Abstract
Objective
In PSO-LONG, long-term proactive management (PAM) of psoriasis with fixed-dose combination calcipotriol 50 µg/g and betamethasone dipropionate 0.5 mg/g (Cal/BD) aerosol foam was superior to conventional reactive management. This post-hoc analysis investigated long-term PAM with Cal/BD foam in PSO-LONG patients who could be more susceptible to corticosteroid-induced hypothalamic-pituitary-adrenal (HPA) axis suppression.
Methods
Efficacy and safety of PAM with Cal/BD foam (twice-weekly) versus reactive management (twice-weekly vehicle foam), with once-daily rescue Cal/BD foam for four weeks following relapse, was assessed in the HPA subgroup (n = 66); patients had moderate-to-severe psoriasis (physician global assessment score ≥3; 10–30% body surface area affected). Primary endpoint was time to first relapse.
Results
PAM with Cal/BD foam was associated with longer median time to first relapse (111 versus 31 days), reduced risk of first relapse (hazard ratio: 0.49; p = .029), greater proportion of days in remission (17%; p = .001) and reduced rate of relapse (60% reduction; p < .001) than reactive management. Adverse events occurred in 37.5% (PAM) and 47.1% (reactive management) of patients, with no new safety signals. No clinically significant HPA-axis suppression was observed.
Conclusion
Efficacy of PAM with Cal/BD foam is maintained in patients with moderate-to-severe psoriasis, with no new safety signals.
Introduction
Topical therapy is the standard of care for treatment of mild-to-moderate psoriasis (Citation1), while in moderate-to-severe psoriasis, topicals are used as an adjunct to phototherapy and systemic treatment, including small molecules and biologic agents (Citation1,Citation2). The chronic nature of psoriasis necessitates long-term treatment with topicals, such as corticosteroids and vitamin D3 analogues. Very few safety concerns around vitamin D3 analogues exist (Citation1,Citation3), although exceeding the maximum dose, for example in patients with psoriatic lesions over a large body surface area (BSA), may rarely cause hypercalcemia and hypercalciuria (Citation4,Citation5).
Beyond any local adverse effects, a systemic, albeit rare, concern with topical steroid use is suppression of the hypothalamic-pituitary-adrenal (HPA) axis (Citation6). Corticosteroids can decrease corticotrophin-releasing hormone (CRH) synthesis and secretion, thus blocking the trophic and adrenocorticotropic hormone (ACTH)-releasing actions of CRH on the anterior pituitary. As a result, the adrenal cortex loses the ability to produce cortisol leading to adrenal insufficiency. Long-term use of systemic corticosteroids has been associated with reductions in calcium absorption from the intestine and calcium reabsorption in the kidney, leading to parathyroid hormone-induced bone loss and an increased risk of fractures (Citation7); this association is relevant for patients with psoriasis using topical treatment (Citation8).
The most potent and efficacious corticosteroids are only approved for use up to 4 weeks (Citation9). Long-term treatment has been primarily reactive, treating relapses rather than proactively aiming to maintain remission (Citation10). Recent data from the PSO-LONG trial has provided evidence for the utility of a proactive approach to long-term management of psoriasis, which is now recognized in guidelines for the treatment of psoriasis with topical therapy (Citation9).
In the Phase III PSO-LONG trial (NCT02899962) proactive, twice-weekly application of fixed-dose combination of calcipotriol 50 µg/g and betamethasone dipropionate 0.5 mg/g (Cal/BD) aerosol foam (Enstilar®; LEO Pharma, Ballerup, Denmark) was shown to be more effective at preventing or delaying disease relapse in patients with mild-to-severe psoriasis than reactive management for up to 52 weeks; reactive management in the trial involved the use of vehicle foam twice weekly, with patients receiving once-daily Cal/BD foam for 4-weeks as rescue treatment only upon relapse (Citation11).
In the proactive group (n = 256), the median time to first relapse was nearly 2-fold higher than for the reactive group (n = 265) (56 vs. 30 days, respectively) and patients in the proactive group had an additional 41 days in remission compared with the reactive group after 1 year (p < .001). Both proactive and reactive management approaches to treatment had favorable safety and tolerability profiles (Citation11).
