG) (Citation12).
There are no known interactions between biologic therapies and contraceptive methods. Advise women of childbearing potential who are starting biologic therapy for psoriasis to use effective contraception and discuss conception plans with the treating physician (Citation12).
Additional considerations relating to the use of specific biological therapies in this population are summarized below and in .
Table 6. Summary of recommendations for biologic therapy options in pregnancy.
TNF- α inhibitors can be used during lactation. They are safe in men attempting conception with their partners. There is a greater theoretical risk with use during the third trimester of pregnancy owing to transplacental transfer of TNF-α inhibitors (Citation13). Certolizumab pegol has shown minimal to no placental transfer, so it is labeled as the best choice for pregnant psoriatic patients (Citation73). Etanercept is considered an alternative to certolizumab if certolizumab is not available (Citation73).
IL-12/IL-23 inhibitors: The safety of IL-12/IL-23 inhibitors during pregnancy and lactation is uncertain. They are acceptable for men attempting conception with their partners (Citation13).
IL-17 inhibitors: There are no studies with these agents in human pregnancy. All IL-17 inhibitors are likely acceptable for men attempting conception with their partners. The presence of IL-17 inhibitors in excreted human milk has not been studied (Citation68).
IL-23 inhibitors: Safety during pregnancy for IL-23 inhibitors is unknown (Citation13). The presence of IL-23 inhibitors in secreted human milk has not been studied (Citation13). However, antibodies are effectively secreted during lactation (Citation13), but generally have no significant impact (Citation74).
Pediatric and adolescent patients
The choice of treatment for pediatrics and adolescents should be made on an individual basis after discussion between the treating clinician and the patient, or their parents or guardian, about the advantages and disadvantages of the treatments available. Among the multiple biologic therapies available for the treatment of psoriasis in adults, adalimumab, etanercept, ixekzumab, secukinumab, and ustekinumab are approved for treating plaque psoriasis in children and young people (Citation12). Recommendations on the choice of biologic therapy for pediatrics and adolescents are summarized in (Citation13,Citation15,Citation75). In addition, infliximab is FDA-approved for the treatment of Crohn’s disease and ulcerative colitis in children 6 years of age and older (Citation13).
Table 7. Summary of recommendations for choice of biologic therapy in pediatrics and adolescents.
Metabolic syndrome and obesity
The prevalence of metabolic syndrome increases with increased BSA affected by psoriasis (Citation14). The relationship between psoriasis and obesity is unclear. Increased levels of pro-inflammatory cytokines (e.g. TNF-α, IL-1b, and IL-6) and adiponectin are detected in the serum of obese patients (Citation14).
When treating a patient with psoriasis, regardless of baseline weight, the effect of treatment on weight management is an important variable to consider (Citation14). Overweight and obese patients frequently require a shorter dose interval or higher doses to achieve a satisfactory response. Infliximab and ustekinumab are dosed based on weight (Citation76).
Malignancy
A correlation between psoriasis and malignancy has been noted in several studies. There may be minimal increased incidence of certain malignancies in patients with psoriasis, particularly cutaneous T-cell lymphoma, head and neck cancers, and digestive tract malignancies (Citation14). Some studies suggest an increased risk for NMSC in patients who have received psoralen UVA phototherapy (Citation77), or have used cyclosporine, or receiving TNF inhibitors (Citation73,Citation78).
With regards to infliximab specifically, an exploratory clinical study evaluating its use in patients with moderate to severe COPD has reported more malignancies in infliximab-treated patients compared with control patients (Citation79). All patients in the study had a history of heavy smoking. Thus, caution should be exercised in considering treatment for patients with increased risk for malignancy due to heavy smoking (Citation14,Citation73).
It is best to avoid all biologic therapy in patients with concurrent malignancy. In cases with a recent history of malignancy, it is recommended to discuss the decision to initiate immunosuppressive therapies with the treating oncologist.
Patients undergoing surgery
For elective high-risk surgery, it is better to discontinue biologic therapy for about 4–5 half-lives before surgeries (Citation36). This is despite the use of biologic therapy does not appear to affect the rates of surgical complications, like infections (Citation80–85). For non-high-risk surgeries, biologics can be continued.
