Abstract
Purpose
Guselkumab, an interleukin (IL)-23 inhibitor, effectively treats moderate-to-severe plaque psoriasis.
Materials and methods
ECLIPSE, was a Phase 3, multicenter, 56-week, double-blinded, active-comparator study of guselkumab vs. secukinumab (IL-17A inhibitor) in patients with moderate-to-severe psoriasis. Patients were treated with guselkumab 100 mg (n = 534) or secukinumab 300 mg (n = 514) through week 44. Efficacy (at least a 90% and 100% improvement from baseline in Psoriasis Area and Severity Index [PASI 90 and PASI 100], Investigator’s Global Assessment [IGA] 0/1, and IGA 0) was analyzed across subpopulations defined by baseline: age (<45, 45 to <65, and ≥65 years old), body weight, body mass index (BMI), psoriasis disease severity (body surface area, disease duration, PASI, and IGA), psoriasis by body regions (head, trunk, upper and lower extremities), and prior psoriasis medication history at week 48.
Results
Overall, 1048 patients were randomized. At week 48, numerically greater proportions of patients achieved PASI 90, PASI 100, IGA 0/1, and IGA 0 with guselkumab vs. secukinumab regardless of baseline age, body weight, BMI, disease severity, body region, and prior medication. The largest differences were in patients ≥65 years old and patients weighing >100 kg.
Conclusions
Guselkumab treatment provided greater efficacy vs. secukinumab at week 48 in most subpopulations of patients with psoriasis.
Introduction
Newer classes of biologic agents, including interleukin (IL)-23 (Citation1–5) and IL-17A inhibitors (Citation6,Citation7), have enabled a greater proportion of patients with moderate-to-severe plaque psoriasis to achieve clear or almost clear skin. Despite these advances, not all biologics work as well in different patient populations, as differences in demographics and disease characteristics, especially weight, can affect the efficacy of biologics (Citation8–12), and these differences may change over time. Therefore, when selecting a therapy for an individual patient with psoriasis, it is often useful to understand the relative performance of each treatment within various clinically relevant and identifiable subpopulations.
Guselkumab, an IL-23p19 subunit inhibitor, has been shown to be highly efficacious and well-tolerated with up to 4 years of continuous treatment for moderate-to-severe psoriasis (Citation13,Citation14). In the recent Phase 3 comparator trial, ECLIPSE (Citation3), guselkumab showed superior long-term efficacy compared with secukinumab (an IL-17A inhibitor) at week 48, the primary endpoint. In the current report, we evaluated the consistency of clinical response across various subpopulations of patients with psoriasis from the ECLIPSE study, including age, body weight, body mass index (BMI), psoriasis disease severity, psoriasis by body regions, and prior psoriasis medication history.
Patients and methods
Patients
Details of the Phase 3 ECLIPSE study (NCT03090100) and eligibility criteria have been previously presented (Citation3). Briefly, ECLIPSE was a multicenter, randomized, double-blinded, comparator-controlled study of guselkumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis. Overall, eligible patients (≥18 years old) had moderate-to-severe plaque-type psoriasis defined as Psoriasis Area and Severity Index (PASI) ≥12, Investigator’s Global Assessment [IGA] score ≥3 (moderate), body surface area (BSA) involvement ≥10%, a history of psoriasis for ≥6 months, and were candidates for phototherapy or systemic therapy (Citation3). The study protocol was approved by Institutional Review Boards at each site and participants provided written informed consent prior to any study-related procedures. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Study design
The study design has been previously presented (Citation3). Patients were randomized in a 1:1 ratio at baseline to receive guselkumab 100 mg (n = 534) subcutaneously at weeks 0, 4, 12, and every-8-weeks thereafter through week 44, or secukinumab 300 mg (n = 514) administered subcutaneously as two 150-mg injections at weeks 0, 1, 2, 3, 4, and every-4-weeks thereafter through week 44. Patients randomized to guselkumab also received placebo injections to match the number and frequency of secukinumab injections to maintain the blind (Citation3).
