Abstract
Objective
The current study aimed to investigate the prognosis and treatment of primary cutaneous angiosarcoma (PCA) and primary subcutaneous angiosarcoma (PSCA), and tried to develop a prognostic nomogram model of them.
Methods
A total of 1763 cases retrieved from the Surveillance, Epidemiology, and End Results (SEER) database were retrospectively analyzed. Survival analyses were performed to explore the prognosis of patients and the effects of different treatment methods. All data were randomly allocated into a training set and a testing set to develop and validate the nomogram model.
Results
The findings showed that age, sex, grade, tumor size, multiple primary malignant tumors, stage, primary site surgery (PSS), radiotherapy (RT), and chemotherapy (CT) were correlated with prognosis (p < .05). The nomogram achieved good accuracy in predicting the prognosis. PSS + RT + CT showed the best prognosis for patients in stages I, II, and III (p < .05).
Conclusion
PCA and PSCA are rare with poor prognoses. Patients undergoing PSS may not gain survival benefits from combining with RT or (and) CT, whereas PSS + RT + CT should be actively performed in earlier stages to improve the prognosis of patients. The nomogram model can be used to predict the overall survival rate and guide better treatment.
Introduction
Angiosarcomas are a subtype of soft-tissue sarcomas, which account for approximately 2% of all sarcoma cases (Citation1). They are rare, aggressive, malignant tumors characterized by poor prognosis and mainly originate from vascular or lymphatic endothelial cells. Angiosarcoma has a definite predilection for cutaneous and subcutaneous soft tissues (Citation2). Studies on the prognosis of primary cutaneous angiosarcoma (PCA) and primary subcutaneous angiosarcoma (PSCA) are limited by a small sample size owing to the rare nature of these tumors.
The Surveillance, Epidemiology, and End Results (SEER) database is an authoritative cancer database in the United States, with survival results showing high accuracy and positive predictive value (Citation3,Citation4). Nomograms are graphical representations of predictors’ effects on the outcome that give the reader a more tangible interpretation (Citation5). Prognostic nomogram models can facilitate better treatment stratification and outcome evaluation to improve health care (Citation6). Therefore, our study retrospectively explored 1763 cases diagnosed with PCA and PSCA from 1975 to 2016 retrieved from the SEER database. We investigated the prognosis and treatment of these tumors and constructed a prognostic nomogram model with high accuracy that might be helpful for clinical decision-making.
Materials and methods
Data sources and selection criteria
Patients who had been diagnosed with PCA and PSCA between1975 and 2016 were retrieved from the SEER database (n = 2885) (ICD-O-3 code: 9120, Primary site code: C44.0-44.9 – Skin, C47.0-47.9 – Peripheral nerves and autonomic nervous system, C49.0-49.9 – Connective, subcutaneous and other soft tissues) and included in the current study. Exclusion criteria: (1) patients without histology confirmation of the sarcomas (n = 45); (2) patients who were younger than 18 years old at diagnosis (n = 21); (3) patients who had been previously diagnosed with other primary malignant tumors (n = 1053); (4) patients whose survival information was not available (n = 3).
Methods
Data of PCA and PSCA patients enrolled in the SEER database were extracted by using SEER*Stat 8.3.6 software. The cutoff points of the year of diagnosis were set as 1996 and 2008 by using X-tile software (Kaplan–Meier method). The cutoff point of tumor size was 5 cm based on the American Joint Committee on Cancer (AJCC) guide for T staging of soft tissue sarcoma. 60 and 70-years-old were set as the cutoff points of age. The tumor stage was determined according to the AJCC seventh edition TNM staging system. R 4.0.2 software was applied to perform statistical analyses and draw survival curves (p by Log-rank test).
Statistical analyses
Univariate and multivariate cox analyses were performed to patients’ baseline characteristics, including the year of diagnosis, primary anatomic tumor site (PATS), age, sex, race, grade (G), tumor size, multiple primary malignant tumors (MPMT), laterality, stage, primary site surgery (PSS), removal of regional lymph nodes (RRLN), non-primary site surgery (NPSS), radiotherapy (RT), chemotherapy (CT) and radiation sequence with PSS. A total of 1763 cases were randomly divided into a training set and a testing set according to 7:3, χ2 tests were used to compare the difference among groups, and then the two datasets were used to develop and validate the prognostic nomogram model of PCA and PSCA. Concordance index (C-index) and calibration curves were used to evaluate the prediction effect of the nomogram model, and internal and external verification were performed separately.