A subgroup of patients from the PSO-LONG trial with more severe disease (moderate–severe vs. mild–severe for the overall patient population) and greater BSA affected (10–30 vs. 2–30% for the overall patient population) at the start of the open-label lead-in period were selected to undergo HPA axis testing. This was undertaken to better define the long-term safety of proactive and reactive approaches to treatment. Data from this group are included in the current post-hoc analysis.
Methods
Trial design
The PSO-LONG trial was a Phase III, multicenter trial, which assessed long-term proactive versus reactive management of psoriasis, the details of which have been described previously (Citation11). Briefly, after a screening and washout phase of up-to four weeks, all eligible patients entered a four-week open-label lead-in phase, during which they applied Cal/BD foam (calcipotriol 50 µg/g and betamethasone dipropionate 0.5 mg/g) once daily to psoriatic lesions on the trunk and/or limbs. Patients who achieved treatment success (physician global assessment [PGA] score of ‘clear’=0 or ‘almost clear’=1, with at least two-grade improvement from baseline; this was the full analysis set [FAS]) were then randomized 1:1 to receive Cal/BD foam (proactive management group) or vehicle foam (reactive management group) twice weekly for 52 weeks in a double-blinded manner.
Patients from either group who relapsed (PGA ≥2) during the maintenance phase received Cal/BD foam, applied to lesions once daily for four weeks, as rescue treatment. Patients who regained PGA <2 following rescue re-started the twice-weekly maintenance treatment per their randomized allocation, while patients who failed to respond were withdrawn from the trial.
Disease rebound was defined as follows: modified Psoriasis Area and Severity Index (m-PASI) ≥12 and an increase in m-PASI ≥ 125% of baseline; or development of new pustular, erythrodermic, or more inflammatory psoriasis within 2 months of treatment discontinuation after the open-label lead in or once-daily rescue medication, or within 2 months after maintenance phase end. For disease rebound, the baseline value was the modified Psoriasis Area and Severity Index (m-PASI) value at the start of the rebound follow-up period. A rebound follow-up period was defined as one of the following: two months after discontinuation of open-label phase treatment; or two months after discontinuation of relapse treatment; or two months after discontinuation of maintenance treatment.
The focus of the current analysis is the subgroup of patients from PSO-LONG with more severe disease who underwent HPA axis testing (herein referred to as the HPA subgroup).
Patients
Detailed inclusion criteria for the PSO-LONG trial have been described previously (Citation11). Briefly, patients were ≥18 years old, with truncal and/or limb psoriasis over 2–30% of BSA, a PGA ≥2 (mild or higher) and m-PASI ≥2 prior to the open-label lead-in phase (referred to as ‘baseline’ hereafter). Prohibited medications included biologic therapies with the potential to effect psoriasis in the 4–16 weeks prior to baseline (depending on therapy) and non-biologic therapies with a potential effect on psoriasis in the four weeks prior to baseline.
Patients included in this HPA subgroup analysis had more severe disease, with an affected BSA of 10–30% and a moderate-to-high PGA score (PGA ≥3) at baseline.
Endpoints
Efficacy and safety endpoints for the HPA subgroup analysis were those from the main trial (Citation11).
The primary endpoint was time to the first relapse (PGA score ‘mild’ or higher) during the maintenance phase. Secondary endpoints were the proportion of days in remission (PGA score ‘clear’/‘almost clear’) and the number of relapses during the 52-week double-blind maintenance phase. A patient-reported outcome for this HPA subgroup analysis was the effect of the patient’s psoriasis on their health-related quality of life (QoL) using the validated, 10-item, patient reported Dermatology Life Quality Index (DLQI) (Citation12). The DLQI was to be completed at all monthly visits and unscheduled visits during the maintenance phase.
Safety assessments included adverse events (AEs), treatment-related AEs, incidence of disease rebound, local safety and tolerability (perilesional assessment at each visit for erythema, erosions, dryness and edema), serum and urine calcium levels and HPA-axis suppression (defined as serum cortisol ≤18 µg/dL at 30 min following ACTH stimulation) (Citation13), which was evaluated at baseline, randomization, midway through (28 weeks) and at the end (52 weeks) of the maintenance phase, or early withdrawal.
