Abstract
Most patients with psoriasis present with localized mild-to-moderate disease. In this case, the application of topical treatments in the first-line setting is recommended in most cases.
Among different topical options, the fixed-dose combination of betamethasone dipropionate (BD) and vitamin D analogue (Cal) aerosol foam (Enstilar®, Leo Pharma) is approved as first-line topical therapy for the treatment of psoriasis in USA and the EU, due to its high efficacy and its favorable administration scheme.
The PSO-LONG was the first trial to report on the long-term efficacy and safety of the Cal/DB foam treatment for the proactive management of psoriasis and now, the indications of Cal/BD foam included its use in the psoriasis maintenance treatment. However, the precise role of this treatment and the potential therapeutic schemes in the long-term management of psoriasis need further clarification.
This Position Paper, authored by a group of Italian Expert Dermatologists, critically discusses the long-term management of psoriasis with Cal/BD foam in clinical practice. In particular, the biological rationale in the proactive treatment with Cal/BD foam and current evidence regarding this therapeutic approach are presented, along with its application also in patients with moderate-to-severe disease, difficult-to-treat lesions, or within combination regimens. In addition, strategies to improve adherence to long-term treatment of psoriasis are discussed,
Introduction
Psoriasis is a chronic skin disease that requires long-term management. Indeed, when the cutaneous lesions are fully resolved, a subclinical inflammation persists, eventually leading to flares of disease (Citation1–3). However, since long-term management requires continuous follow-up and adaptation of therapeutic strategies, this approach is challenging in clinical practice and many patients remain untreated or undertreated (Citation1,Citation2,Citation4).
The wide majority of patients with psoriasis present with localized disease of mild-to-moderate severity. In this case, current guidelines and recommendations are consistent in proposing the application of topical treatments in the first-line setting (Citation5–7). Topical treatments are widely used in clinical practice (Citation8). Currently approved topical treatments include corticosteroids, vitamin D analogs, combined corticosteroid/vitamin D (calcipotriol – Cal) formulations, vitamin A derivatives, anthralin and newer formulations of tar (Citation2). Remarkably, the vehicle plays a major role in the selection of treatment since it can greatly influence efficacy and potency (Citation9–11). Available vehicles include creams, lotions, gels, ointments, sprays, powders and, more recently, foams.
Despite the plethora of topical treatments available for the management of psoriasis, at present, clinical data on the long-term use of this therapeutic strategy are scant (Citation2,Citation3). In clinical practice, long-term management with topical treatment is usually based on a reactive approach, and it is started as soon as psoriasis relapses (Citation12). However, a proactive approach, consisting of the regular application of maintenance therapy with calcineurin inhibitors, has been shown to prevent and delay disease exacerbation in another chronic dermatological condition (atopic dermatitis) and, in line of principle, can also be effective in psoriasis (Citation3,Citation13).
The fixed-dose combination of corticosteroid (betamethasone dipropionate – BD) and vitamin D analogue (Cal) is currently recommended as first-line topical therapy in patients with psoriasis, due to its high efficacy and its favorable administration scheme (once-daily application), which is associated with increased adherence (Citation1). Cal/BD aerosol foam (Enstilar®, Leo Pharma) is currently approved for the treatment of psoriasis in USA and the EU. In the recent PSO-LONG trial, long-term proactive management with Cal/BD foam applied twice weekly for up to 52 weeks prolonged the time to first relapse, increased time in remission and reduced the number of relapses compared with the vehicle (Citation14). To date, this is the only trial investigating proactive topical treatment for psoriasis.
The recent revision of the indications of Cal/BD foam included its use in the maintenance treatment of psoriasis. However, the precise role of Cal/BD foam and the potential therapeutic schemes in the long-term management of psoriasis in clinical practice need further clarification. Furthermore, strategies to improve adherence to long-term treatment of psoriasis are eagerly awaited.
This Position Paper is authored by a group of Italian Expert Dermatologists, with the aim to critically discuss the long-term management of psoriasis with Cal/BD foam in clinical practice and to provide an expert opinion on this topic, since papers on this aspect are very limited.
Long-term, proactive treatment of psoriasis with cal/BD foam: rationale and current evidence
Long-term management of psoriasis: state of the art
Different therapeutic strategies have been proposed for the long-term management of psoriasis: biological agents, conventional systemic drugs, small molecules and topical treatment (Citation2). Systemic agents, however, are not usually indicated for mild disease, which affects the majority of psoriasis patients; in particular, conventional systemic therapies (e.g. methotrexate, acitretin, cyclosporine, fumaric acid esters) may be associated with poor safety/tolerability over the long-term, leading to treatment discontinuation (Citation2,Citation15). Topical therapy is not associated with any particular safety concern and is less costly than biological agents, small molecules or conventional systemic drugs (Citation2). With specific reference to topical treatment, a number of consensus documents have pointed out that the fixed-dose combination of a steroid and a vitamin D analogue can be considered the preferred topical approach in both the initial treatment phase (with daily applications) and maintenance phase (with twice weekly or on a weekend regimen) due to its favorable efficacy profile, good tolerability and favorable cost–effectiveness (Citation16–18). Among different combinations, at present, the best level of evidence on the long-term therapy of psoriasis is available for the combination of Cal and BD foam (Citation3).