Results
Baseline characteristics of patients
Baseline characteristics of PCA and PSCA patients included in the current study were presented in . The number of PCA and PSCA patients slightly increased from 1975 to 1999 and showed a significant increase in 2000 then maintained a mildly growing tendency up to 2016 (Figure S(A)). Approximately 80.0% of patients were diagnosed from 1997 to 2016. Head, face, and neck were the common sites of these tumors. Age ranged from 18 to 102-years-old and the median age was 70-years-old. Old patients (≥60-years-old) represented 72.3% of the total patients with a peak incidence at 70–80-years-old (Figure S(B)). Male patients were slightly more compared with female patients and the male-female ratio was approximately 1.4:1. Patients with high grade (G3 and G4) accounted for 35.7%. Patients with tumor size >5 cm comprised 23.0% of the total patients and patients who were at stage IV accounted for 9.4%. The percentage of patients who underwent PSS, RT, and CT were 67.5%, 40.5%, and 28.5%, respectively, whereas 26.7% of patients received postoperative RT.
Table 1. Baseline characteristics of PCA and PSCA patients (%).
Survival analysis
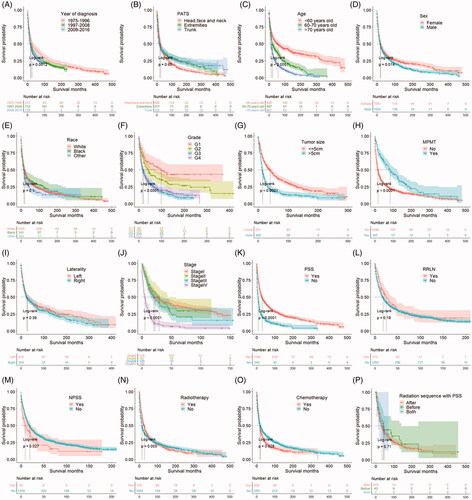
All patients were on active follow-up from diagnosis to the end of the study (December 31, 2016). Out of the selected 1763 cases, 24.7% of patients were alive throughout the study period (n = 436), 55.3% of patients died of PCA or PSCA (n = 974), 19.3% of patients died of other causes (n = 340), and 0.7% of patients died of unknown reasons (n = 13). The overall survival time was 0 to 479 months and the median survival time was 16 months. The findings showed that the 0.5-, 1-, 3- and 5-year overall survival rates were 74.6%, 59.6%, 32.4%, and 22.7%, respectively. Analyses of survival curves showed a survival difference in year of diagnosis, age, sex, grade, tumor size, MPMT, stage, PSS, NPSS, RT, and CT separately (p < .05), while no survival difference was observed in several variables including PATS (p = .650), race (p = .100), laterality (p = .390), RRLN (p = .190), and radiation sequence with PSS (p = .710, ).
Figure 1. Survival curves of different variables. (A) Year of diagnosis. (B) PATS: Primary anatomic tumor site. (C) Age. (D) Sex. (E) Race. (F) Grade. (G) Tumor size. (H) MPMT: Multiple primary malignant tumors. (I) Laterality. (J) Stage. (K) PSS: Primary site surgery. (L) RRLN: Removal of regional lymph nodes. (M) NPSS: Non-primary site surgery. (N) Radiotherapy. (O) Chemotherapy. (P) Radiation sequence with PSS.