Statistical methods
Detailed statistical methods have been described in detail previously (Citation11). Briefly, time to first relapse between groups was analyzed using a Cox proportional hazard model, with the following as factors: treatment group, pooled sites and PGA score at randomization. The proportion of days in remission was analyzed using an analysis of variance model, with the following factors: treatment group, pooled sites and PGA score at randomization. Multiple imputation was used for handling missing data for withdrawn participants. The number of relapses was analyzed using a Poisson regression model with treatment group, pooled sites and PGA at randomization as fixed factors, patient as a random effect, and time at risk as an offset variable.
Results
Demographics and baseline characteristics
The HPA subgroup population included 66 randomized patients, although three were randomized in error after failing to achieve treatment success at the end of the 4-week open-label lead in phase. These patients were not analyzed for efficacy but were included in the safety assessments.
At baseline, patients in the HPA subgroup had a mean (standard deviation [SD]) m-PASI score of 11.4 (3.7) and a mean (SD) BSA of 15.2% (6.2%). The majority were male (59.1%), White (93.9%) and had a PGA score of moderate (97.0%) ().
Table 1. Patient demographics and baseline characteristics.
Efficacy
During the maintenance phase, the difference in median time to the first relapse between the proactive and reactive management groups was 80 days: 111 (95% confidence interval [CI]: 29, 253) and 31 days (95%: CI 29, 55), respectively in the HPA subgroup (); this was numerically higher than the 26 days reported for the FAS (Citation11).
Figure 1. Time to first relapse during the maintenance phase with proactive management or reactive management with Cal/BD in the HPA subgroup. Cal/BD: calcipotriol 50 µg/g and betamethasone dipropionate 0.5 mg/g; HPA: hypothalamic-pituitary-adrenal. Censoring is not displayed for clarity.
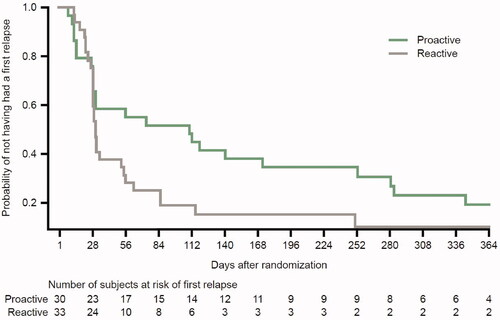
The risk of experiencing the first relapse in the HPA subgroup was significantly reduced in patients in the proactive versus reactive management group (hazard ratio [HR]: 0.49; 95% CI: 0.26, 0.93; p = .029); this reduction was numerically greater in the HPA subgroup than that previously reported for the FAS (HR: 0.57; 95% CI: 0.47, 0.69; p < .001) (Citation11).
In the HPA subgroup, like the FAS, patients on proactive treatment spent a greater proportion of days in remission (mean [SD]: 73.8% [22.4%]; observed number) compared with those on reactive treatment (60.3% [22.6%]; observed number; ). The estimated difference in the proportion of days in remission between proactive and reactive treatment was 17% (95% CI: 7, 27%; p = .001) in the HPA subgroup, corresponding to 61.5 additional days in remission per year; in the FAS, the estimated difference in the proportion of days in remission between proactive and reactive treatment was 11% (95% CI: 8, 14%; p < .001), corresponding to 41 additional days in remission per year (Citation11).
Table 2. Number of relapses and time in remission: summary.
In line with the FAS (Citation11), patients on proactive treatment in the HPA subgroup had fewer relapses compared with reactive treatment during the maintenance phase (mean [SD]: 2.2 [2.0] versus 3.7 [2.7], respectively; observed numbers; ). The estimated reduction in the rate of relapse between proactive and reactive treatment was 60% (95% CI: 34, 76%, p < .001) in the HPA subgroup and 46% (95% CI: 37, 54%; p < .001) in the FAS (Citation11) (corresponding to 2.4 and 1.7 fewer relapses per year, respectively).
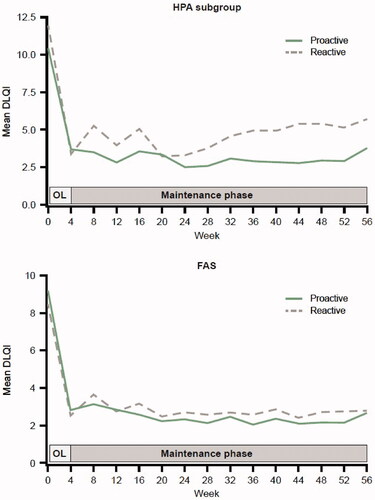
The mean DLQI scores decreased rapidly in the proactive and reactive groups during the open-label phase, then stabilized at a lower level throughout the maintenance phase. The mean DLQI score was, in general, numerically slightly lower in the proactive compared with the reactive group throughout the maintenance phase in the HPA subgroup and the FAS ().