Cal/BD foam: rationale in the long-term management of psoriasis
It is now widely accepted that successfully treated lesions, after complete resolution, tend to recur within months (Citation19,Citation20). This recurrent pattern is due to the residual subclinical inflammation, with the presence of pro-inflammatory cytokines and cells. Therefore, there is a major need to continue treating the resolved lesions, with the aim to diminish, or even abolish, the degree of residual inflammation.
It has been shown that the fixed-combination Cal/BD retains higher efficacy when compared with the individual components (Citation21,Citation22). The synergic efficacy relies upon a profound mechanistic rationale. Indeed, Cal, as a vitamin D analogue, primarily acts on epidermal dysregulation, reducing epidermal hyperproliferation and promoting the differentiation of keratinocytes and also presents immunomodulatory properties (Citation23,Citation24). On the other hand, BD targets pro-inflammatory cytokines and chemokines and also enhances keratinocyte differentiation, complementing the action of Cal (Citation23,Citation24). Studies specifically investigating the immunomodulatory properties of Cal/BD showed a decreased expansion of Th1 and Th17 cells into the psoriatic lesions, also suggesting a positive modulation of dendritic cells (Citation25,Citation26). The administration of Cal/BD is also able to downmodulate the expression of pro-inflammatory cytokines, such as IFN-γ, TNF-α, IL-17 and IL-23, and to upregulate the expression of anti-inflammatory cytokines, such as IL-4 and IL-6 (Citation25,Citation26). These data were recently sustained by the results of another study on a skin inflammation model, specifically conducted with Cal/BD foam (Citation27). These data suggest a peculiar immunomodulation on the IL-23/Th17 axis through the combined activity of Cal and BD and provide a rationale to the maintenance of a relapse-free state with Cal/BD foam, which prevent the onset of new psoriatic lesions (Citation1,Citation27) ().
Figure 1. After the base treatment of psoriatic lesion (A), a subclinical inflammation persists under the resolved lesion (B). The proactive treatment with Cal/BD foam (C) is able to modulate the subclinical inflammation counteracting the disease recurrence. TRMs: tissue resident memory; LC: Langerhans cells.
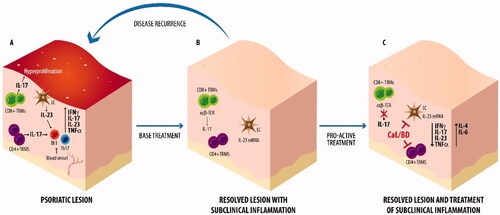
Furthermore, local skin reactions potentially associated with Cal administration are mitigated by the addition of the steroid, and in turn Cal restores the skin atrophy induced by BD; these actions improve the safety profile of Cal/BD combination over monotherapies (Citation23,Citation24,Citation28).
The efficacy of topical therapy is dependent upon the penetration of the active compounds through the skin (Citation9). Therefore, research is active in the development of new drug delivery systems. Foam vehicles show increased penetration at a faster rate than traditional topical formulations (i.e. creams, gels and ointments) (Citation29), likely due to their ability to alter the stratum corneum and deliver drug via an intracellular route, in contrast to a slower hydration-dependent process observed with traditional vehicles. With the use of Cal/BD foam, a stable supersaturated solution of the active compounds is formed, leading to increased bioavailability and superior efficacy over ointment formulation (Citation29,Citation30).
The efficacy and safety of this formulation of Cal/BD in aerosol foam was shown by the results of multiple clinical trials in patients with psoriasis of different severity, the detailed description of which goes beyond the scopes of the present paper (Citation29,Citation31–40). Real-life studies and case reports also confirmed the effectiveness and safety of Cal/BD aerosol foam in daily-practice settings (Citation41–48). An indirect comparison using pooled data showed that Cal/BD foam is associated with improved efficacy compared with apremilast, methotrexate or acitretin also in patients with moderate-to-severe disease (Citation49). The favorable efficacy and safety profiles also resulted in an improved quality of life of treated patients (Citation32,Citation33,Citation36,Citation50). Moreover, the possibility of an aerosolized formulation with a once-daily administration may be, in line with principle, associated with improved compliance (Citation46).
A pharmacoeconomic analysis also showed that Cal/BD foam can be considered as the most cost-effective Cal/BD formulation for the topical treatment of psoriasis (Citation51), and, according to a recent analysis, it is also cost-effective when compared with non-biological systemic therapies (Citation52).