Efficacy assessments
Efficacy was assessed at week 48 using data from ECLIPSE in the following baseline subpopulations: (1) age; (2) body weight and BMI; (3) psoriasis disease severity; (4) psoriasis by body regions (head, trunk, upper extremities, and lower extremities); and (5) prior psoriasis medication history. Endpoints included at least a 90% improvement from baseline in PASI (PASI 90), PASI 100, IGA 0/1 (cleared or minimal), and IGA 0 (cleared) at week 48 compared with secukinumab.
PASI measures psoriasis severity with investigator-determined regional subscores for erythema, induration, scaling, and percentage of BSA affected (Citation15). The regional PASI components of head, trunk, and upper, and lower extremities were assessed. IGA captures the investigator’s assessment of the patient’s psoriasis lesions (induration, erythema, and scaling) with a score of clear (0), minimal (1), mild (2), moderate (3), or severe (4).
Statistical analyses
Analyses were performed using descriptive statistics data for the identified subpopulations in the ECLIPSE study for the endpoints described above. Differences in the proportion of patients achieving a clinical response and the associated 95% confidence intervals (CIs) for the between group differences were calculated for the analyses of PASI 90, PASI 100, IGA 0/1, and IGA 0 by subpopulation. The 95% CIs were calculated by Wald asymptotic confidence limits with continuity correction. Data handling rules were the same as those used for the primary and major secondary analyses (Citation3). The analyses of PASI 90 and PASI 100 at week 48 by baseline body weight were post-hoc analyses. Testing hypotheses were not performed for subgroup analyses due to multiplicity adjustment considerations and lack of sufficient power to detect subgroup effects for some categories of baseline factors.
Results
Baseline demographics and disease characteristics
Baseline demographics and disease characteristics were generally comparable between patients treated with guselkumab and secukinumab (). Overall, most patients were White (93.4%), men (67.5%), approximately 46 years old, with a mean BMI of 29.9. Patients had approximately 24% BSA involvement, 99.9% had moderate to severe IGA scores, and mean PASI was 20.0. At baseline, prior psoriasis medication history was comparable between the treatment groups ().
Table 1. Baseline demographics and disease characteristics from ECLIPSE study (Citation3).
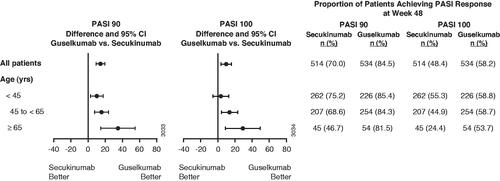
Efficacy by age at baseline
PASI 90 and PASI 100 response rates were numerically higher at week 48 in the guselkumab group than the secukinumab group across all 3 age categories: <45 years, 45 to <65 years, and ≥65 years (). Similarly, IGA 0/1 responses (<45 years: 85.8% [guselkumab] vs. 81.3% [secukinumab], between group difference 95% CI: [-2.4, 11.5]; 45 to <65 years: 85.4 vs. 73.4%, 95% CI: [4.1, 19.9]; ≥65 years: 79.6 vs. 44.4%, 95% CI: [15.1, 55.3]) and IGA 0 responses (<45 years: 64.6 vs. 58.0%, 95% CI: [-2.5, 15.6]; 45 to <65 years: 61.4 vs. 46.4%, 95% CI: [5.5, 24.5]; ≥65 years: 55.6 vs. 24.4%, 95% CI: [10.8, 51.4]) were also numerically higher at week 48 for guselkumab-treated patients compared with secukinumab-treated patients, respectively.