Figure 2. Mean DLQI score by time during the maintenance phase for proactive and reactive Cal/BD management groups in the HPA subgroup and FAS. Cal/BD: calcipotriol 50 µg/g and betamethasone dipropionate 0.5 mg/g; DLQI: Dermatology Life Quality Index; FAS: full analysis set; HPA: hypothalamic-pituitary-adrenal; OL: open-label.
Safety
During the maintenance phase, 52 AEs were reported in total in the HPA subgroup, comprising 23 AEs in 12 patients (37.5%) in the proactive group and 29 AEs in 16 patients (47.1%) in the reactive group (). In the reactive group, two patients (5.9%) experienced serious AEs (venous thrombosis and appendicitis, both assessed as not related to treatment by the investigator) and one patient (2.9%) experienced an AE that led to the withdrawal from the trial (reported as pregnancy). No serious AEs or AEs leading to withdrawal were reported with proactive treatment in the HPA subgroup. For comparison, in the overall population, 48.9% of patients in the proactive group and 47.6% in the reactive group experienced AEs, with similar reports for serious AEs (5.1 vs. 4.0% patients, respectively) and AEs leading to withdrawal (0.7 vs. 0.4% patients, respectively) (Citation11).
Table 3. Summary of AEs during the maintenance phase for the HPA subgroup.
In the HPA subgroup, treatment-related AEs were experienced by two patients (6.3%) from the proactive group (2 events) and two patients (5.9%) from the reactive group (3 events) (). Moderate bronchitis was the most commonly reported AE with two patients from the reactive group experiencing one event each. All other treatment-related AEs were reported in no more than one patient each.
Table 4. Treatment-related AEs during the maintenance phase for the HPA subgroup.
In the HPA subgroup, four patients in each of the proactive and reactive groups had rebounds. All occurred during the maintenance phase as determined by m-PASI score; none were reported as AEs.
HPA-axis suppression was observed in four patients in the HPA subgroup after 30 min (two in the reactive group, two in the proactive group) and one patient after 60 min (proactive group) (); none of the patients had HPA-axis suppression at both time points. Most patients in the HPA subgroup were within the normal ranges for serum and urinary calcium from randomization to end of the trial, similar to the results for the overall population (Citation11).
Table 5. Effect on corticosteroid metabolism.
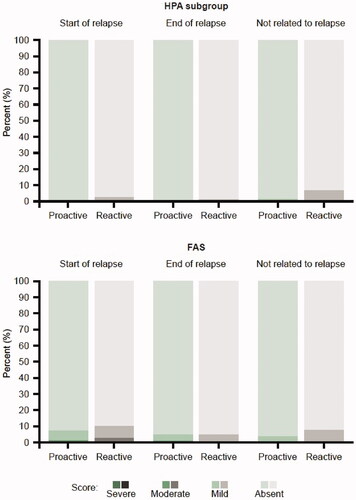
Local safety and tolerability of Cal/BD foam after relapse was favorable across all four parameters (redness, dryness, edema, and erosion) in the HPA subgroup and the FAS ().
Figure 3. Patient’s perilesional assessment of local safety and tolerability (redness, dryness, edema, and erosion) relative to relapse in the HPA subgroup and FAS. Percentages are taken out of the total number of assessments done at visits where a relapse has started, a relapse has ended or where a relapse has not started or ongoing (not related to relapse). FAS: full analysis set; HPA: hypothalamic-pituitary-adrenal.
Discussion
The PSO-LONG trial demonstrated that long-term (up to 56 weeks including the open-label lead-in) proactive management with twice-weekly Cal/BD foam was superior to conventional reactive management (vehicle foam twice weekly, with once-daily Cal/BD foam for four weeks as rescue medication on relapse) in patients with psoriasis (Citation11). This subgroup analysis suggests that the efficacy and safety of long-term proactive management with Cal/BD foam is maintained in patients from the PSO-LONG trial with more severe disease (PGA ≥3 vs. PGA ≥2) that affects a greater BSA (10–30 vs. 2–30%).