The PSO-LONG trial
The recently-published, international, phase III, randomized, double blind, vehicle controlled PSO-LONG trial evaluated the long-term efficacy and safety of proactive psoriasis management with twice-weekly Cal/DB aerosol foam over a 52-week period (Citation15). Patients who achieved treatment success after a 4-week open-label lead-in phase period were randomized to proactive management with Cal/BD foam (n = 272) or reactive management with vehicle foam (n = 273). Treatment was administered twice weekly (every 2 or 3 days, on fixed days) on psoriatic lesions that had cleared/almost cleared during the open-label lead-in phase or after treatment of a relapse. Upon relapse, patients from both treatment groups received rescue treatment with Cal/BD foam, applied to lesions once-daily for 4 weeks.
The median time to first relapse, primary endpoint of the trial, was 56 days with the proactive treatment with Cal/BD foam compared with 30 days with the reactive strategy, with a 43% reduction in the risk of experiencing a first relapse in patients on proactive therapy (HR: 0.57; 95% CI: 0.47–0.69; p < .001). After 52 weeks, patients assigned to proactive Cal/BD foam had experienced additional 41 days in remission when compared with those on the reactive regimen (p < .001). The number of relapses was lower with the proactive regimen (3.1 vs. 4.8) and the rate of relapse was also 46% lower (95% CI: 37–54%; p < .001). Both groups responded to rescue treatment with Cal/BD foam. The tolerability profile and the incidence of adverse events were similar in the two groups, with the wide majority of events of mild severity and no signs of skin atrophy.
Some ancillary analyses of the PSO-LONG trial have been presented at the EADV meeting 2020 and are available to date in abstract form. Calzavara-Pinton et al. showed that the impact of initial flare treatment with Cal/BD foam for 4 weeks, which significantly improved DLQI, EQ-5D and PSI, was maintained at 52 weeks. Patients assigned to proactive management had significantly better DLQI and PSI scores versus patients assigned to reactive management (Citation53). In another analysis focusing on QoL, patients in relapse had poorer health-related quality of life and patient-perceived symptoms than patients in remission, suggesting that the reduction in the number of relapses and the increased time in remission over a year of exposure could also improve HRQoL and patient-perceived symptoms in patients receiving proactive management compared with patients on reactive management (Citation54). Papp et al. showed that in a subgroup of patients with 10–30% BSA (body surface area) and disease severity of at least moderate (psoriasis global assessment, PGA ≥3) who underwent hypothalamic–pituitary–adrenal (HPA) axis testing, there were no new safety signals, non-clinically significant HPA axis suppression and no signs of clinically relevant hypercalcemia with the proactive treatment (Citation55). Last, long-term proactive management was associated with sustained and significantly lower mean mPASI/PGA scores (Citation56). Clinical evidence on the proactive treatment with Cal/BD foam are summarized in .
Table 1. Clinical evidence on the proactive treatment with Cal/BD foam.
The PSO-LONG was the first trial to report on the efficacy and safety of long-term proactive treatment with Cal/BD foam. Only few studies have assessed long-term treatment with Cal/BD ointment or gel, but they focused either on intermittent or rescue regimen (Citation57,Citation58), and not on proactive management, a strategy that holds promise to change the therapeutic approach to psoriasis.
Long-term, proactive treatment of psoriasis with cal/BD foam: application in clinical practice
According to the results of the PSO-LONG trial, long-term proactive treatment with Cal/BD foam twice weekly can help avoid the worsening of mild-to-moderate psoriasis and prevent the onset of relapse without relevant safety concerns. Of note, the possibility of a twice-weekly regimen is of particular relevance in clinical practice, since this administration schedule is highly preferred by physicians and patients, thus potentially maximizing adherence (Citation2). While specific data on the long-term treatment with Cal/BD foam are being collected, research is also exploring the use of this compound in other settings, such as patients with moderate-to-severe disease, difficult-to-treat lesions, or within combination regimens. It is possible to speculate that the results reported in these settings may well translate to long-term treatment, likely providing a further rational to the use of Cal/BD foam over a long-term in different types of patients and clinical situations.
In the current era characterized by the mounting use of biological agents, the topical combination of Cal/BD can also have a role in patients with moderate-to-severe psoriasis even if they are receiving systemic treatments (Citation14). In a recent post-hoc subgroup analysis, Iversen et al. used pooled data from phase II/III trials to investigate the efficacy and safety of Cal/BD foam in patients with moderate-to-severe psoriasis, showing that topical Cal/BD foam is also well tolerated and effective in this setting (Citation59). A rapid onset of action and high efficacy on skin lesions has been reported also in patients with modified Psoriasis Area Severity Index (PASI) >10 at baseline (Citation60). Furthermore, topical treatment with Cal/BD foam allows to treat specific sites on which biological agents are less effective. The efficacy of Cal/BD foam in difficult-to-treat areas was specifically investigated in a recent pilot study conducted in 20 patients, all with moderate disease at baseline (Citation61). After 4-week treatment, the majority of patients showed improvements in all outcome measures (PGA, lesion size, BSA, erythema, induration, scaling; being clear or almost clear in 75% of patients), with a significant improvement already by week 2 of treatment (clear or almost clear in 30% of patients p < .0001). In two phase II studies, Cal/BD foam proved to be effective also in patients with scalp psoriasis, one of the most challenging form of this disease (Citation62). In a post-hoc analysis on one of these studies, Cal/BD foam resulted in a greater improvement than Cal or BD foam monotherapies in redness, scaliness and thickness of scalp lesions (Citation63). The effectiveness of Cal/BD foam was also confirmed in a series of three patients with scalp psoriasis, two of whom were resistant to previous treatment (Citation45).
