Abstract
Background
The European Prospective Observational Study in Patients Eligible for Systemic Therapy for Atopic Dermatitis (EUROSTAD) is an ongoing observational study aiming to describe characteristics of patients with atopic dermatitis (AD) treated with systemic therapy over time and the management of their disease in a real-world setting.
Methods
Data from patients enrolled in EUROSTAD between March 2017 and April 2019 were analyzed for systemic therapy use and treatment change over 12 months.
Results
288 patients reported taking systemic medications; 42.7% received cyclosporine, 35.3% dupilumab, 28.1% methotrexate, 25.4% oral corticosteroids, 6.8% azathioprine, 6.1% injectable corticosteroids, and 3.4% mycophenolate. The median duration of treatment was 1.1 months for oral systemic corticosteroids, 3.2 months for injectable corticosteroids, 4.8 months for cyclosporine, 7.3 months for methotrexate, and 14.9 months for dupilumab. The most frequent reasons for stopping treatment included lack of efficacy, patient decision, adverse events, and disease well controlled.
Conclusion
The 12-month interim EUROSTAD study analysis highlights the current trends and outcomes of systemic treatments for moderate-to-severe AD. Among all systemic treatments for AD, dupilumab was the least likely to be discontinued, whereas cyclosporine and corticosteroids, whilst effective, were primarily limited to episodic flare management consistent with treatment guidelines.
© 2022 Sanofi. Published with license by Taylor & Francis Group, LLC.
Introduction
Atopic dermatitis (AD), a predominantly type 2 inflammatory skin disease characterized by pruritus (itch) and eczematous lesions, is often associated with other type 2 (atopic/allergic) comorbidities such as asthma, certain chronic sino-nasal conditions, and allergic conjunctivitis (Citation1,Citation2). AD affects approximately 2%–7% of adults worldwide, with the highest rates observed in Europe and the USA (Citation3–5). In moderate-to-severe AD, lesions can be extensive with intense pruritus. Sleep and mental health disturbances can occur, which might impact the quality of life (QoL) (Citation6–8). Due to the chronic and relapsing nature of moderate-to-severe AD, patients often require long-term, systemic treatments (Citation9–11). Insight into real-world treatments and disease burdens are needed to help inform clinical and health policy decisions.
The European Prospective Observational Study in Patients Eligible for Systemic Therapy for Atopic Dermatitis (EUROSTAD) is an ongoing observational study aiming to inform physician treatment choices by describing characteristics over time of patients with AD treated with systemic therapy and the management of their disease in a real-world setting (Citation12).
The objective of this paper is to describe the patient characteristics, outcomes, and the median duration of use of different systemic therapies (drug survival) in real-world conditions in adult AD patients from an interim 1-year analysis of the EUROSTAD observational study.
Methods
EUROSTAD was a prospective observational study of patients with moderate-to-severe AD receiving systemic treatment in various European countries. The study design and baseline characteristics have been previously published (Citation12,Citation13). Briefly, EUROSTAD was designed to characterize the real-world demographics and medical history of patients receiving systemic therapies, their disease activity, symptoms, and QoL, and lastly, real-world effectiveness and safety of systemic AD therapy. The study aimed to enroll 500 patients at 51 sites in ten European countries.
Eligible patients were aged ≥ 18 years and were eligible for systemic treatment and had started or switched to a new systemic treatment on Day 1 or in the 30 days before enrollment. Patients were intended to continue for 60 months in total, with follow-up visits every 3–4 months; however, the study was terminated early due to the impact of COVID-19. This analysis includes interim data at 12 months, collected between March 2017 and April 2019 with a database lock of April 4, 2019.
Study outcomes
Patients enrolled in EUROSTAD were analyzed for both clinical and patient-reported outcomes. The treating clinician assessed the disease status using the Investigator’s Global Assessment (IGA) scale (0 = clear, 1 = almost clear, 2 = mild, 3 = moderate, 4 = severe) (Citation14) and Eczema Area and Severity Index (EASI; range 0 − 72) (Citation15). Patient-reported data included Peak Pruritus Numeric Rating Scale (NRS; range 1–10) (Citation16), Dermatology Life Quality Index (DLQI; range 0–30) (Citation17); Patient-Oriented Eczema Measure (POEM; range 0–28) (Citation18); Hospital Anxiety and Depression Scale (HADS; 0–7 = normal; 8–10 = borderline abnormal, 11–21 = abnormal) (Citation19); sleep quality Visual Analog Scale (VAS; 0–100mm [0 to <40mm indicating none or mild impairment, 40 to <70mm moderate, 70 to <90mm severe, and ≥90mm very severe impairment]); and the 5-dimension EuroQoL 3-level questionnaire (EQ-5D-3L) VAS (range 0–100) (Citation20). For each of these metrics, higher scores represent greater severity. IGA 0 or 1, or 2-point improvement was also recorded.