Patients with hepatitis
It is generally accepted that biologics should not be initiated in patients with active hepatitis B infection (Citation22,Citation23,Citation31). The risk of developing severe hepatitis due to reactivation of HBV infection with biologic agents cannot be excluded, therefore management of psoriatic patients with a hepatologist should be considered in the cases of chronic carriers of HBV or those with positive serology and positive symptoms, such as nausea, appetite loss, and pruritus (Citation86). The infliximab insert warns about HBV reactivation and recommends monitoring of HBV carriers during and several months after therapy (Citation22).
There is no clear consensus regarding the management of patients with HCV. However, the risk of developing severe hepatitis is not as critical for patients with HCV as for those with HBV (Citation10). If the HCV-infected patient has already been successfully treated with antiviral therapy, the risk seems to be even lower (Citation86,Citation87). There are several published reports of successful treatment of HCV-infected psoriatic patients with adalimumab and etanercept (Citation10).
The safety profile of ustekinumab in patients with hepatitis is controversial (Citation73). IL-17 inhibitors appear to have a favorable safety profile, but the available data are limited (Citation73). Data are also limited on IL-23 inhibitor use in patients with hepatitis (Citation73).
Tuberculosis
Patients who receive anti -TNF biologic therapy are at increased risk of LTBI reactivation (Citation10,Citation19); the risk may be greater with the monoclonal antibodies (infliximab and adalimumab) than etanercept. Also, ustekinumab may facilitate the reactivation of tuberculosis (Citation12). The atypical clinical presentation of infection with extrapulmonary and disseminated disease in patients treated with TNF antagonist’s incidence is higher (Citation10,Citation12). There are no reported LTBI cases with IL-17 and IL-23 so far (Citation73).
If patients with LTBI have normal chest radiographs and no symptoms or signs of active TB, treatment of LTBI is indicated. Treatment with isoniazid (INH) for 9 months or rifampin for 4 months (only when INH regimen is not feasible and after consulting with a TB specialist due to high risk of rifampicin-resistance) (Citation10,Citation19), aim to complete one month of treatment before starting the biologic therapy (Citation10).
If active TB is suspected, treatment with biologics should be deferred, and chest x-ray, sputum AFB stain, and culture must be repeated to rule out any new infection or reactivation (Citation10). Referral to a TB expert is indicated in the case of LTBI or active TB (Citation78).
Patients with demyelinating diseases/multiple sclerosis (MS)
Do not use TNF-α antagonists in patients with demyelinating diseases and review alternative interventions in patients who have an affected first-degree relative with the demyelinating disease (Citation76,Citation88). Stop treatment and seek specialist advice if neurological symptoms suggestive of the demyelinating disease develop during TNF-α antagonist therapy (Citation89).
IL 12/23 inhibitor may be used in patients with MS as it does not improve or worsen MS (Citation76,Citation90). IL-17 inhibitors can be used with some benefit in MS symptoms (Citation76,Citation91). Data are limited for the IL-23 inhibitors, but there are no reports of MS worsening with these drugs (Citation76).
Patients at elevated cardiovascular risk
TNF-α inhibitors are preferred systemic agents for the treatment of psoriasis in patients with coexisting cardiovascular risk factors (Citation76,Citation92,Citation93). IL-12/23 inhibitor has some potential cardioprotective benefit, but more long-term data are needed (Citation76,Citation94). More data are needed for the use of IL-17 and IL-23 inhibitors (Citation76).
Congestive heart failure (CHF)
Avoid TNF-α antagonist therapy in people with severe cardiac failure (New York Health Association [NYHA] class III and IV) (Citation76,Citation95,Citation96), and discontinue TNF-α antagonist therapy in the event of new or worsening preexisting heart failure and seek specialist advice (Citation76,Citation95,Citation96). IL 12/23, IL-17, and IL-23 inhibitors appear to be safe to use in patients with CHF (Citation97).
Inflammatory bowel disease (IBD)
Patients with a history of concomitant IBD might benefit from TNF-α inhibitor therapy. In fact, adalimumab, infliximab, and certolizumab are approved by the US FDA for the treatment of IBD (Citation98–101). Etanercept is not as effective as other TNF-α inhibitors for Crohn’s Disease (Citation102).
IL 12/23 inhibitor is also approved for Crohn’s disease but not ulcerative colitis (Citation103). IL-23 inhibitor use in Crohn’s disease has promising results in preliminary studies, but more data are needed to draw definite conclusions (Citation104). Exercise caution and consult a gastroenterology specialist before using IL-17 inhibitors in patients with IBD, or those with first-degree relatives with IBD (Citation105–108).