Univariate and multivariate cox analysis
The results of univariate and multivariate cox analysis were presented in . Univariate cox analysis showed that several factors were associated with the prognosis of PCA and PSCA patients (p < .05), including the year of diagnosis, age, sex, race, grade, tumor size, MPMT, stage, PSS, NPSS, RT, and CT. Multivariate cox analysis based on variables with p-value <.05 from univariate cox analysis, indicated that age, sex, grade, tumor size, MPMT, stage, PSS, RT, and CT were independent prognostic factors of PCA and PSCA (p < .05). The findings showed that patients between 60 and 70-years-old (p < .001, HR = 1.766, 95%CI = 1.491–2.092) and patients >70-years-old (p < .001, HR = 2.666, 95%CI = 2.296–3.096) had a higher risk of death compared with patients <60-years-old. Males had a worse prognosis compared with females (p = .007, HR = 1.168, 95%CI = 1.044–1.308). The prognoses of patients who were in G3 (p < .001, HR = 1.877, 95%CI = 1.340–2.629) and G4 (p < .001, HR = 1.947, 95%CI = 1.394–2.720) were poorer compared with patients in G1 group. The risk of death in patients with tumor size was >5 cm was higher compared with patients whose tumor size was ≤5 cm (p < .001, HR = 1.593, 95%CI = 1.357–1.870). Patients diagnosed with PCA and PSCA combined with other malignant tumors showed a lower risk of death compared with those who were diagnosed only with PCA and PSCA (p < .001, HR = 0.554, 95%CI = 0.455–0.676). Patients with stage IV tumors had a worse prognosis compared with patients who were at stage I (p < .001, HR = 2.631, 95%CI = 2.098–3.299). The prognosis of patients who received PSS (p < .001, HR = 2.041, 95%CI = 1.784–2.336), RT (p < .001, HR = 1.246, 95%CI = 1.108–1.401), and CT (p = .001, HR = 1.252, 95%CI = 1.091–1.437) was better compared with patients who did not receive these therapies.
Table 2. The results of univariate and multivariate cox analysis.
Development and validation of the prognostic nomogram model
All data variables were randomly divided into a training set (n = 1234) and a testing set (n = 529). χ2 test showed that the basic characteristics of the three groups reached inter-group equilibrium as shown in (p >.05). Variables including age, sex, grade, tumor size, MPMT, stage, PSS, RT, and CT were used to construct the prognostic nomogram model. The findings showed that a higher total score of independent prognostic factors was correlated with a better 0.5-, 1-, 3-, and 5-year survival rate (). The prediction model developed in this study showed good prediction accuracy with an internal verification C-index of 0.707 and an external verification C-index of 0.719. The calibration curves showed that the 0.5-, 1-, 3-, and 5-year survival rates predicted by the nomogram were consistent with the actual survival rate ().
Figure 2. The prognostic nomogram model of PCA and PSCA patients. MPMT: multiple primary malignant tumors; PSS: primary site surgery; RT: radiotherapy; CT: chemotherapy.
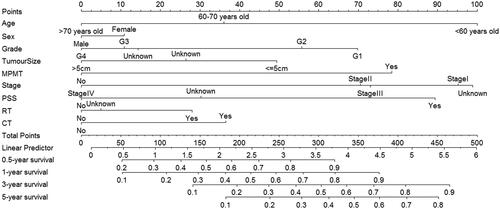
Figure 3. Calibration curves of the training set and the testing set. (A, B, C, D) Calibration curves that are created in the light of the training set sample compare predicted and actual survival proportions at (A) 0.5-year, (B) 1-year, (C) 3-year and (D) 5-year, separately. (a, b, c, d) Calibration curves that are created in the light of the testing set sample compare predicted and actual survival proportions at (a) 0.5-year, (b) 1-year, (c) 3-year and (d) 5-year, separately.