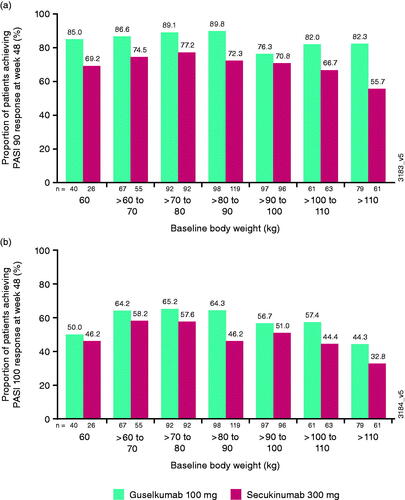
Efficacy by baseline body weight and BMI
The proportions of patients achieving PASI 90 or PASI 100 responses at week 48 by baseline body weight (from <60 to >110 kilograms [kg]) were numerically higher for guselkumab-treated vs. secukinumab-treated patients, especially in the heaviest patients (>100 kg) (). Similarly, the proportion of patients achieving IGA 0/1 responses at week 48 were generally numerically higher for guselkumab-treated vs. secukinumab-treated patients, respectively (≤60 kg: 77.5% [guselkumab] vs. 76.9% [secukinumab]; >60 to 70 kg: 85.1 vs. 74.5%; >70 to 80 kg: 90.2 vs. 79.3%; >80 to 90 kg: 91.8 vs. 79.8%; >90 to 100 kg: 79.4 vs. 81.3%; >100 to 110 kg: 78.7 vs. 69.8%; >110 kg: 86.1 vs. 55.7%) and for IGA 0, respectively (≤60 kg: 52.5 vs. 50.0%; >60 to 70 kg: 65.7 vs. 60.0%; >70 to 80 kg: 68.5 vs. 59.8%; >80 to 90 kg: 71.4 vs. 48.7%; >90 to 100 kg: 57.7 vs. 53.1%; >100 to 110 kg: 62.3 vs. 44.4%; >110 kg: 50.6 vs. 34.4%).
Figure 2. Proportion of patients achieving (a) at least a 90% improvement in Psoriasis Area and Severity Index (PASI 90) or (b) PASI 100 response at week 48 by baseline body weight deciles. kg: kilograms.
Likewise, the proportions of patients achieving PASI 90 at week 48 by baseline BMI were numerically higher for guselkumab-treated vs. secukinumab-treated patients (normal [<25 kg/m2]: 88.1 vs. 75.2%; overweight [≥25 to <30 kg/m2]: 84.1 vs. 73.4%; obese [≥30 kg/m2]: 82.5 vs. 65.3%). Similar results were reported for PASI 100 (normal: 64.2 vs. 57.8%; overweight: 61.4 vs. 53.7%; obese: 52.5 vs. 40.4%), IGA 0/1 (normal: 85.8 vs. 77.1%; overweight: 86.9 vs. 81.9%; obese: 83.0 vs. 69.3%), and IGA 0 (normal: 68.7 vs. 60.6%; overweight: 64.2 vs. 54.2%; obese: 57.0 vs. 43.1%).