Lupus erythematosus
There is a concern for the development of de novo lupus or flare-up of lupus during treatment with TNF- α blockers, also known as anti TNF- α induced lupus (ATIL) (Citation109,Citation110). IL-12/23 inhibitor is the safest treatment option for concomitant lupus and psoriasis as it reportedly improves SLE symptoms, specifically oral ulcerations, anemia or thrombocytopenia, and lupus arthritis (Citation111,Citation112). There are not enough data regarding the use of IL-17 and IL-23 inhibitors in patients with SLE, but no new cases of lupus induction or flare have been reported yet (Citation76).
Conclusions
This consensus statement represents a summary of the scientific evidence currently available on the efficacy of the biologic treatments indicated for psoriasis on the Saudi market at the time of publication and the selection criteria for the use of these drugs. The choice of biologic therapy should always be based on knowledge of the published response rates in clinical trials and take into account the disease course at the time of the prescription and the characteristics of the patient.
Acknowledgments
A medical writer (Dr. Mike Gwilt, GT Communications) edited the manuscript prepared by the authors for submission to the journal. Sahar Shami (Itkan Health Consulting) provided medical writing support, including drafting the proceedings of the four workshops, development of the first draft, and collation/incorporation of author comments.
Disclosure statement
Issam Hamadah received research grants from AbbVie, Novartis, Janssen; received honoraria speaking for AbbVie, Janssen, Novartis, Sanofi, Pfizer; received honoraria serving as a consultant for AbbVie, Janssen, Newbridge, Novartis, Sanofi, and Biologix; received travel support from AbbVie, Janssen, Galderma, and Sanofi. Mohammed Fatani received research grants from Sanofi; received honoraria speaking for AbbVie, Janssen, Novartis, Sanofi, Eli Lilly; received honoraria serving as a consultant for Sanofi, AbbVie, Novartis, Janssen, Newbridge; received travel support from AbbVie, Sanofi, Novartis, Janssen. Yousef Binamer received speaker honorarium, honoraria serving as a consultant, and travel support from AbbVie, Janssen, Novartis, Sanofi, and Eli Lilly; received research grants from Novartis and Sanofi. Maysa Eshmawi received travel support from Novartis. Bedor Al Omari received honoraria serving as a consultant/advisory board member for Janssen, AbbVie, Eli Lilly. All other authors declared no conflicts of interest regarding the publications of these recommendations.
Additional information
Funding
References
- Christophers E. Psoriasis-epidemiology and clinical spectrum. Clin Exp Dermatol. 2001;26(4):314–320.
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377–385.
- Rendon A, Schakel K. Psoriasis pathogenesis and treatment. IJMS. 2019;20(6):1475.
- Rachakonda TD, Schupp CW, Armstrong AW. Armstrong AW psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70(3):512–516.
- Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(suppl_2):ii18–23.
- Kim BR, Kang D, Kang M, et al. Risk of acute infections in patients with psoriasis: a nationwide population-based cohort study. J Am Acad Dermatol. 2020;82(3):764–766.
- Furue M, Kadono T. Psoriasis: behind the scenes. J Dermatol. 2016;43(1):4–8.
- Armstrong AW. Psoriasis. JAMA Dermatol. 2017;153(9):956.
- Navarini AA, Trueb RM. [Psoriasis]. Ther Umsch. 2010;67(4):153–165.
- Hamadah IR, Al Raddadi AA, Bahamdan KA, et al. Saudi practical guidelines on biologic treatment of psoriasis. J Dermatolog Treat. 2015;26(3):223–229.
- Nast A, Spuls PI, van der Kraaij G, et al. European S3-Guideline on the systemic treatment of psoriasis vulgaris – update Apremilast and Secukinumab – EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol. 2017;31(12):1951–1963.
- Smith CH, Jabbar-Lopez ZK, Yiu ZZ, et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2017. Br J Dermatol. 2017;177(3):628–636.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–1072.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80(4):1073–1113.
- NICE Pathway Systemic biological therapy for psoriasis 2019. Available from: https://pathways.nice.org.uk/pathways/psoriasis (accessed December 2020).
- Amatore F, Villani AP, Tauber M, et al. Recommandations françaises sur l’utilisation des traitements systémiques chez les patients adultes atteints de psoriasis modéré à sévère [French guidelines on the use of systemic treatments for moderate-to-severe psoriasis in adults]. Ann Dermatol Venereol. 2019;146(6–7):429–439.