Systemic therapy and treatment change (including the reasons for starting, stopping, or changing systemic treatment), as well as the most common reason for treatment discontinuation during the follow-up period, were also recorded. All patient data was pseudo-anonymized.
Statistical analyses
The enrollment and safety populations included patients who completed the enrollment visit (Visit 1). Demographic, clinical characteristics, and outcomes data were summarized using descriptive statistics and measures of central tendency. No imputation of missing data was performed. All statistical analyses were performed using SAS version 9.2 or higher (SAS Institute, Cary, NC, USA). Kaplan–Meier curve analysis was used to illustrate the median duration of treatment for each systemic therapy until the day of discontinuation of therapy. If the discontinuation day was not reached (for example, due to the data cutoff for the interim analysis), the patient was censored at the date of the last available information. If multiple treatment durations were present, the longest treatment cycle was used in the illustration.
EUROSTAD is being conducted in accordance with the principles defined by the 18th World Medical Association General Assembly Declaration of Helsinki and all subsequent amendments. The EUROSTAD protocol was reviewed and approved by institutional review boards before patient recruitment. All patients provided written informed consent before any EUROSTAD procedures began.
Results
Patient disposition
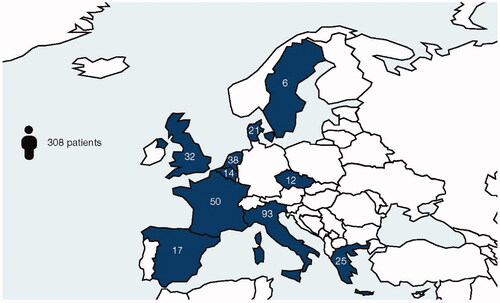
In total, 308 patients were included in EUROSTAD, with baseline demographics and disease characteristics as shown in . A breakdown by country of enrollment is shown in . Patients had a mean age of 37 years, with a mean duration of AD of ∼25 years. Disease burden at baseline was relatively high, with a mean EASI of 16.2 and IGA score of 3.1. QoL metrics at baseline showed a moderate impact on sleep, anxiety, and depression. At database lock, follow-up 3-month data were available for 290 patients, 6-month data for 269 patients, 9-month data for 235 patients, 12-month data for 192 patients, and more-than-12 months’ data for 95 patients.
Table 1. Baseline demographics and disease characteristics.
Systemic treatments
At baseline, most patients (286 [92.9%]) reported taking systemic medication, of whom 39.2% received cyclosporine, 23.1% methotrexate, 19.6% dupilumab,14.0% oral corticosteroids, 5.6% azathioprine, 3.8% injectable corticosteroids, and 2.8% mycophenolate (). During the 12-month treatment follow-up, 288 patients used systemic medications, of whom 42.7% received cyclosporine, 35.3% dupilumab, 28.1% methotrexate, 25.4% oral corticosteroids, 6.8% azathioprine, 6.1% injectable corticosteroids, and 3.4% mycophenolate ().
Table 2. Systemic treatment at enrollment and during 12-month follow-up.
Treatment sequence and overlap analysis
Common treatment sequences of patients included, but were not limited to, cyclosporine -> dupilumab; oral corticosteroids -> injectable corticosteroids -> dupilumab; and methotrexate and oral corticosteroids -> methotrexate -> dupilumab. Patients may have received more than one systemic treatment concurrently, and data are shown for combinations of methotrexate, cyclosporine, and dupilumab (). Treatment sequences visualized per patient by initiating or switching medication are shown in Supplementary Figure S1. The percentage of patients who had 2 or more sequential overlapping systemic treatments (overlap of fewer than 4 weeks) in this study was 20.7%. Overlap of 2 or more systemic treatments of 4 weeks or more occurred in 7.1% of patients.