Although the HPA subgroup patients are expected to be at higher risk of corticosteroid-induced systemic toxicity, due to their higher BSA and PGA (greater amount of product used and potentially greater absorption), no clinically significant HPA-axis suppression was observed in either treatment group. Four patients (3 proactive; 1 reactive) met the threshold for HPA axis suppression at 30 min after ACTH challenge (defined in the trial protocol as serum cortisol ≤18 µg/dL at 30 min following ACTH stimulation); the lowest recorded value between them was 13 µg/dL, and all achieved normal cortisol levels by 60 min. The lowest post-ACTH challenge serum cortisol level recorded (11.3 µg/dL) among the HPA subgroup was for one patient in the proactive treatment group. Although it met the criteria for HPA axis suppression according to trial criteria with regards to serum cortisol level (but not time), caution is advised with interpreting this result as suppression was only seen at the 60 min timepoint, and the initial measurement (0 min) was higher, indicating a labeling or sampling error likely occurred.
The low rate of HPA axis suppression observed for the HPA subgroup is consistent with prior studies of short-term Cal/BD treatment. Previously, only 3/43 (7%) patients (BSA affected 15–30%) had adrenal suppression after 4 weeks’ treatment with once-daily Cal/BD suspension/gel and none of the remaining patients had a 30 min serum cortisol ≤18 μg/dL, despite continuing treatment for a further 4 weeks (Citation14). In addition, no clinically relevant HPA axis suppression was observed with once-daily Cal/BD foam treatment over 4 weeks in patients with moderate-to-severe, extensive psoriasis (15–30% of BSA, including ≥30% of scalp) (Citation15) and no HPA axis suppression was observed following once-daily Cal/BD foam treatment for 4 weeks in adolescent patients (12–17 years) with mean affected BSA of approximately 19% (Citation16).
Although systemic absorption of calcipotriol, a synthetic vitamin D analogue, following topical overdose, can affect calcium metabolism leading to hypercalcemia (Citation17), there have been no reported clinically relevant changes in serum and urine calcium in adolescent (Citation16) and adult patients (Citation14,Citation15) following Cal/BD short-term use (4–8 weeks). Similarly, and despite the HPA subgroup having higher affected BSA, there was no indication that calcium homeostasis was affected by long-term proactive management in these patients.
The efficacy of proactive Cal/BD foam appeared to be improved in the HPA subgroup compared with the FAS, based only on a comparison of numerical values between the two groups (no statistical analyses undertaken). The time to the first relapse and time spent in remission were numerically longer in the HPA subgroup than the treatment differences in the FAS; there was also a numerically greater reduction in the number of relapses per year in the HPA subgroup than the FAS (Citation11). This may be secondary to the small sample size sample resulting in greater variability, or that it is easier to observe a greater improvement in cases of more severe disease, i.e. an improvement from more severe disease at baseline to less severe disease during treatment.
The notable improvements in DLQI scores in response to daily Cal/BD treatment during the 4-week open label lead-in phase are consistent with several other studies that have assessed the effect of Cal/BD foam on health-related quality of life over the short term (Citation18). Importantly, the improvement was largely maintained throughout the 52-week maintenance period for the HPA subgroup and the FAS, suggesting that proactive management of psoriasis with Cal/BD foam might have a positive long-term impact on patients’ quality of life.
The safety profile was similar for proactive and reactive treatment within the HPA subgroup. In keeping with observations for the overall population (Citation11), similar proportions of patients in each group experienced AEs, with few considered treatment-related or serious, collectively supporting that having more severe disease and higher affected BSA does not translate to an increased risk of Cal/BD-related AEs.
Limitations of the study
This subgroup analysis was not powered to analyze differences between the treatment groups and owing to the small sample size, caution is advised when interpreting the data.
Conclusions
The results of this analysis suggest that even in patients with more severe psoriasis, long-term proactive management with fixed-dose Cal/BD foam has the potential to offer improved disease control over conventional reactive treatment, with no new safety signals or clinically significant suppression of the HPA axis.
Acknowledgments
Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Gregor Fyfe, PhD, of Ashfield MedComms, an Ashfield Health company, and funded by LEO Pharma.