Topical treatment with Cal/BD foam can also be administered in association with systemic therapy, for instance, in case of poor response, with the aim to improve control of symptoms. Bagel et al. conducted a pilot trial in 25 psoriasis patients with poor response to therapy with a biological agent (Citation64). Over the study period, adjunctive treatment with Cal/BD foam resulted in significant improvements in PGA score, BSA involvement, and PGA × BSA measures by week 4, and these improvements were maintained through week 16. A significantly higher number of patients achieved treatment success with PGA ≤1 and BSA ≤1% with Cal/BD foam at both week 4 (both 76 vs. 12% and 4%, respectively; p < .01) and week 16 (both 68 vs. 12% and 4%, respectively; p < .01), as compared with biologic monotherapy at baseline. Treatment satisfaction was high, and only non-serious adverse events were reported. In a very recent randomized study, conducted in a small sample of patients (n = 28), Cal/BD foam was evaluated as an adjunctive therapy to apremilast (Citation65). At week 4, patients on Cal/BD plus apremilast showed a greater improvement than those on apremilast alone in terms of PASI 75 response (50 vs 7%; p = .003), achievement of PGA score of ‘clear’ or ‘almost clear’ (43 vs 7%; p = .001) and score of pruritus evaluated through a visual analog scale (2 vs 5; p = .0079). Another study, showed that complete clearance with Cal/BD foam, in patients not cleared on their initial biologic, was a less costly approach compared to the lowest cost dose escalation of or switching to a different biological therapy (Citation64,Citation66).
Last, a very recent study, conducted on 187 consecutive patients with moderate-to-severe disease, compared the efficacy of Cal/BD foam plus phototherapy versus phototherapy alone (Citation67). At 12 weeks, the combined regimen was more effective in clearing psoriasis (modified PASI: 2.1 vs 4.4; p < .01) and in the reduction of itching than phototherapy alone, without any acute adverse event.
The importance of a correct adherence
Over the long-term, poor adherence to therapy is a major barrier to treatment success (Citation1,Citation2,Citation68,Citation69). Indeed, rates of adherence to topical treatments for psoriasis lies between 50 and 70%, with lower figures (40%) for corticosteroids (Citation70).
Optimization of adherence is, therefore, crucial in clinical practice. First, it is widely accepted that treatment goals should be agreed and shared between the patient and treating clinician (Citation71,Citation72). Strategies to improve adherence include educational interventions, and reminder programs (Citation1,Citation70,Citation73). The use of apps can also improve short-term adherence to topical treatment (Citation74,Citation75).
The use of simplified treatment regimens and proper vehicles also plays a major role in ensuring adherence (Citation1). The ideal agent should be easy to apply, show, high cosmetic acceptance, present fast onset of action, high efficacy and optimal safety (). Cal/BD foam appears to be endowed with all these properties and is associated with a high degree of patients’ preferences (Citation1,Citation35,Citation76–78). Indeed, in a recent ‘field-practice’ study by the Italian Association of Private Dermatologists, most patients considered Cal/BD foam as superior over other topical treatments in terms of efficacy, easiness of use and tolerability (Citation46). In another 4-week field-practice study, 312/381 patients (82%) showed complete adherence to Cal/BD foam therapy and were willing to continue this treatment thereafter (Citation41).
Table 2. Treatment adherence impact factors.
Expert opinion
The PSO-LONG trial has represented an unprecedented milestone in the treatment of psoriasis, since it is the first study demonstrating the efficacy of proactive long-term therapy of mild-to-moderate disease. Indeed, proactive treatment with Cal/BD foam has prolonged time to first relapse, has reduced the number of relapses and prolonged time free in remission as compared with a reactive regimen, also showing a favorable safety profile and a positive effect on quality of life. Of note, its good cosmetic appearance, without greasy residues, might further contribute to patients’ acceptance to this long-term proactive therapy. Given the benefits shown by proactive long-term therapy, it is of utmost importance that proper educational interventions for patients are established to ensure optimal adherence.
Furthermore, multiple pieces of evidence can suggest that proactive treatment with Cal/BD foam may be an effective tool also in combination with conventional systemic therapies, for instance in patients where the systemic treatment is able to control comorbidities but not the psoriasis plaques (e.g. patients with psoriatic arthritis responsive to methotrexate but still with skin manifestations). Another example can be represented by the combination with biological drugs in patients with comorbidities potentially manageable by these agents and incomplete clearance of skin lesions manifestations. Patients affected by comorbidities that make biological drugs contraindicated, such as neoplasm or immunodepression, or those with paradoxical psoriasis (patients suffering from other conditions responsive to biological drugs also indicated for psoriasis, in which the skin disease arises or worsens during such treatments) may also have major benefits from the use of proactive treatment. We believe that dedicated studies in these setting are needed to further clarify the role of proactive long-term management, which still results a neglected topic.