- Humphrey-Murto S, Varpio L, Gonsalves C, et al. Using consensus group methods such as Delphi and Nominal Group in medical education research. Med Teach. 2017;39(1):14–19.
- Mahil SK, Wilson N, Dand N, et al. Psoriasis treat to target: defining outcomes in psoriasis using data from a real-world, population-based cohort study (the British Association of Dermatologists Biologics and Immunomodulators Register, BADBIR). Br J Dermatol. 2020;182(5):1158–1166.
- Al Jahdali HH, Baharoon S, Abba AA, et al. Saudi guidelines for testing and treatment of latent tuberculosis infection. Ann Saudi Med. 2010;30(1):38–49.
- WHO. WHO LTBI FAQs 2018 [cited 2020 Jan 26]. Available from: https://wwwwhoint/tb/publications/2018/ltbi_faqspdf?ua=1
- Nast A, Jacobs A, Rosumeck S, et al. Methods report: European S3-guidelines on the systemic treatment of psoriasis vulgaris-update 2015-EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol. 2015;29(12):e1–e22.
- Product Information. REMICADE lyophilized concentrate for intravenous injection, infliximab lyophilized concentrate for intravenous injection. Horsham (PA): Janssen Biotech, Inc (per FDA); 2011.
- Product Information. HUMIRA subcutaneous injection, adalimumab subcutaneous injection. North Chicago (IL): AbbVie Inc (per FDA); 2015.
- Product Information. CIMZIA subcutaneous injection, Certolizumab pegol subcutaneous injection. Smyrna (GA): UCB Inc (per manufacturer); 2019.
- Product Information. STELARA subcutaneous injection, intravenous injection, ustekinumab subcutaneous injection, intravenous injection. Horsham (PA): Janssen Biotech Inc (per FDA); 2019.
- Product Information. COSENTYX subcutaneous injection, secukinumab subcutaneous injection. East Hanover (NJ): Novartis Pharmaceuticals Corporation (per FDA); 2018.
- Product Information. TALTZ subcutaneous injection, ixekizumab subcutaneous injection. Indianapolis (IN): Eli Lilly and Company (per manufacturer); 2019.
- Product Information. TREMFYA subcutaneous injection, guselkumab subcutaneous injection. Horsham (PA): Janssen Biotech Inc (per FDA); 2019.
- Product Information. ILUMYA subcutaneous injection, tildrakizumab-ASMN subcutaneous injection. Whitehouse Station (NJ): Merck & Co Inc (per manufacturer); 2018.
- Product Information. SKYRIZI subcutaneous injection, risankizumab-RZAA subcutaneous injection. North Chicago (IL): AbbVie Inc (per FDA); 2019.
- Product Information. Enbrel subcutaneous injection solution, etanercept subcutaneous injection solution. Thousand Oaks (CA): Amgen Inc (per FDA); 2015.
- Series, Micromedex Healthcare Internet database USA Thomson Reuters (Healthcare) Inc. Available from: http://www.micromedexcom/products/hcs/ (accessed 2019).
- National Institute for Health and Care Excellence (NICE). Clinical guideline on psoriasis – assessment and management 2012 (updated 2017).
- Hsu S, Papp KA, Lebwohl MG, et al. Consensus guidelines for the management of plaque psoriasis. Arch Dermatol. 2012;148(1):95–102.
- Furer V, Rondaan C, Heijstek MW, et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2020;79(1):39–52.
- Poelman SM, Keeling CP, Metelitsa AI. Practical guidelines for managing patients with psoriasis on biologics: an update. J Cutan Med Surg. 2019;23(1_suppl):3S–12s.
- Al-Tawfiq JA, Memish ZA. The Hajj 2019 vaccine requirements and possible new challenges. J Epidemiol Glob Health. 2019;9(3):147–152.
- Gelfand JM, Armstrong AW, Bell S, et al. National Psoriasis Foundation COVID-19 Task Force guidance for management of psoriatic disease during the pandemic: version 2-advances in psoriatic disease management, COVID-19 vaccines, and COVID-19 treatments. J Am Acad Dermatol. 2021;84(5):1254–1268.