Subgroup analyses based on different treatment modalities
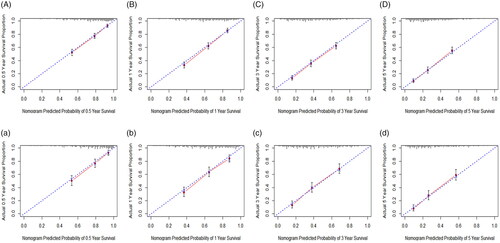
Findings from multivariate cox analysis indicated that PSS, RT, and CT could improve the prognosis of PCA and PSCA patients. Moreover, the prognosis of patients in stage I was different from that of patients in stage IV (p < .05). However, there was no prognostic difference among stage I, stage II, and stage III patients (p > .05). Therefore, patients who had not accepted any treatment (n = 219) and patients whose treatment modalities were unknown (n = 48) were excluded to regroup. Subgroup analyses were performed to investigate the effects of different treatment modalities in the total population group (n = 1496), stage IV group (n = 126), and stages I, II, and III groups (n = 436). As shown in , survival curves of different treatment modalities in the total population group and stages I, II, and III groups were statistically significant (p < .0001). However, there was no survival difference of patients undergoing various treatment modalities in the stage IV group (p = .540). As shown in , there was no difference among PSS, PSS + RT, and PSS + RT + CT (p > .05). Notably, PSS showed the best prognosis followed by PSS + CT (p = .005, HR = 1.408, 95%CI = 1.112–1.784), CT + RT (p < .001, HR = 1.606, 95%CI = 1.250–2.063), CT (p < .001, HR = 2.177, 95%CI = 1.765–2.685), and RT (p < .001, HR = 2.689, 95%CI = 2.128–3.399) in total population group. Patients who received PSS + RT + CT showed a better prognosis (p = .010, HR = 0.557, 95%CI = 0.357–0.868) compared with those who underwent PSS alone in stages I, II, and III groups. However, there was no obvious prognostic difference among various treatment modalities for patients in the stage IV group (p > .05).
Figure 4. Survival curves of different treatment modalities in various groups. (A) Survival curves of different treatment modalities in total population group. (B) Survival curves of different treatment modalities in stage I, II and III group. (C) Survival curves of different treatment modalities in stage IV group. PSS: primary site surgery; RT: radiotherapy; CT: chemotherapy.
Table 3. Univariate cox analysis of different treatment modalities in various groups.
Discussion
Approximately 0.5/1,000,000 persons in the US have been diagnosed with angiosarcoma, with about 200 new cases reported every year (Citation7). Although the causes of most cases are unknown, risk factors related to this sarcoma, include prior radiation, chemicals exposure, foreign material retention, chronic lymphedema, familial syndromes, arteriovenous fistula, ultraviolet ray exposure, and immunodeficiency (Citation1,Citation3,Citation8). PCA and PSCA are rare. However, the number of cases was steadily growing, which might be attributed to enhanced diagnostic levels and more risk factors exposure. A sharp increase was observed in 2000 which was likely attributed to the 2000 International Classification of Diseases for Oncology version 3 (ICD-O-3). ICD-O-3 probably improved the diagnosis of patients that previously ignored. Although the number of patients was increasing yearly, our study found that the prognosis of patients in different years had no significant difference. Therefore, it is necessary to explore this tumor.
Angiosarcomas can occur in any part of the body, but they are commonly found in the head, face, and neck, followed by extremities and trunk (Citation9,Citation10). The findings of the current study were similar to previous findings. The findings showed that PATS probably had no effect on the prognosis of patients and tumors grew in one side of a paired site whether the left or the right hardly affected the prognosis. No significant difference in the incidence of the tumor occurred on the left and those occurring on the right. However, most cases grew in the non-paired site. Analysis showed no significant correlation between race and prognosis. Most of the patients in the SEER database are white, thus this conclusion should be explored further.
Angiosarcoma is a malignant tumor with a poor prognosis (Citation1). Several studies report that PCA and PSCA commonly occur in elderly males (Citation1,Citation3,Citation11). In the current study, the old and male patients showed higher incidence and poor prognosis, which could be attributed to the health status of elderly patients, occupations, and hormones. Fayette et al. (Citation12) report that tumor size and histological grade are prognostic factors for angiosarcoma. Large lesions (tumor size > 5 cm) and high grade were related to a worse prognosis. Poor therapeutic outcomes for patients with large lesions and high grades may be associated with poor prognosis. Effects of other malignant tumors which are secondary to PCA and PSCA remain uncertain. In our study, patients diagnosed with PCA and PSCA only showed a worse prognosis. However, MPMT cases were few and there was no information on subsequent primary malignant tumors in the SEER database, thus this finding should be further explored. The tumor stage is an important factor that determines the prognosis (Citation13). Patients diagnosed with distant metastasis showed a poor prognosis (Citation1,Citation14). The findings showed that stage IV characterized by distant metastasis had higher mortality.