While those studies appear awaited, we believe that the use of proactive long-term therapy of mild-to-moderate psoriasis with Cal/BD foam will translate into major benefits for patients, reducing the number of relapses and improving QoL.
Author contributions
All authors have contributed to literature research and critical revision, have drafted the manuscript, and have approved the final version of the paper for submission.
Acknowledgments
Editorial assistance was provided by Luca Giacomelli, PhD, Simonetta Papa, PhD and Aashni Shah (Polistudium SRL, Milan, Italy). Graphical assistance was provided by Massimiliano Pianta (Polistudium SRL, Milan, Italy).
Disclosure statement
CDS served as a speaker and consultant for Almirall, Abbvie, Janssen, Leopharma, Novartis, Eli Lilly, UCB Pharma. No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Megna M, Cinelli E, Camela E, et al. Calcipotriol/betamethasone dipropionate formulations for psoriasis: an overview of the options and efficacy data. Expert Rev Clin Immunol. 2020;16(6):599–620.
- Segaert S, Calzavara-Pinton P, de la Cueva P, et al. Long-term topical management of psoriasis: the road ahead. J Dermatolog Treat. 2020;1–10. doi: 10.1080/09546634.2020.1729335.
- Carrascosa JM, Theng C, Thaçi D. Spotlight on topical long-term management of plaque psoriasis. Clin Cosmet Investig Dermatol. 2020;13:495–498.
- Armstrong AW, Robertson AD, Wu J, et al. Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: findings from the national psoriasis foundation surveys, 2003–2011. JAMA Dermatol. 2013;149(10):1180–1185.
- National Institute for Health and Care Excellence. Psoriasis: assessment and management; 2012 [cited 2019 Nov 1]. Available from: https://www.nice.org.uk/Guidance/CG153.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60(4):643–659.
- Thaçi D, de la Cueva P, Pink AE, et al. Calzavara-Pinton P. General practice recommendations for the topical treatment of psoriasis: a modified-Delphi approach. BJGP Open. 2020;4(5):bjgpopen20X101108.
- Svendsen MT, Jeyabalan J, Andersen KE, et al. Worldwide utilization of topical remedies in treatment of psoriasis: a systematic review. J Dermatolog Treat. 2017;28(5):374–383.
- Girolomoni G, Calzavara Pinton P, Cristaudo A, et al. Back to the future: a new topical approach for mild-to-moderate psoriasis. G Ital Dermatol Venereol. 2018;153(3):375–382.
- Piaserico S, Manfredini S, Borghi A, et al. How to improve adherence to treatment in patients with mild-to-moderate psoriasis. G Ital Dermatol Venereol. 2018;153(5):692–697.
- Kuehl B, Shear NH. The evolution of topical formulations in psoriasis. Skin Therapy Lett. 2018;23(4):5–9.
- Bonnekoh B, Fernández García JJ, Surber C, et al. Innovation that drives your dermatological future. EMJ Dermatol. 2017;5(1):36–43.
- Wollenberg A, Bieber T. Proactive therapy of atopic dermatitis-an emerging concept. Allergy. 2009;64(2):276–278.
- Lebwohl M, Kircik L, Lacour JP, et al. Twice-weekly topical calcipotriene/betamethasone dipropionate foam as proactive management of plaque psoriasis increases time in remission and is well tolerated over 52 weeks (PSO-LONG trial). J Am Acad Dermatol. 2021;84(5):1269–1277.
- Smith D. Fumaric acid esters for psoriasis: a systematic review. Ir J Med Sci. 2017;186(1):161–177.
- Imafuku S, Zheng M, Tada Y, et al. Asian consensus on assessment and management of mild to moderate plaque psoriasis with topical therapy. J Dermatol. 2018;45(7):805–811.
- Paul C, Gallini A, Archier E, et al. Evidence-based recommendations on topical treatment and phototherapy of psoriasis: systematic review and expert opinion of a panel of dermatologists. J Eur Acad Dermatol Venereol. 2012; 26(Suppl 3):1–10.
- Augustin M, Mrowietz U, Bonnekoh B, et al. Topical longterm therapy of psoriasis with vitamin D3 analogues, corticosteroids and their two compound formulations: position paper on evidence and use in daily practice. J Dtsch Dermatol Ges. 2014;12(8):667–682.
- Benezeder T, Wolf P. Resolution of plaque-type psoriasis: what is left behind (and reinitiates the disease). Semin Immunopathol. 2019;41(6):633–644.
- Suárez-Fariñas M, Fuentes-Duculan J, Lowes MA, et al. Resolved psoriasis lesions retain expression of a subset of disease-related genes. J Invest Dermatol. 2011;131(2):391–400.
- Papp KA, Guenther L, Boyden B, et al. Early onset of action and efficacy of a combination of calcipotriene and betamethasone dipropionate in the treatment of psoriasis. J Am Acad Dermatol. 2003;48(1):48–54.