Drug survival analysis
The median duration of treatment was 1.1 months for oral systemic corticosteroids, 3.2 months for injectable corticosteroids, 4.8 months for cyclosporine, 7.3 months for methotrexate, and 14.9 months for dupilumab. Kaplan–Meier plots of drug survival showed dupilumab treatment persistence was highest and declined slowly over 12 months (). The most frequent reasons for initiating systemic treatment are shown in , with the exacerbation of disease being the most common reason for starting. Of note, methotrexate was recorded as being used for treatment induction in 33.7% of patients receiving it. Reasons for stopping treatment are also shown in for each therapy, the most common reasons across all drugs were as follows: disease well-controlled, lack of efficacy, adverse events, and patient decision. Among the patients who discontinued cyclosporine, less than half (40.2%) did so because of good disease control. In patients who discontinued systemic corticosteroids, good disease control was reported as the reason for discontinuation in 72.3% of them.
Figure 2. Treatment duration: Kaplan–Meier analysis of time to treatment discontinuation. Values underneath the graphs show patients with available measurements at each visit. Month 0 is the therapy start time. The X-axis represents the time (month) since the start of therapy.
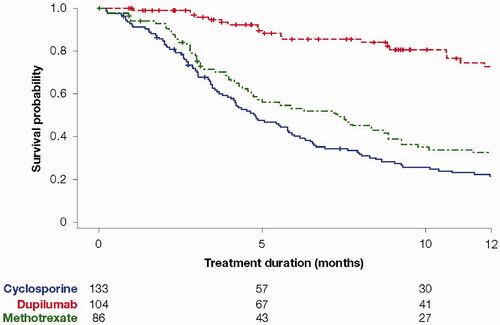
Table 3. Most common reasons for initiating or discontinuing systemic therapy.
Disease severity
shows the overall change in EASI and IGA score over the study, with both metrics trending toward better disease severity over time.
Table 4. Change in EASI and IGA score over time from the start of EUROSTAD.
Discussion
This 12-month interim analysis from the EUROSTAD study highlights the current real-world medical trends and outcomes in the treatment of moderate-to-severe AD. Patients enrolled in EUROSTAD had moderate-to-severe disease, with a mean baseline EASI of 16.2, and >85% of patients having an IGA score of 3 or 4. The burden of AD experienced in the enrolled patients is consistent with other real-world studies from around the world (Citation21–24). A previous study of 1467 patients at multiple centers in Europe and Canada reported that moderate and severe AD disease (as classified by the IGA) was accompanied by a substantial and significantly high burden across multiple domains, including sleep, anxiety, depression, pain, and overall QoL measured by HADS, DLQI, and components of Scoring of Atopic Dermatitis (SCORAD) and POEM (Citation21). Data from analysis of the Optum research database, including 801 AD patients in the USA receiving systemic therapies, showed that 81.3% of patients experienced AD flares over 12 months, despite being on systemic treatment (Citation25).
Another facet of disease burden can be measured in terms of the lost time and productivity of AD patients, and an earlier study by Zuberbier et al. discussed the high costs of untreated allergic conditions, including dermatitis, with indirect costs of up to €2405/year (2014) (Citation26). Similarly, the patient burden of inadequately controlled AD is a significant limitation for patients and has been published previously for the EUROSTAD patient cohort, with time lost from work or activities of 4.1 and 16.8 days/year, respectively (Citation12).
In this study the highest drug persistence was with dupilumab: 14.9 months. Of note, analysis of the reasons for starting and stopping treatment with cyclosporine and corticosteroids is consistent with their use for acute flare management, as a rescue and not as a maintenance treatment, in line with treatment guidelines (Citation11). For example, with cyclosporine, 64% started therapy due to exacerbation, and 40% discontinued due to disease under control; with injectable corticosteroids, 72% started due to AD exacerbation, and 59% discontinued due to disease well controlled. Methotrexate was recorded in this study to be used for treatment induction which seems at odds with its relatively slow onset of action to improve AD from 2 weeks to 3 months onwards (Citation11).