Surgical resection of primary lesion improves the survival rate of angiosarcoma (Citation1,Citation3,Citation8,Citation11,Citation15–17). Most patients had access to PSS, which could be attributed to the high number of patients in earlier stages. Radical surgery with complete resection is the primary treatment approach for angiosarcoma (Citation1). However, negative resection margins are difficult to achieve owing to the invasive and multifocal nature of angiosarcomas and the correlation of lesions with other anatomical structures (Citation1,Citation8,Citation10–13,Citation16,Citation18,Citation19). As a result, although patients can receive PSS, the prognosis is still poor. Previous studies report that lymph node involvement of angiosarcoma comprises approximately 20% and two-thirds of these cases are isolated metastases without synchronous hematogenous spread (Citation12,Citation14). Approximately 20–30% of patients present with metastatic disease at diagnosis owing to the aggressive nature of angiosarcoma and approximately a half of the patients with the localized disease develop metastases after diagnosis (Citation8,Citation12,Citation18–22). The most common sites of metastasis include lung, bone, and liver (Citation12,Citation23). Surgical removal of the metastatic lesion is not effective (Citation10). The findings showed that RRLN and NPSS hardly improved the prognosis. The number of patients who received RRLN or NPSS was few, thus further studies should be conducted to explore the relationship between these treatment approaches and prognosis.
Previous studies report that RT can improve the prognosis of patients (Citation8,Citation12,Citation13,Citation24). Although radiation performed prior to surgical resection is well-tolerated, Mark et al. report that it has no survival benefit (Citation25). Studies report that surgical resection with radiation may improve local control and overall survival (Citation8,Citation10,Citation13,Citation14,Citation18,Citation26–28). However, other studies report that outcomes are not different when patients undergo postoperative RT or not (Citation12,Citation24). In the current study, RT could improve the prognosis of PCA and PSCA patients. However, radiation sequence with PSS possibly showed no effect on prognosis even though postoperative RT was conducted more often. Moreover, patients who received PSS showed no survival benefits from combining with RT. Several studies report that CT is beneficial for patients with metastatic disease (Citation1,Citation10,Citation11,Citation14,Citation20). Mainly used CT drugs include anthracyclines, ifosfamide, and taxanes (Citation1,Citation20). Neoadjuvant CT is performed for unresectable tumors (Citation20). Adjuvant CT is associated with a good prognosis (Citation29–31), can reduce the risk of metastasis (Citation1), and is better for patients with large tumors (Citation32,Citation33). These analyses found a lower risk of death in patients treated with CT. However, CT probably did not improve the prognosis of patients undergoing PSS. No data on CT sequence with surgery and CT regimen was unavailable in the SEER database, thus this study could not further explore the effects of these.
Previous studies report that surgery can improve survival rates compared with RT or CT (Citation15,Citation22). Similar findings were observed in the current study that PSS might be the primary treatment. It is challenging to obtain negative surgical margins (Citation27), frequent local recurrences (Citation13,Citation24,Citation34), and the risk of metastasis (Citation1), thus postoperative RT and adjuvant CT should be performed following surgery in local lesions to improve prognosis (Citation11,Citation13,Citation20,Citation33). PSS + RT + CT should be actively performed when patients were in earlier stages. Notably, analyses showed no significant prognostic difference among various treatment modalities for patients in stage IV. The overall prognosis is poor, especially for patients with metastasis (Citation8,Citation10,Citation12). Therefore, novel therapies including molecular targeted therapies, immunotherapy, and the beta-blocker propranolol should be developed (Citation3,Citation11,Citation35–41).
Conclusion
PCA and PSCA are rare with poor prognoses. Patients undergoing PSS may not gain survival benefits from combining with RT or (and) CT, whereas PSS + RT + CT should be actively performed in earlier stages to improve the prognosis of patients. The nomogram model can be used to predict the overall survival rate and guide better treatment.
Ethical approval
This study is exempt from the approval processes of the Institutional Review Boards because the SEER database patient information is available in a public database. A patient consent form was also not applicable.