- Fleming C, Ganslandt C, Guenther L, et al. Calcipotriol plus betamethasone dipropionate gel compared with its active components in the same vehicle and the vehicle alone in the treatment of psoriasis vulgaris: a randomised, parallel group, double-blind, exploratory study. Eur J Dermatol. 2010;20(4):465–471.
- Segaert S, Ropke M. The biological rationale for use of vitamin d analogs in combination with corticosteroids for the topical treatment of plaque psoriasis. J Drugs Dermatol. 2013;12(8):e129-37–e137.
- Segaert S, Shear NH, Chiricozzi A, et al. Optimizing anti-Inflammatory and immunomodulatory effects of corticosteroid and vitamin D analogue fixed-dose combination therapy. Dermatol Ther. 2017;7(3):265–279.
- Fujiyama T, Ito T, Umayahara T, et al. Topical application of a vitamin D3 analogue and corticosteroid to psoriasis plaques decreases skin infiltration of TH17 cells and their ex vivo expansion. J Allergy Clin Immunol. 2016;138(2):517–528.e5.
- Lovato P, Norsgaard H, Tokura Y, et al. Calcipotriol and betamethasone dipropionate exert additive inhibitory effects on the cytokine expression of inflammatory dendritic cell-Th17 cell axis in psoriasis. J Dermatol Sci. 2016;81(3):153–164.
- Lovato P, Jiang L, Hebsgaard J, et al. Calcipotriol/betamethasone dipropionate foam inhibits Th17 cytokine secretion and improves epidermal barrier markers in a human Th17 skin inflammation model. Dermatol Ther. 2021;11(1):265–274.
- Norsgaard H, Kurdykowski S, Descargues P, et al. Calcipotriol counteracts betamethasone-induced decrease in extracellular matrix components related to skin atrophy. Arch Dermatol Res. 2014;306(8):719–729.
- Lind M, Nielsen KT, Schefe LH, et al. Supersaturation of calcipotriene and betamethasone dipropionate in a novel aerosol foam formulation for topical treatment of psoriasis provides enhanced bioavailability of the active ingredients. Dermatol Ther. 2016;6(3):413–425.
- Kim ES, Frampton JE. Calcipotriol/betamethasone dipropionate foam: a review in plaque psoriasis. Drugs. 2016;76(15):1485–1492.
- Queille-Roussel C, Olesen M, Villumsen J, et al. Efficacy of an innovative aerosol foam formulation of fixed combination calcipotriol plus betamethasone dipropionate in patients with psoriasis vulgaris. Clin Drug Investig. 2015;35(4):239–245.
- Leonardi C, Bagel J, Yamauchi P, et al. L. Efficacy and safety of calcipotriene plus betamethasone dipropionate aerosol foam in patients with psoriasis vulgaris–a randomized phase III study (PSO-FAST). J Drugs Dermatol. 2015;14(12):1468–1477.
- Lebwohl M, Tyring S, Bukhalo M, et al. Fixed combination aerosol foam calcipotriene 0.005% (cal) Plus betamethasone dipropionate 0.064% (BD) is more efficacious than cal or BD aerosol foam alone for psoriasis vulgaris: a randomized, double-blind, multicenter, three-arm, phase 2 study. J Clin Aesthet Dermatol. 2016;9(2):34–41.
- Koo J, Tyring S, Werschler WP, et al. Superior efficacy of calcipotriene and betamethasone dipropionate aerosol foam versus ointment in patients with psoriasis vulgaris-a randomized phase II study. J Dermatolog Treat. 2016;27(2):120–127.
- Paul C, Stein Gold L, Cambazard F, et al. Calcipotriol plus betamethasone dipropionate aerosol foam provides superior efficacy vs. gel in patients with psoriasis vulgaris: randomized, controlled PSO-ABLE study. J Eur Acad Dermatol Venereol. 2017;31(1):119–126.
- Leonardi C, Bagel J, Yamauchi P, et al. L. The aerosol foam formulation of the fixed combination calcipotriene plus betamethasone dipropionate improves the health-related quality of life in patients with psoriasis vulgaris: results from the randomized PSO-FAST study. J Drugs Dermatol. 2016;15(8):981–987.
- Queille-Roussel C, Bang B, Clonier F, et al. Enhanced vasoconstrictor potency of the fixed combination calcipotriol plus betamethasone dipropionate in an innovative aerosol foam formulation vs. other corticosteroid psoriasis treatments. J Eur Acad Dermatol Venereol. 2016;30(11):1951–1956.
- Stein Gold L, Lebwohl M, Menter A, et al. Aerosol foam formulation of fixed combination calcipotriene plus betamethasone dipropionate is highly efficacious in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. J Drugs Dermatol. 2016;15(8):951–957.
- Menter A, Gold LS, Koo J, et al. Fixed-Combination calcipotriene plus betamethasone dipropionate aerosol foam is well tolerated in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. Skinmed. 2017;15(2):119–124.