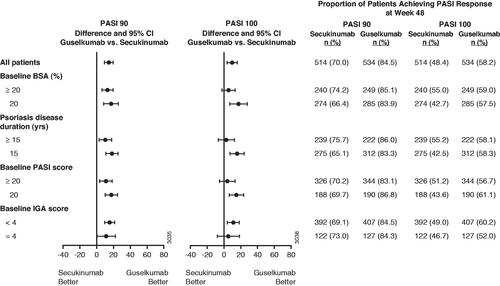
Efficacy by baseline disease severity
At week 48, the proportions of patients achieving PASI 90 or PASI 100 responses were numerically higher for guselkumab-treated vs. secukinumab-treated patients for all subgroups defined by baseline disease severity including: baseline BSA, disease duration, baseline PASI, and baseline IGA score (). Likewise, the proportion of patients achieving IGA 0/1 responses by baseline disease characteristics were numerically higher for guselkumab-treated vs. secukinumab-treated patients, respectively (BSA involvement <20%: 85.9 vs. 80.8%, 95% CI: [-1.9, 12.1]; BSA involvement ≥20%: 84.2 vs. 69.7%, 95% CI: [7.3, 21.8]; psoriasis disease duration <15 years: 84.7 vs. 77.0%, 95% CI: [0.1, 15.3]; psoriasis disease duration ≥15 years: 85.3 vs. 73.1%, 95% CI: [5.3, 19.1]; PASI <20: 83.1 vs. 78.8.%, 95% CI: [-1.9, 10.5]; PASI ≥20: 88.4 vs. 68.1%, 95% CI: [11.7, 28.9]; IGA <4: 86.2 vs. 75.8%, 95% CI: [4.8, 16.1]; IGA = 4: 81.1 vs. 72.1%, 95% CI: [-2.3, 20.2]), and IGA 0 responses (BSA involvement <20%: 64.3 vs. 57.5%, 95% CI: [-2.3, 15.8]; BSA involvement ≥20%: 60.4 vs. 44.2%, 95% CI: [7.7, 24.7]; psoriasis disease duration <15 years: 63.5 vs. 57.3%, 95% CI: [-3.2, 15.5]; psoriasis disease duration ≥15 years: 61.2 vs. 44.4%, 95% CI: 8.5, 25.2]; PASI <20: 61.3 vs. 53.1%, 95% CI: [0.5, 16.0]; PASI ≥20: 63.7 vs. 45.7%, 95% CI: [7.5, 28.3]; IGA <4: 64.1 vs. 51.3%, 95% CI: [5.8, 19.9]; IGA = 4: 55.9 vs. 47.5%, 95% CI: [-4.8, 21.5]).
Efficacy by baseline psoriasis per body region
At week 48, numerically greater improvements in PASI component scores were reported for the following body regions: head, trunk, upper extremities, and lower extremities in guselkumab-treated patients compared with secukinumab-treated patients ().
Table 2. Efficacy of Psoriasis Area and Severity Index component responses by body region at week 48.
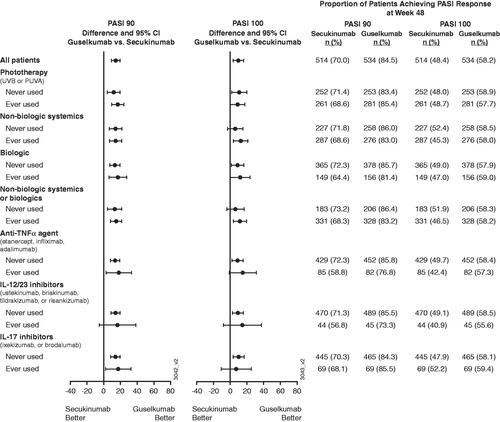
Efficacy by prior psoriasis medication history
Regardless of previous psoriasis medication history, a numerically greater proportion of guselkumab-treated patients achieved PASI 90 or PASI 100 responses at week 48 compared with secukinumab-treated patients (). Similar results were observed for IGA 0/1 responses (phototherapy: 86.1 vs. 77.0%, 95% CI: [2.2, 16.0]; non-biologic systemics or biologics: 84.8 vs. 75.5%, 95% CI: [2.9, 15.6]; non-biologic systemics: 85.1 vs. 76.3%, 95% CI: [2.0, 15.7]; biologic: 84.0 vs. 71.8%, 95% CI: [2.3, 22.1]; tumor necrosis factor-alpha (TNFα) inhibitor: 81.7 vs. 64.7%, 95% CI: [2.6, 31.4]; IL-12/23 inhibitors: 77.8 vs. 63.6%, 95% CI: [-6.8, 35.1]; IL-17 inhibitors: 87.0 vs. 75.4%, 95% CI: [-2.8, 25.9]) and for IGA 0 (phototherapy: 61.6 vs. 49.8%, 95% CI: [3.1, 20.4]; non-biologic systemics or biologics: 61.3 vs. 47.7%, 95% CI: [-1.9, 18.7]; non-biologic systemics: 61.2 vs. 46.7%, 95% CI: [6.0, 23.0]; biologic: 60.9 vs. 47.7%, 95% CI: [1.5, 25.0]; TNFα inhibitor: 58.5 vs. 43.5%, 95% CI: [-1.2, 31.2]; IL-12/23 inhibitors: 57.8 vs. 40.9%, 95% CI: [-5.9, 39.6]; IL-17 inhibitors: 60.9 vs. 52.2%, 95% CI: [-9.2, 26.6]) at week 48, with guselkumab-treated patients having numerically greater responses compared with secukinumab-treated patients, respectively.