Authors’ contributions
Jinqian Mao and Jin Hu conceptualized and designed the study, performed analysis, interpreted data, and drafted the article. Yunfei Chen performed analysis and interpreted data.Yiqing Li and Xiaoqian Run interpreted and revised the manuscript. All authors approved the final article and accept accountability for all aspects of the work.
Acknowledgment
Authors thank the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries for providing high-quality open resources for researchers.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Original data presented in the study are publicly available. The data can be found at the site: https://seer.cancer.gov/data/
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11(10):983–991.
- Yu J, Steiner FA, Muench JP, et al. Juxtathyroidal neck soft tissue angiosarcoma presenting as an undifferentiated thyroid carcinoma. Thyroid. 2002;12(5):427–432.
- Conic RRZ, Damiani G, Frigerio A, et al. Incidence and outcomes of cutaneous angiosarcoma: a SEER population-based study. J Am Acad Dermatol. 2020;83(3):809–816.
- Sharma A, Jung MK, Polce SA, et al. Surveillance epidemiology and end results (SEER) reported survival outcomes have a high accuracy and positive predictive value when tested using randomized controlled trials (RCTs) as gold standard. Int J Radiat Oncol Biol Phys. 2019;105(1):E467–E468.
- Park SY. Nomogram: an analogue tool to deliver digital knowledge. J Thorac Cardiovasc Surg. 2018;155(4):1793.
- Wang S, Yang L, Ci B, et al. Development and validation of a nomogram prognostic model for SCLC Patients. J Thorac Oncol. 2018;13(9):1338–1348.
- Rouhani P, Fletcher CD, Devesa SS, et al. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: an analysis of 12,114 cases. Cancer. 2008;113(3):616–627.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors: a 25-year single institution experience. Am J Clin Oncol. 2014;37(5):473–479.
- Florou V, Rosenberg AE, Wieder E, et al. Angiosarcoma patients treated with immune checkpoint inhibitors: a case series of seven patients from a single institution. J Immunother Cancer. 2019;7(1):213.
- Schlemmer M, Reichardt P, Verweij J, et al. Paclitaxel in patients with advanced angiosarcomas of soft tissue: a retrospective study of the EORTC soft tissue and bone sarcoma group. Eur J Cancer. 2008;44(16):2433–2436.
- Ishida Y, Otsuka A, Kabashima K. Cutaneous angiosarcoma: update on biology and latest treatment. Curr Opin Oncol. 2018;30(2):107–112.
- Fayette J, Martin E, Piperno-Neumann S, et al. Angiosarcomas, a heterogeneous group of sarcomas with specific behavior depending on primary site: a retrospective study of 161 cases. Ann Oncol. 2007;18(12):2030–2036.
- Pawlik TM, Paulino AF, McGinn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98(8):1716–1726.
- Lahat G, Dhuka AR, Lahat S, et al. Outcome of locally recurrent and metastatic angiosarcoma. Ann Surg Oncol. 2009;16(9):2502–2509.
- Shin JY, Roh SG, Lee NH, et al. Predisposing factors for poor prognosis of angiosarcoma of the scalp and face: systematic review and meta-analysis. Head Neck. 2017;39(2):380–386.
- Sinnamon AJ, Neuwirth MG, McMillan MT, et al. A prognostic model for resectable soft tissue and cutaneous angiosarcoma. J Surg Oncol. 2016;114(5):557–563.
- Trofymenko O, Curiel-Lewandrowski C. Surgical treatment associated with improved survival in patients with cutaneous angiosarcoma. J Eur Acad Dermatol Venereol. 2018;32(1):e29–e31.
- Abraham JA, Hornicek FJ, Kaufman AM, et al. Treatment and outcome of 82 patients with angiosarcoma. Ann Surg Oncol. 2007;14(6):1953–1967.
- Fury MG, Antonescu CR, Van Zee KJ, et al. A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J. 2005;11(3):241–247.
- Florou V, Wilky BA. Current and future directions for angiosarcoma therapy. Curr Treat Options Oncol. 2018;19(3):14.
- Lahat G, Dhuka AR, Hallevi H, et al. Angiosarcoma: clinical and molecular insights. Ann Surg. 2010;251(6):1098–1106.
