The rate of breakthrough infections is twice as high with the PfizerBioNTech COVID-19 as with the Moderna COVID-19 vaccine () (Citation1). Accurate reporting of risk is important in providing the general public with information to properly make a decision. Hearing the breakthrough infection rate is twice as high with the PfizerBioNTech vaccine may seem alarming; however, as the baseline risk of the outcome is low, the absolute risk difference is small (Citation2). For example, when comparing the PfizerBioNTech and Moderna vaccines in absolute terms, the difference in breakthrough infections (absolute risk increase) is about 1 case per 100 person-years (). Perhaps even more reassuring is that the incidence of vaccinated individuals not acquiring COVID-19 is 98 and 99 per 100 person-years for PfizerBioNTech and Moderna, respectively () (Citation1). Accurate statistical reporting can still be misleading; clearly communicating the magnitude of risk is important to avoid unwarranted anxiety and to facilitate wise decision making. In dermatology, reporting relative risk is common; communicating absolute risk is also needed (Citation3).
Figure 1. (A) Incidence rate of COVID-19 breakthrough infections in vaccinated individuals with the PfizerBioNTech and Moderna vaccines. The incidence rate of breakthrough infections with the PfizerBioNTech vaccine is about twice what the rate is for the Moderna vaccine. (B) Absolute risk increase in COVID-19 breakthrough infections in vaccinated individuals with the PfizerBioNTech and Moderna vaccines. Over one year, for every 100 people treated with PfizerBioNTech and Moderna vaccines, one additional breakthrough infections would be expected with the PfizerBioNTech vaccine than with the Moderna vaccine.
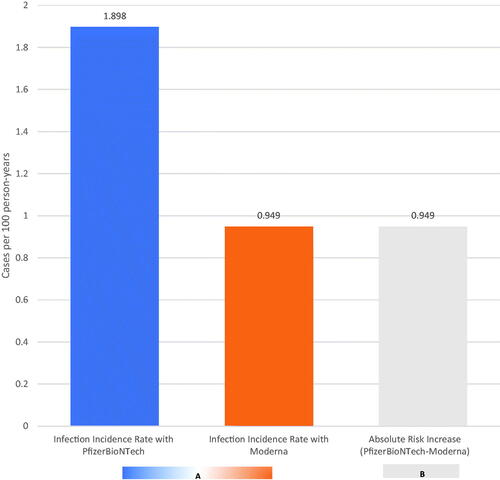
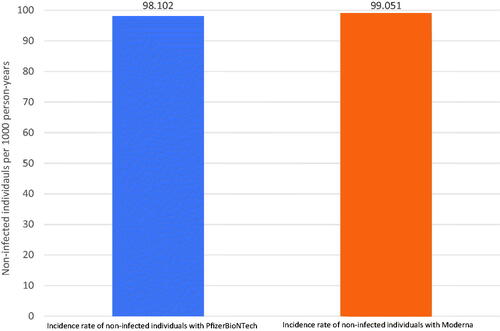
Figure 2. Incidence rate of individuals not acquiring COVID-19 breakthrough infections with the PfizerBioNTech and Moderna vaccine. The rate of vaccinated individuals not having a breakthrough infection is 98 per 100 person-years for PfizerBioNTech and 99 per 100 person-years for Moderna.
Medical journals have pushed for the inclusion of absolute risk, and guidelines such as STROBE and CONSORT encourage absolute risk reporting (Citation4,Citation5). However, even with these guidelines, many studies only report relative risk (Citation6–11). In dermatology, risk is commonly discussed when reporting comorbidities associated with various immune-mediated inflammatory conditions such as psoriasis or rosacea. The absolute risk of patients acquiring said comorbidities is valuable information for patients to not only understand their illness, but monitor for signs of progression and whether to seek further intervention and treatment.
In a study analyzing the risk of acquiring certain comorbidities, such as diabetes or melanoma, patients with psoriasis were twice as likely to develop diabetes and six times as likely of having melanoma compared with the general population (Citation12). It may not be immediately apparent from these numbers that the risk of developing diabetes is greater than that for melanoma in psoriasis patients or that the risks of both are rather small. The number needed to harm (NNH), a measure of absolute risk, was 580 and 20,000 for diabetes and melanoma, respectively: (Citation12) 580 patients with psoriasis would need to be seen before one more case of diabetes is diagnosed due to psoriasis, and 20,000 patients with psoriasis for one more case of melanoma (Citation12). Reporting both absolute and relative risk helps providers and patients to communicate and assess the risk of comorbidities.