Figure 4. Proportion of patients achieving at least a 90% improvement in Psoriasis Area and Severity Index (PASI 90) or PASI 100 response at week 48 by prior psoriasis medication history at baseline. 95% CI: 95% confidence interval; IL: interleukin; PUVA: psoralen plus ultraviolet A; TNFα: tumor necrosis factor alpha; UVB: ultraviolet B.
Discussion
With the approval of newer biologic agents, including the IL-23 and IL-17 inhibitors, increased efficacy with complete skin clearance is often attainable (Citation1,Citation2,Citation4–6,Citation16–18). The ECLIPSE study (Citation3) provided direct efficacy comparisons between therapies from the two newest and most effective psoriasis drug classes available. Distinct domains of psoriasis, such as scalp, nail, and hand/feet (Citation17,Citation19–21), and different subgroups, defined by weight (Citation9,Citation22,Citation23) and previous therapy, may respond differently to targeted treatments. Here, we showed that guselkumab treatment resulted in numerically higher levels of efficacy compared with secukinumab treatment in most subpopulations of patients described above, including in patients with more severe baseline disease (baseline BSA >20%, PASI >20) and in patients previously-treated with systemics or biologics.
Notably, guselkumab was more effective in heavier patients, which is important because most patients with psoriasis are overweight (Citation24,Citation25), and obesity may affect a patient’s response to treatment (Citation26). Perhaps the most striking difference occurred in patients 65 years of age and older; patients in this age subgroup who received guselkumab had much better clinical responses than patients in this age group who received secukinumab. Patients with longer disease duration at baseline treated with guselkumab also responded significantly better than those treated with secukinumab. In real-world practice, despite the variety of treatment options for psoriasis, some patients continue to experience psoriasis activity (Citation27). When considering available treatment options, it is useful for clinicians to have comparative data, like those presented here, when making treatment decisions for patients of varying demographics and disease characteristics.
A limitation of this analysis was that efficacy data were reported only through 48 weeks; longer term efficacy comparisons would be useful, but the ECLIPSE study was limited to 1 year. In addition, this report was a subgroup analysis. Also, when considering prior medication history, there could be recall bias. We also did not present comparisons between these drugs in terms of their efficacy on psoriatic joint disease. Of note, both guselkumab and secukinumab are now approved to treat active psoriatic arthritis (Citation28,Citation29). Safety comparisons within each subpopulation were also not assessed between the two treatments, as the safety of each of treatment was relatively similar in the full set of patients through week 48 (Citation3).
In conclusion, guselkumab treatment provided greater efficacy compared with secukinumab at week 48 across different psoriasis patient subpopulations defined by baseline characteristics, including age, body weight, BMI, disease severity, affected body region, and prior psoriasis medication history. These findings should optimize patient care by guiding clinicians in treatment selection for diverse patient populations.
Ethical approval
The study protocol for the ECLIPSE study (NCT03090100) was approved by Institutional Review Boards at each site. All participants provided written informed consent.
Acknowledgments
The authors wish to thank Kristin Ruley Sharples, PhD, of Janssen Scientific Affairs, LLC, Horsham, PA for her writing and editorial support in the preparation of this manuscript.