- Product Information. REMSIMA lyophilized concentrate for intravenous injection, infliximab lyophilized concentrate for intravenous injection. Incheon, South Korea: Celltrion Healthcare Co Ltd; 2016.
- Pharmaceutical and herbal products list, Saudi food and drug authority. Available from: https://wwwsfdagovsa/ar/drug/search/Pages/defaultaspx
- Menter A, Tyring SK, Gordon K, et al. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol. 2008;58(1):106–115.
- Gordon K, Papp K, Poulin Y, et al. Long-term efficacy and safety of adalimumab in patients with moderate to severe psoriasis treated continuously over 3 years: results from an open-label extension study for patients from REVEAL. J Am Acad Dermatol. 2012;66(2):241–251.
- Gottlieb AB, Blauvelt A, Thaçi D, et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: results through 48 weeks from 2 phase 3, multicenter, randomized, double-blinded, placebo-controlled studies (CIMPASI-1 and CIMPASI-2). J Am Acad Dermatol. 2018;79(2):302–314.e6.
- Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349(21):2014–2022.
- Leonardi C, Strober B, Gottlieb AB, et al. Long-term safety and efficacy of etanercept in patients with psoriasis: an open-label study. J Drugs Dermatol. 2010;9(8):928–937.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–417.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76(3):418–431.
- Reich K, Nestle FO, Papp K, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005;366(9494):1367–1374.
- Gottlieb AB, Masud S, Ramamurthi R, et al. Pharmacodynamic and pharmacokinetic response to anti-tumor necrosis factor-alpha monoclonal antibody (infliximab) treatment of moderate to severe psoriasis vulgaris. J Am Acad Dermatol. 2003;48(1):68–75.
- Menter A, Feldman SR, Weinstein GD, et al. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2007;56(1):31.e1–31.e315.
- Barker J, Hoffmann M, Wozel G, et al. Efficacy and safety of infliximab vs. methotrexate in patients with moderate-to-severe plaque psoriasis: results of an open-label, active-controlled, randomized trial (RESTORE1). Br J Dermatol. 2011;165(5):1109–1117.
- Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386(9993):541–551.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392(10148):650–661.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338.
- Langley RG, Lebwohl M, Krueger GG, et al. Long-term efficacy and safety of ustekinumab, with and without dosing adjustment, in patients with moderate-to-severe psoriasis: results from the PHOENIX 2 study through 5 years of follow-up. Br J Dermatol. 2015;172(5):1371–1383.
- Loos AM, Liu S, Segel C, et al. Comparative effectiveness of targeted immunomodulators for the treatment of moderate-to-severe plaque psoriasis: A systematic review and network meta-analysis. J Am Acad Dermatol. 2018;79(1):135–144.e7.
- Mansouri Y, Goldenberg G. Biologic safety in psoriasis: review of long-term safety data. J Clin Aesthet Dermatol. 2015;8(2):30–42.
- Loft ND, Vaengebjerg S, Halling AS, et al. Adverse events with IL-17 and IL-23 inhibitors for psoriasis and psoriatic arthritis: a systematic review and meta-analysis of phase III studies. J Eur Acad Dermatol Venereol. 2020;34(6):1151–1160.
- Curtis JR, Mariette X, Gaujoux-Viala C, et al. Long-term safety of certolizumab pegol in rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis, psoriasis and Crohn's disease: a pooled analysis of 11,317 patients across clinical trials. RMD Open. 2019;5(1):e000942.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (efficacy and safety trial evaluating the effects of apremilast in psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73(1):37–49.
- Rich P, Gooderham M, Bachelez H, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with difficult-to-treat nail and scalp psoriasis: results of 2 phase III randomized, controlled trials (ESTEEM 1 and ESTEEM 2). J Am Acad Dermatol. 2016;74(1):134–142.
- Bissonnette R, Pariser DM, Wasel NR, et al. Apremilast, an oral phosphodiesterase-4 inhibitor, in the treatment of palmoplantar psoriasis: results of a pooled analysis from phase II PSOR-005 and phase III efficacy and safety trial evaluating the effects of apremilast in psoriasis (ESTEEM) clinical trials in patients with moderate to severe psoriasis. J Am Acad Dermatol. 2016;75(1):99–105.
- Product Information. OTEZLA oral tablets, apremilast. Thousand Oaks (CA): Amgen Inc; 2014.
- Kerdel F, Zaiac M. An evolution in switching therapy for psoriasis patients who fail to meet treatment goals. Dermatol Ther. 2015;28(6):390–403.