Rosacea is associated with an increased risk of developing cancers and Alzheimer’s disease (AD) (Citation13,Citation14). In a study, the relative risk of thyroid cancer, glioma, and hepatic cancer was 1.6, 1.43, and 1.42, respectively. The 60, 43, and 42% increased risk of thyroid cancer, glioma, and hepatic cancer, respectively, might sound concerning; the absolute risk increases present a different picture (Citation13). The absolute risk of these malignancies, the number of rosacea patients needed to see one more of each of these malignancies, is 7080, 6963, and 21,645 for thyroid cancer, glioma, hepatic cancer, respectively (Citation13). Rosacea patients have a 28% increased risk of acquiring AD compared to those without rosacea; the absolute risk increase for acquiring AD in rosacea patients over non-rosacea patients was 0.19 per 1000 individuals, meaning approximately 5,000 rosacea patients must be seen before one more case of AD can be attributed to rosacea (Citation14). Even if this increased risk is due to AD (and not to some unidentified bias), the risk does not appear to be clinically meaningful.
Absolute risk and relative risk have roles in providing an accurate depiction of differences in risk. Relative risk is easy to report; however, it may be misleading and cause unnecessary worry when the difference (even if statistically significant and not due to unidentified bias) may be of little to no clinical significance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Puranik A, Lenehan PJ, Silvert E, et al. Comparison of two highly-effective mRNA vaccines for COVID-19 during periods of alpha and delta variant prevalence.medRxiv. 2021. DOI:10.1101/2021.08.06.21261707
- Schechtman E. Odds ratio, relative risk, absolute risk reduction, and the number needed to treat-which of these should we use? Value Health. 2002;5(5):431–436.
- Ranganathan P, Pramesh CS, Aggarwal R. Common pitfalls in statistical analysis: absolute risk reduction, relative risk reduction, and number needed to treat. Perspect Clin Res. 2016;7(1):51–53.
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. J Pharmacol Pharmacother. 2010;1(2):100–107.
- von Elm E, Altman DG, Egger M, STROBE Initiative, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLOS Med. 2007;4(10):e296.
- Shamseer L, Hopewell S, Altman DG, et al. Update on the endorsement of CONSORT by high impact factor journals: a survey of journal “‘Instructions to Authors’ in 2014”. Trials. 2016;17(1):301.
- Schwartz LM, Woloshin S, Dvorin EL, et al. Ratio measures in leading medical journals: structured review of accessibility of underlying absolute risks. BMJ. 2006;333(7581):1248.
- Jaeschke R, Guyatt G, Shannon H, et al. Basic statistics for clinicians: 3. Assessing the effects of treatment: measures of association. CMAJ. 1995;152(3):351–357.
- King NB, Harper S, Young ME. Use of relative and absolute effect measures in reporting health inequalities: structured review. BMJ. 2012;345:e5774.
- Alonso-Coello P, Carrasco-Labra A, Brignardello-Petersen R, et al. Systematic reviews experience major limitations in reporting absolute effects. J Clin Epidemiol. 2016;72:16–26.
- Vinkers CH, Lamberink HJ, Tijdink JK, et al. The methodological quality of 176,620 randomized controlled trials published between 1966 and 2018 reveals a positive trend but also an urgent need for improvement. PLoS Biol. 2021;19(4):e3001162.
- Saleem MD, Kesty C, Feldman SR. Relative versus absolute risk of comorbidities in patients with psoriasis. J Am Acad Dermatol. 2017;76(3):531–537.
- Tjahjono LA, Cline A, Huang WW, et al. Rosacea: relative risk versus absolute risk of malignant comorbidities. J Am Acad Dermatol. 2019;81(2):623–624.
- Egeberg A, Hansen PR, Gislason GH, et al. Patients with rosacea have increased risk of dementia. Ann Neurol. 2016;79(6):921–928.