Disclosure statement
A. Blauvelt has served as a scientific adviser and/or clinical study investigator for AbbVie, Almirall, Arena, Athenex, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Eli Lilly, Evommune, Forte, Galderma, Incyte, Janssen, LEO Pharma, Novartis, Pfizer, Rapt, Regeneron, Sanofi Genzyme, Sun Pharmaceutical, and UCB Pharma. A. W. Armstrong has served as a research investigator and/or scientific advisor to LEO Pharma, AbbVie, UCB, Janssen, Eli Lilly, Novartis, Ortho Dermatologics, Sun Pharmaceutical, Dermavant, Bristol Myers Squibb, Sanofi, Regeneron, Dermira, and Modmed. R. G. Langley has served/received compensation in the form and or Honoria as Principle I investigator for/or on the scientific advisory board/or served as a speaker for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Janssen, LEO Pharma, Eli Lilly, Merck, Novartis, Pizer, Sun Pharmaceutical, and UCB Pharma. K. Gebauer has served as an advisory board member and investigator for AbbVie, Janssen, LEO Pharma, Sun Pharmaceutical, and UCB, and has received educational grants from AbbVie, Janssen, Sanofi, and Sun Pharmaceutical. L. C. Guenther has served as a consultant, investigator, and speaker for AbbVie, Amgen, Bausch Health, Celgene, Eli Lilly, GlaxoSmithKline, Janssen, LEO Pharma, Merck, Novartis, Pfizer, and Sun Pharmaceutical; has been a speaker and consultant for Actelion; and has been an investigator for Boehringer Ingelheim, and UCB. C. Paul has served as an investigator or consultant for AbbVie, Almirall, Astellas, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Galderma, Janssen Cilag, LEO Pharma, Merck, Novartis, Pfizer, Regeneron, Sanofi, and UCB Pierre Fabre; K. Reich has served as advisor and/or paid speaker for and/or participated in clinical trials sponsored by AbbVie, Affibody, Almirall, Amgen, Avillion, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Covagen, Dermira, Eli Lilly, Forward Pharma, Fresenius Medical Care, Galapagos, GlaxoSmithKline, Janssen, Janssen-Cilag, Kyowa Kirin, LEO Pharma, Medac, Merck Sharp & Dohme, Novartis, Miltenyi Biotec, Ocean Pharma, Pfizer, Regeneron, Samsung Bioepis, Sanofi, Sun Pharmaceutical, Takeda, UCB, Valeant, and Xenoport; B. Randazzo, S. Flavin, M.-C. Hsu, and Y. You are all employees of Janssen Research & Development, LLC, and own stock in Johnson & Johnson, of which Janssen is a subsidiary.
Data availability statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Additional information
Funding
References
- Blauvelt A, Papp KA, Griffiths CEM, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–407.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76(3):418–431.
- Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394(10201):831–839.
- Reich K, Griffiths CEM, Gordon KB, et al. Maintenance of clinical response and consistent safety profile with up to 3 years of continuous treatment with guselkumab: results from the VOYAGE 1 and VOYAGE 2 trials. J Am Acad Dermatol. 2020;82(4):936–945.
- Reich K, Gooderham M, Thaçi D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394(10198):576–586.
- Langley RG, Elewski BE, Lebwohl M, et al. ERASURE and FIXTURE Study Groups. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338.
- Gordon KB, Blauvelt A, Papp KA, et al. UNCOVER-1, UNCOVER-2, and UNCOVER-3 Study Groups. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375(4):345–356.
- Kaushik SB, Lebwohl MG. Psoriasis: Which therapy for which patient: psoriasis comorbidities and preferred systemic agents. J Am Acad Dermatol. 2019;80(1):27–40.
- Gordon KB, Blauvelt A, Foley P, et al. Efficacy of guselkumab in subpopulations of patients with moderate-to-severe plaque psoriasis: a pooled analysis of the phase III VOYAGE 1 and VOYAGE 2 studies. Br J Dermatol. 2018;178(1):132–139.