- Seyger M, Abramovits W, Liljedahl M, et al. Safety and efficacy of fixed-dose combination calcipotriol (50 μg/g) and betamethasone dipropionate (0.5 mg/g) cutaneous foam in adolescent patients (aged 12 to <17 years) with plaque psoriasis: results of a phase II, open-label trial. J Eur Acad Dermatol Venereol. 2020;34(9):2026–2034.
- Gerdes S, Krakor M, Anger T, et al. Prospective, observational, Non-Interventional, multicentre study on the efficacy and tolerability of a new calcipotriol/betamethasone aerosol foam (enstilar®) in patients with plaque psoriasis under daily practice conditions. Dermatology. 2017;233(6):425–434.
- Wu JJ, Veverka KA, Lu M, et al. Real-world experience of calcipotriene and betamethasone dipropionate foam 0.005%/0.064% in the treatment of adults with psoriasis in the United States. J Dermatolog Treat. 2019;30(5):454–460.
- Pinter A, Thormann H, Angeletti F, et al. Calcipotriol/betamethasone dipropionate aerosol foam for the treatment of psoriasis vulgaris: case series and review of the literature. Clin Cosmet Investig Dermatol. 2018;11:451–459.
- Gallo L, Megna M, Cirillo T, et al. Psoriasis and skin pain: real-life effectiveness of calcipotriol plus betamethasone dipropionate in aerosol foam formulation. J Eur Acad Dermatol Venereol. 2019;33(7):1312–1315.
- Anderko M, Navarro Triviño FJ, Sharples CL. Calcipotriol plus betamethasone dipropionate aerosol foam for scalp psoriasis. Clin Cosmet Investig Dermatol. 2019;12:699–705.
- Giovene GL, Giacomelli L, AIDA (Italian Association of Outpatient Dermatologists) Working Group. Calcipotriene plus betamethasone dipropionate in aerosol foam formulation: will this effective treatment for mild-to-moderate psoriasis change clinical practice? G Ital Dermatol Venereol. 2018;153(6):872–876.
- Amat-Samaranch V, Puig L. Safety of calcipotriene and betamethasone dipropionate foam for the treatment of psoriasis. Expert Opin Drug Saf. 2020;19(4):423–432.
- Dattola A, Silvestri M, Bennardo L, et al. A novel vehicle for the treatment of psoriasis. Dermatol Ther. 2020;33(1):e13185.
- Bewley AP, Shear NH, Calzavara-Pinton PG, et al. Calcipotriol plus betamethasone dipropionate aerosol foam vs. apremilast, methotrexate, acitretin or fumaric acid esters for the treatment of plaque psoriasis: a matching-adjusted indirect comparison. J Eur Acad Dermatol Venereol. 2019;33(6):1107–1115.
- Griffiths CE, Stein Gold L, Cambazard F, et al. Greater improvement in quality of life outcomes in patients using fixed-combination calcipotriol plus betamethasone dipropionate aerosol foam versus gel: results from the PSO-ABLE study. Eur J Dermatol. 2018;28(3):356–363.
- Foley P, Garrett S, Ryttig L. A cost-effectiveness analysis of calcipotriol plus betamethasone dipropionate aerosol foam versus gel for the topical treatment of plaque psoriasis. Curr Med Res Opin. 2018;34(7):1277–1283.
- Balak DMW, Carrascosa JM, Gregoriou S, Calzavara-Pinton P, et al. Cost per PASI-75 responder of calcipotriol plus betamethasone dipropionate cutaneous foam versus nonbiologic systemic therapies for the treatment of plaque psoriasis in seven european countries. J Dermatolog Treat. 2020;6:1–8.
- Calzavara-Pinton P, Kircik L, Jalili A, et al. Patients with psoriasis receiving long-term proactive or reactive management with fixed-dose combination CAL/BD foam showed sustained improvement in DLQI, PSI and EQ-5D scores, with significantly greater improvement in the proactive group. Presented at EADV; 2020. p. 29–31, Virtual.
- Thaçi D, Tyring S, de la Cueva P, et al. Patient-reported outcomes in remission versus relapse in patients with psoriasis receiving treatment with fixed-dose combination CAL/BD foam from the PSO-LONG trial. Presented at EADV, Virtual; 2020 October. p. 29–31.
- Papp K, Guenther L. Liljedahl M. Proactive treatment of plaque psoriasis with twice-weekly topical CAL/BD foam is safe in patients undergoing HPA-AXIS testing. Presented at EADV 2020, virtual. 2020 October. p. 29–31.
- Stein-Gold L, Alonso-Llamazares J, Laws P, et al. Proactive management with twice-weekly topical cal/BD foam prolongs treatment efficacy versus reactive management in patients with plaque psoriasis. Presented at EADV, Virtual. 2020 October. p. 29–31.
- Kragballe K, Austad J, Barnes L, et al. A 52-week randomized safety study of a calcipotriol/betamethasone dipropionate two-compound product (dovobet/daivobet/taclonex) in the treatment of psoriasis vulgaris. Br J Dermatol. 2006;154(6):1155–1160.