- Mrowietz U, de Jong EM, Kragballe K, et al. A consensus report on appropriate treatment optimization and transitioning in the management of moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2014;28(4):438–453.
- Torii H, Nakano M, Yano T, et al. Efficacy and safety of dose escalation of infliximab therapy in Japanese patients with psoriasis: results of the SPREAD study. J Dermatol. 2017;44(5):552–559.
- Phung M, Georgakopoulos JR, Ighani A, et al. Secukinumab dose optimization in adult psoriasis patients: a retrospective, multicenter case series. JAAD Case Rep. 2018;4(4):310–313.
- Langley RG, Papp K, Gooderham M, et al. Efficacy and safety of continuous every-2-week dosing of ixekizumab over 52 weeks in patients with moderate-to-severe plaque psoriasis in a randomized phase III trial (IXORA-P). Br J Dermatol. 2018;178(6):1315–1323.
- van Bezooijen JS, van Doorn MBA, Schreurs MWJ, et al. Prolongation of biologic dosing intervals in patients with stable psoriasis: a feasibility study. Ther Drug Monit. 2017;39(4):379–386.
- Umezawa Y, Torisu-Itakura H, Morisaki Y, Japanese Ixekizumab Study Group, et al. Long-term efficacy and safety results from an open-label phase III study (UNCOVER-J) in Japanese plaque psoriasis patients: impact of treatment withdrawal and retreatment of ixekizumab. J Eur Acad Dermatol Venereol. 2019;33(3):568–576.
- Murase JE, Chan KK, Garite TJ, et al. Hormonal effect on psoriasis in pregnancy and post partum. Arch Dermatol. 2005;141(5):601–606.
- Porter C, Armstrong-Fisher S, Kopotsha T, et al. Certolizumab pegol does not bind the neonatal Fc receptor (FcRn): consequences for FcRn-mediated in vitro transcytosis and ex vivo human placental transfer. J Reprod Immunol. 2016;116:7–12.
- Kaushik SB, Lebwohl MG. Psoriasis: Which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80(1):43–53.
- Flint J, Panchal S, Hurrell A, et al. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding-part I: standard and biologic disease modifying anti-rheumatic drugs and corticosteroids. Rheumatology. 2016;55(9):1693–1697.
- Product Information. STELARA subcutaneous injection, intravenous injection, ustekinumab subcutaneous injection, intravenous injection. Janssen Biotech Inc (per EMA); 2020. Available from: https://wwwemaeuropaeu/en/medicines/human/EPAR/stelara
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: psoriasis comorbidities and preferred systemic agents. J Am Acad Dermatol. 2019;80(1):27–40.
- Pouplard C, Brenaut E, Horreau C, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013;27(Suppl 3):36–46.
- Nast A, Gisondi P, Ormerod AD, et al. European S3-Guidelines on the systemic treatment of psoriasis vulgaris-update 2015-short version-EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol. 2015;29(12):2277–2294.
- Rennard SI, Fogarty C, Kelsen S, et al. The safety and efficacy of infliximab in moderate to severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175(9):926–934.
- Hernandez C, Emer J, Robinson JK. Perioperative management of medications for psoriasis and psoriatic arthritis: a review for the dermasurgeon. Dermatol Surg. 2008;34(4):446–459. 200
- Bibbo C, Goldberg JW. Infectious and healing complications after elective orthopaedic foot and ankle surgery during tumor necrosis factor-alpha inhibition therapy. Foot Ankle Int. 2004;25(5):331–335.
- Busti AJ, Hooper JS, Amaya CJ, et al. Effects of perioperative antiinflammatory and immunomodulating therapy on surgical wound healing. Pharmacotherapy. 2005;25(11):1566–1591.
- Colombel JF, Loftus EV Jr, Tremaine WJ, et al. Early postoperative complications are not increased in patients with Crohn's disease treated perioperatively with infliximab or immunosuppressive therapy. Am J Gastroenterology. 2004;99(5):878–883.
- Pieringer H, Stuby U, Biesenbach G. Patients with rheumatoid arthritis undergoing surgery: how should we deal with antirheumatic treatment? Semin Arthritis Rheum. 2007;36(5):278–826.