Disclosure statement
Kim Papp: Consultant for AbbVie, Akros, Amgen, Arcutis, Atellas, Avillion, Bausch Health/Valeant, Baxalta, Boehringer Ingelheim, Can-Fite Biopharma, Celgene, Coherus, Dermavant, Dermira, Dow Pharma, Eli Lilly and Company, Evelo, Galapagos, Galderma, Genentech, Incyte, Janssen, Kyowa Hakko Kirin, Leo Pharma A/S, Merck (MSD), Merck Serono, Novartis, Pfizer, PRCL Research, Regeneron, Roche, Sanofi-Aventis/Genzyme, Takeda, UCB. Received research grants from Anacor, Gilead, GSK, MedImmune, Moberg Pharma and Sun Pharma. Scientific officer for Akros, Anacor, Arcutis, Dice Pharmaceuticals and Kyowa Hakko Kirin. Consultant for Dice Pharmaceuticals, Meiji Seika Pharma and Mitsubishi Pharma. Speaker, received honoraria and participated in steering committees and advisory boards for AbbVie, Amgen, Bausch Health/Valeant, Celgene, Eli Lilly and Company, Janssen, Merck (MSD), Novartis, Pfizer and Sanofi-Aventis/Genzyme. Speaker for Astellas, Galderma, Incyte, Kyowa Hakko Kirin and Leo Pharma A/S. Participated in steering committees for Boehringer Ingelheim, Kyowa Hakko Kirin, Merck Serono and Regeneron. Participated in advisory boards for Astellas, Boehringer Ingelheim, Bristol-Myers Squibb, Dow Pharma, Galderma, Regeneron, Sun Pharma and UCB. Received honoraria from Akros, Boehringer Ingelheim, Coherus, Galderma, Kyowa Hakko Kirin, Merck Serono, Mitsubishi Pharma, PRCL Research, Takeda and UCB.
Zygmunt Adamski: Investigator in clinical trials sponsored by LEO Pharma.
Lyn Guenther: Consultant, investigator, and speaker for AbbVie, Amgen, Bausch Health, Celgene, Eli Lilly and Company, Janssen, Leo Pharma, Merck, Pfizer and UCB. Speaker and consultant for Miravo.
Monika Liljedahl: Is an employee of LEO Pharma.
Agnieszka Miasik-Pogodzinska: Investigator in clinical trials sponsored by LEO Pharma.
Anna Szponar-Bojda: Investigator in clinical trials sponsored by LEO Pharma.
Charles Lynde: Consultant, speaker and investigator for AbbVie, Amgen, AnaptysBio, Bausch Health, Bristol Meyers Squibb, Boehringer Ingelheim, Celgene, Eli Lilly and Company, Galderma, Genentech, GSK, Janssen, Leo Pharma, L'Oreal, Merck, Novartis, Pfizer, Sanofi Genzyme, and UCB. Investigator for Asana Biosciences, Concert, Dermira, Glenmark, Incyte, and Kyowa.
Terri Nutt: Investigator in clinical trials sponsored by LEO Pharma.
Marie Holst Mørch: Is an employee of LEO Pharma.
Stephen Tyring: Investigator in clinical trials sponsored by LEO Pharma.
William Werschler: Consultant, independent contractor or speaker for Abbvie, Allergan, Celgene, Eskata, Leo Pharma, Merz, Prescriber’s Choice, Pfizer, Suneva, Ulthera, and Xoft. Received honoraria from Abbvie, Celgene, Continued Med, llergan, Leo Pharma, Merz, Prescriber’s Choice and Suneva.
Adam Reich: Principal Investigator or Subinvestigator in clinical trials sponsored by AbbVie, Drug Delivery Solutions Ltd, Galderma, Genentech, Janssen, Kymab Limited, Leo Pharma, Menlo Therapeutics, MetrioPharm, MSD, Novartis, Pfizer, and Trevi. Consultant or speaker for AbbVie, Bioderma, Celgene, Chema Elektromet, Eli Lilly and Company, Galderma, Janssen, Leo Pharma, Medac, Menlo Therapeutics, Novartis, Pierre-Fabre, Sandoz, and Trevi.
Neil Sadick: Consultant for Allergan Inc., Cutera Inc., Lypolysis Robotics, Lumisque, Prescriber’s Choice and Venus Concept; has received honoraria from Allergan, Endo International plc, Storz and Venus Concept.