- Saraceno R, Camplone G, D'Agostino M, et al. Efficacy and maintenance strategies of two-compound formulation calcipotriol and betamethasone dipropionate gel (xamiol® gel) in the treatment of scalp psoriasis: results from a study in 885 patients. J Dermatolog Treat. 2014;25(1):30–33.
- Iversen L, Kurvits M, Snel-Prentø AM, et al. Calcipotriol/betamethasone dipropionate cutaneous foam treatment for psoriasis in patients with BSA 5–15% and PGA ≥ 3: post-hoc analysis from three randomized controlled trials. Dermatol Ther. 2020;10(5):1111–1120.
- Pink AE, Jalili A, Berg P, et al. Rapid onset of action of calcipotriol/betamethasone dipropionate cutaneous foam in psoriasis, even in patients with more severe disease. J Eur Acad Dermatol Venereol. 2019;33(6):1116–1123.
- Del Rosso JQ, Kircik LH. The effect of calcipotriene-betamethasone dipropionate aerosol foam versus vehicle on target lesions in moderate severity plaque psoriasis: focus on elbows and knees. J Drugs Dermatol. 2019;18(4):358–361.
- Petersen B, Lebwohl M. Treating scalp psoriasis with calcipotriene/betamethasone dipropionate fixed-dose combination cutaneous foam: review of phase 2 data. J Drugs Dermatol. 2020;19(8):784–786.
- Patel DS, Veverka KA, Hansen JB, et al. Efficacy of fixed-combination calcipotriene 0.005% and betamethasone dipropionate 0.064% foam for scalp plaque psoriasis: additional analysis of a phase II, randomized clinical study. J Clin Aesthet Dermatol. 2020;13(5):18.
- Bagel J, Zapata J, Nelson E. A prospective, open-label study evaluating adjunctive calcipotriene 0.005%/betamethasone dipropionate 0.064% foam in psoriasis patients with inadequate response to biologic therapy. J Drugs Dermatol. 2018;17(8):845–850.
- Kircik LH, Schlesinger TE, Tanghetti E. Efficacy and safety of calcipotriene 0.005%/betamethasone dipropionate 0.064% foam with apremilast for moderate plaque psoriasis. J Drugs Dermatol. 2020;19(9):874–880.
- Haidari W, Pona A, Feldman SR. Management of residual psoriasis in patients on biologic treatment. J Drugs Dermatol. 2020;19(2):188–194.
- Rovati C, Arisi M, Venturini M, et al. Combination with an aerosol foam containing calcipotriene and betamethasone is a simple way to strongly improve treatment results of narrow-band UVB phototherapy. Eur J Dermatol. 2021; in press.
- WHO. 2003. Adherence to long-term therapies: evidence for action [cited 2021 Feb 14]. Available from: https://www.who.int/chp/knowledge/publications/adherence_report/en/.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176(3):759–764.
- Reich K, Zschocke I, Bachelez H, PSO-TOP study group, et al. A topical treatment optimization programme (TTOP) improves clinical outcome for calcipotriol/betamethasone gel in psoriasis: results of a 64-week multinational randomized phase IV study in 1790 patients (PSO-TOP). Br J Dermatol. 2017;177(1):197–205.
- Strober BE, van der Walt JM, Armstrong AW, et al. Clinical goals and barriers to effective psoriasis care. Dermatol Ther. 2019;9(1):5–18.
- Eicher L, Knop M, Aszodi N, et al. A systematic review of factors influencing treatment adherence in chronic inflammatory skin disease – strategies for optimizing treatment outcome. J Eur Acad Dermatol Venereol. 2019;33(12):2253–2263.
- Caldarola G, De Simone C, Moretta G, et al. Role of personalized medication training in improving efficacy and adherence to a topical therapy in psoriatic patients. J Dermatolog Treat. 2017;28(8):722–725.
- Svendsen MT, Andersen F, Andersen KH, et al. A smartphone application supporting patients with psoriasis improves adherence to topical treatment: a randomized controlled trial. Br J Dermatol. 2018;179(5):1062–1071.
- Armitage LC, Kassavou A, Sutton S. Do mobile device apps designed to support medication adherence demonstrate efficacy? A systematic review of randomised controlled trials, with meta-analysis. BMJ Open. 2020;10(1):e032045.
- Hong CH, Papp KA, Lophaven KW, et al. Patients with psoriasis have different preferences for topical therapy, highlighting the importance of individualized treatment approaches: randomized phase IIIb PSO-INSIGHTFUL study. J Eur Acad Dermatol Venereol. 2017;31(11):1876–1883.
- Vender R, Gooderham MJ, Guenther LC, et al. Psoriasis patients' preference for an aerosol foam topical formulation. J Eur Acad Dermatol Venereol. 2018;32(11):e400–e401.
- Velasco M, González-Fernández D, Rodriguez-Martín M, et al. Patient and physician satisfaction with calcipotriol and betamethasone dipropionate aerosol foam in the treatment of plaque psoriasis on the Body. Actas Dermosifiliogr. 2019;110(9):752–758.