- Singla S, Papavasiliou P, Powers B, et al. Challenges in the treatment of angiosarcoma: a single institution experience. Am J Surg. 2014;208(2):254–259.
- Katsurahara M, Horiki N, Takei Y. Duodenal metastasis from subcutaneous angiosarcoma of the head: rare cause of obscure gastrointestinal bleeding. Dig Endosc. 2014;26(2):291.
- Mark RJ, Poen JC, Tran LM, et al. Angiosarcoma. A report of 67 patients and a review of the literature. Cancer. 1996;77(11):2400–2406.
- Oxenberg J, Khushalani NI, Salerno KE, et al. Neoadjuvant chemotherapy for primary cutaneous/soft tissue angiosarcoma: determining tumor behavior prior to surgical resection. J Surg Oncol. 2015;111(7):829–833.
- Bernstein JM, Irish JC, Brown DH, et al. Survival outcomes for cutaneous angiosarcoma of the scalp versus face. Head Neck. 2017;39(6):1205–1211.
- Morrison WH, Byers RM, Garden AS, et al. Cutaneous angiosarcoma of the head and neck. A Therapeutic Dilemma. Cancer. 1995;76(2):319–327.
- Ogawa K, Takahashi K, Asato Y, et al. Treatment and prognosis of angiosarcoma of the scalp and face: a retrospective analysis of 48 patients. Br J Radiol. 1019;85:e1127–e1133.
- Adjuvant chemotherapy for localised resectable soft tissue sarcoma in adults. Sarcoma meta-analysis collaboration (SMAC). Cochrane Database Syst Rev. 2000;2:Cd001419.
- Naka N, Ohsawa M, Tomita Y, et al. Prognostic factors in angiosarcoma: a multivariate analysis of 55 cases. J Surg Oncol. 1996;61(3):170–176.
- Woll PJ, Reichardt P, Le Cesne A, et al. Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. Lancet Oncol. 2012;13(10):1045–1054.
- Fujisawa Y, Nakamura Y, Kawachi Y, et al. Comparison between taxane-based chemotherapy with conventional surgery-based therapy for cutaneous angiosarcoma: a single-center experience. J Dermatolog Treat. 2014;25(5):419–423.
- Fujisawa Y, Yoshino K, Kadono T, et al. Chemoradiotherapy with taxane is superior to conventional surgery and radiotherapy in the management of cutaneous angiosarcoma: a multicentre, retrospective study. Br J Dermatol. 2014;171(6):1493–1500.
- Holden CA, Spittle MF, Jones EW. Angiosarcoma of the face and scalp, prognosis and treatment. Cancer. 1987;59(5):1046–1057.
- Chow W, Amaya CN, Rains S, et al. Growth attenuation of cutaneous angiosarcoma with Propranolol-Mediated β-Blockade. JAMA Dermatol. 2015;151(11):1226–1229.
- Donghi D, Dummer R, Cozzio A. Complete remission in a patient with multifocal metastatic cutaneous angiosarcoma with a combination of paclitaxel and sorafenib. Br J Dermatol. 2010;162(3):697–699.
- Fujiwara S, Nagai H, Nakamachi Y, et al. Refractory metastasis of cutaneous angiosarcoma showing complete response to o pazopanib. Eur J Dermatol. 2015;25(1):71–73.
- Lu HJ, Chen PC, Yen CC, et al. Refractory cutaneous angiosarcoma successfully treated with sunitinib. Br J Dermatol. 2013;169(1):204–206.
- Ulrich L, Krause M, Brachmann A, et al. Successful treatment of angiosarcoma of the scalp by intralesional cytokine therapy and surface irradiation. J Eur Acad Dermatol Venereol. 2000;14(5):412–415.
- Wada M, Horinaka M, Yasuda S, et al. PDK1 is a potential therapeutic target against angiosarcoma cells. J Dermatol Sci. 2015;78(1):44–50.
- Young RJ, Woll PJ, Staton CA, et al. Vascular-targeted agents for the treatment of angiosarcoma. Cancer Chemother Pharmacol. 2014;73(2):259–270.