Irina Turchin: Consultant, speaker or investigator for Abbvie, Amgen, Arcutis, Bausch Health, Celgene, Eli Lilly and Company, Galderma, Janssen, Leo Pharma, Novartis, Pfizer, Sanofi, Sun Pharma, and UCB.
Jean-Philippe Lacour: Received grant and consulting fees from AbbVie, Amgen, Boehringer-Ingelheim, Celgene, Eli Lilly and Company, Galderma International, Janssen, LEO Pharma, Merck, Novartis, Pfizer, Pierre Fabre, and Sanofi.
Additional information
Funding
References
- Kim WB, Jerome D, Yeung J. Diagnosis and management of psoriasis. Can Fam Physician. 2017;63(4):278–285.
- Feldman SR, Goffe B, Rice G, et al. The challenge of managing psoriasis: unmet medical needs and stakeholder perspectives. Am Health Drug Benefits. 2016;9(9):504–513.
- Stein Gold LF. Topical therapies for psoriasis: improving management strategies and patient adherence. Semin Cutan Med Surg. 2016;35(2 Suppl 2):S36–S44, quiz S45.
- Georgiou S, Tsambaos D. Hypercalcaemia and hypercalciuria after topical treatment of psoriasis with excessive amounts of calcipotriol. Acta Derm Venereol. 1999;79(1):86.
- Bourke JF, Mumford R, Whittaker P, et al. The effects of topical calcipotriol on systemic calcium homeostasis in patients with chronic plaque psoriasis. J Am Acad Dermatol. 1997;37(6):929–934.
- Hengge UR, Ruzicka T, Schwartz RA, et al. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol. 2006;54(1):1–15, quiz 16-8.
- Nicolaides NC, Pavlaki AN, Maria Alexandra MA, et al. Glucocorticoid therapy and adrenal suppression. In: Feingold KR, Anawalt B, Boyce A, et al. editors. Endotext. South Dartmouth (MA): MDText.com, Inc. Copyright © 2000-2021, MDText.com, Inc.; 2000.
- Sirufo MM, De Pietro F, Bassino EM, et al. Osteoporosis in skin diseases. Int J Mol Sci. 2021;21(13):4749.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84(2):432–470.
- Bonnekoh B, Fernández García JJ, Surber C, et al. Innovation that drives your dermatological future. EMJ Dermatology. 2017;5(1):36–43.
- Lebwohl M, Kircik L, Lacour JP, et al. Twice-weekly topical calcipotriene/betamethasone dipropionate foam as proactive management of plaque psoriasis increases time in remission and is well tolerated over 52 weeks (PSO-LONG trial). J Am Acad Dermatol. 2020;S0190-9622(20):32625–32626.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)-a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216.
- Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(2):364–389.
- Silver S, Tuppal R, Gupta AK, et al. Effect of calcipotriene plus betamethasone dipropionate topical suspension on the hypothalamic-pituitary-adrenal axis and calcium homeostasis in subjects with extensive psoriasis vulgaris: an open, non-controlled, 8-week trial. J Drugs Dermatol. 2013;12(8):882–887.
- Taraska V, Tuppal R, Olesen M, et al. A novel aerosol foam formulation of calcipotriol and betamethasone has no impact on HPA axis and calcium homeostasis in patients with extensive psoriasis vulgaris. J Cutan Med Surg. 2016;20(1):44–51.
- Seyger M, Abramovits W, Liljedahl M, et al. Safety and efficacy of fixed-dose combination calcipotriol (50 μg/g) and betamethasone dipropionate (0.5 mg/g) cutaneous foam in adolescent patients (aged 12 to <17 years) with plaque psoriasis: results of a phase II, open-label trial. J Eur Acad Dermatol Venereol. 2020;34(9):2026–2034.
- Bourke JF, Berth-Jones J, Hutchinson PE. Hypercalcaemia with topical calcipotriol. BMJ. 1993;306(6888):1344–1345.
- Jalili A, Yosipovitch G. Fixed-dose combination calcipotriol/betamethasone dipropionate foam provides a rapid onset of action, effective itch relief and improves patient quality of life. J Eur Acad Dermatol Venereol. 2021;35 Suppl 1(1):20–27.