- Xie KK, Braue A, Martyres R, et al. Baseline patients' characteristics as predictors for therapeutic survival and response in patients with psoriasis on biological treatments. Australas J Dermatol. 2018;59(4):e247–e252.
- Karczewski J, Poniedziałek B, Rzymski P, et al. Factors affecting response to biologic treatment in psoriasis. Dermatol Ther. 2014;27(6):323–330.
- Poulin Y, Ramon M, Rosoph L, et al. Efficacy of tildrakizumab by patient demographic and disease characteristics across a phase 2b and 2 phase 3 trials in patients with moderate-to-severe chronic plaque psoriasis. J Eur Acad Dermatol Venereol. 2020;34(7):1500–1509.
- Griffiths CEM, Papp KA, Song M, et al. Continuous treatment with guselkumab maintains clinical responses through 4 years in patients with moderate-to-severe psoriasis: results from VOYAGE 1. J Dermatol Treat. 2020;13(1):1–9.
- Reich K, Armstrong AW, Foley P, et al. Maintenance of response through up to 4 years of continuous guselkumab treatment of psoriasis in the VOYAGE 2 phase 3 study. Am J Clin Dermatol. 2020;21(6):881–890.
- Fredriksson T, Pettersson U. Severe psoriasis-oral therapy with a new retinoid. Dermatologica. 1978;157(4):238–244.
- Reich K, Puig L, Szepietowski JC, et al. Secukinumab dosing optimization in patients with moderate-to-severe plaque psoriasis: results from the randomized, open-label OPTIMISE study. Br J Dermatol. 2020;182(2):304–315.
- Lebwohl MG, Gordon KB, Gallo G, et al. Ixekizumab sustains high level of efficacy and favourable safety profile over 4 years in patients with moderate psoriasis: results from UNCOVER-3 study. J Eur Acad Dermatol Venereol. 2020;34(2):301–309.
- Sawyer LM, Malottki K, Sabry-Grant C, et al. Assessing the relative efficacy of interleukin-17 and interleukin-23 targeted treatments for moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response. PLOS One. 2019;14(8):e0220868.
- Elewski BE, Baker CS, Crowley JJ, et al. Adalimumab for nail psoriasis: efficacy and safety over 52 weeks from a phase-3, randomized, placebo-controlled trial. J Eur Acad Dermatol Venereol. 2019;33(11):2168–2178.
- Foley P, Gordon K, Griffiths CEM, et al. Efficacy of guselkumab compared with adalimumab and placebo for psoriasis in specific body regions: a secondary analysis of 2 randomized clinical trials. JAMA Dermatol. 2018;154(6):676–683.
- Armstrong AW, Vender R, Kircik L. Secukinumab in the treatment of palmoplantar, nail, scalp, and pustular psoriasis. J Clin Aesthet Dermatol. 2016;9(6 Suppl 1):S12–S16.
- Pinter A, Gerdes S, Papavassilis C, et al. Characterization of responder groups to secukinumab treatment in moderate to severe plaque psoriasis. J Dermatolog Treat. 2020;31(8):769–775.
- Menter A, Gordon KB, Leonardi CL, et al. Efficacy and safety of adalimumab across subgroups of patients with moderate to severe psoriasis. J Am Acad Dermatol. 2010;63(3):448–456.
- Snekvik I, Smith CH, Nilsen TIL, et al. Obesity, waist circumference, weight change, and risk of incident psoriasis: prospective data from the HUNT Study. J Invest Dermatol. 2017;137(12):2484–2490.
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2(12):e54.
- Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: implications for management. J Am Acad Dermatol. 2017;76(3):393–403.
- Wolf P, Weger W, Legat F, et al. Quality of life and treatment goals in psoriasis from the patient perspective: results of an Austrian cross-sectional survey. J Dtsch Dermatol Ges. 2018;16(8):981–990.
- TREMFYA® [guselkumab] [package insert]. Horsham, PA: Janssen Biotech Inc.; 2021.
- COSENTYX® [secukinumab] [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2021.