- den Broeder AA, Creemers MC, Fransen J, et al. Risk factors for surgical site infections and other complications in elective surgery in patients with rheumatoid arthritis with special attention for anti-tumor necrosis factor: a large retrospective study. J Rheumatol. 2007;34(4):689–695.
- McIntyre N. Clinical presentation of acute viral hepatitis. Br Med Bull. 1990;46(2):533–547.
- Aslanidis S, Vassiliadis T, Pyrpasopoulou A, et al. Inhibition of TNFalpha does not induce viral reactivation in patients with chronic hepatitis C infection: two cases. Clin Rheumatol. 2007;26(2):261–264.
- Gomez-Gallego M, Meca-Lallana J, Fernandez-Barreiro A. Multiple sclerosis onset during etanercept treatment. Eur Neurol. 2008;59(1–2):91–93.
- Kemanetzoglou E, Andreadou E. CNS demyelination with TNF-α Blockers. Curr Neurol Neurosci Rep. 2017;17(4):36.
- Segal BM, Constantinescu CS, Raychaudhuri A, et al. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 2008;7(9):796–804.
- Havrdová E, Belova A, Goloborodko A, et al. Activity of secukinumab, an anti-IL-17A antibody, on brain lesions in RRMS: results from a randomized, proof-of-concept study. J Neurol. 2016;263(7):1287–1295.
- Armstrong EJ, Harskamp CT, Armstrong AW. Psoriasis and major adverse cardiovascular events: a systematic review and meta-analysis of observational studies. JAMA. 2013;2:e000062.
- Gulliver WP, Young HM, Bachelez H, et al. Psoriasis patients treated with biologics and methotrexate have a reduced rate of myocardial infarction: a collaborative analysis using international cohorts. J Cutan Med Surg. 2016;20(6):550–554.
- Papp K, Gottlieb AB, Naldi L, et al. Safety surveillance for ustekinumab and other psoriasis treatments from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Drugs Dermatol. 2015;14:706–714.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58(5):826–850.
- Desai SB, Furst DE. Problems encountered during anti-tumour necrosis factor therapy. Best Pract Res Clin Rheumatol. 2006;20(4):757–790.
- Bai F, Li GG, Liu Q, et al. Short-term efficacy and safety of IL-17, IL-12/23, and IL-23 inhibitors brodalumab, secukinumab, ixekizumab, ustekinumab, guselkumab, tildrakizumab, and risankizumab for the treatment of moderate to severe plaque psoriasis: a systematic review and network meta-analysis of randomized controlled trials. J Immunol Res. 2019;2019:2546161.
- Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359(9317):1541–1549.
- Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004;350(9):876–885.
- Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006;130(2):323–591.
- Sandborn WJ, Lee SD, Randall C, et al. Long-term safety and efficacy of certolizumab pegol in the treatment of Crohn's disease: 7-year results from the PRECiSE 3 study. Aliment Pharmacol Ther. 2014;40(8):903–916.
- Sandborn WJ, Hanauer SB, Katz S, et al. Etanercept for active Crohn's disease: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2001;121(5):1088–1094.
- Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2016;375(20):1946–1960.
- Feagan BG, Sandborn WJ, D'Haens G, et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn's disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2017;389(10080):1699–1709.
- Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61(12):1693–1700.
- An de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75(1):83–98.e4.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375(4):345–356.
- Eich K, Leonardi C, Langley RG, et al. Inflammatory bowel disease among patients with psoriasis treated with ixekizumab: a presentation of adjudicated data from an integrated database of 7 randomized controlled and uncontrolled trials [published correction appears in J Am Acad Dermatol. 2017 Aug;77(2):390–390.e1]. J Am Acad Dermatol. 2017;76(3):441–448.e2.
- Wetter DA, Davis MD. Lupus-like syndrome attributable to anti-tumor necrosis factor alpha therapy in 14 patients during an 8-year period at Mayo Clinic. Mayo Clin Proc. 2009;84(11):979–984.
- Williams EL, Gadola S, Edwards CJ. Anti-TNF-induced lupus. Rheumatology. 2009;48(7):716–720.
- Winchester D, Duffin KC, Hansen C. Response to ustekinumab in a patient with both severe psoriasis and hypertrophic cutaneous lupus. Lupus. 2012;21(9):1007–1010.
- Varada S, Gottlieb AB, Merola JF, et al. Treatment of coexistent psoriasis and lupus erythematosus. J Am Acad Dermatol. 2015;72(2):253–260.