With respect to treatment persistence, a retrospective cohort study of 1963 adult patients in the USA who received dupilumab treatment showed the persistence at 6 and 12 months was 91.9% (95% CI: 90.7%–93.2%) and 77.3% (75.0%–79.7%), respectively (Citation27). Similarly, in the BioDay registry, which included 402 adult patients receiving dupilumab across multiple centers in the Netherlands, the overall drug survival rates for dupilumab were 91% and 88% after 1 and 2 years, respectively (Citation28). This is in contrast to the drug persistence seen with other systemic treatments − 4.8 months for cyclosporine and 7.3 months for methotrexate observed in the present study, and of 7.9 and 7.3 months for cyclosporine and methotrexate, respectively, in the BioDay study (Citation28). Studies prior to the introduction of biologics also showed poor drug persistence of immunosuppressants as a class, of less than 32% over 12 months (Citation29). In another small retrospective study of 56 patients in France, drug survival was 12 months with methotrexate compared with two months with cyclosporine (Citation30). Taken together, the real-world persistence of dupilumab appears to offer stable treatment for AD patients.
Limitations of the current study include that this is an observational study with limited patient numbers and that the patient population is relatively young, with a high disease burden, including patients receiving systemic treatments, which is not representative of all AD patients. However, we feel that real-world data collected in a practice setting, rather than a clinical trial, adds an additional source of data to inform physician treatment choices more reflective of everyday practice. EUROSTAD will continue to follow this patient cohort over time, allowing for more insight into ongoing treatment choices and disease course of patients with relatively severe AD in a real-world setting.
Conclusions
In conclusion, this 12-month interim EUROSTAD study analysis highlights the current trends and duration of use in systemic treatments for moderate-to-severe AD. Among all systemic treatments for AD, dupilumab was the least likely to be discontinued, while cyclosporine and corticosteroids were primarily limited to episodic flare management, consistent with current treatment guidelines.
Author contribution
MdBW, MA contributed to study concept and design, analysis, and interpretation, provided critical feedback on the manuscript, approved the final manuscript for submission, and were accountable for the accuracy and integrity of the manuscript. SJ conducted statistical analyses on the data. All authors interpreted the data, provided critical feedback on the manuscript, approved the final manuscript for submission, and were accountable for the accuracy and integrity of the manuscript.
Supplemental Material
Download JPEG Image (2.9 MB)Acknowledgments
Research sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. Medical writing/editorial assistance was provided by Yunyu Huang, Ph.D. of Excerpta Medica, funded by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc. according to the Good Publication Practice guideline.
Disclosure statement
MdBW is a consultant, advisory board member, and/or speaker for AbbVie, Almirall, Aslan Pharmaceuticals, Eli Lilly, Galderma, Janssen, LEO Pharma, Pfizer, Regeneron Pharmaceuticals, Inc., Sanofi Genzyme, and UCB. AEP is an advisory board member, speaker or investigator for AbbVie, Almirall, Bristol Myers Squibb, Eli Lilly, La Roche-Posay, LEO Pharma, Novartis, Pfizer, Sanofi, and UCB. SMF is a speaker and consultant for Drex Pharma, Menarini, Novartis, Pierre Fabre Laboratories, Sanofi Genzyme, and SVR; Principal Investigator for Eli Lilly, Novartis, Regeneron Pharmaceuticals, Inc., and Sanofi Genzyme. AP is an advisory board member, investigator for, and received grants from Eli Lilly; advisory board member, and/or investigator, and/or speaker for, and/or has received grants from AbbVie; and advisory board member for and received grants from Almirall, Eli Lilly, La Roche-Posay, LEO Pharma, Menarini, Novartis, Pierre Fabre, Roche, Sanofi Genzyme, Pfizer, and UCB. AS is a Principal Investigator for Regeneron Pharmaceuticals, Inc. and Sanofi Genzyme. MLS is an advisory board member and consultant for Sanofi Genzyme; investigator for AbbVie, Novartis, and Sanofi Genzyme; and consultant for Regeneron Pharmaceuticals, Inc. MT is an investigator, speaker, and consultant for Regeneron Pharmaceuticals, Inc. and/or Sanofi Genzyme; investigator, speaker and consultant for AbbVie; investigator and consultant for Lilly; consultant for Medac. MA is an employee and shareholder for Regeneron Pharmaceuticals, Inc. SJ and MD are employees of Sanofi Genzyme and may hold stock and/or stock options in the company.
Additional information
Funding
References
- Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13(5):425–437.
- Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132(5):1132–1138.
- Brandt EB, Sivaprasad U. Th2 cytokines and atopic dermatitis. J Clin Cell Immunol. 2011;2(3):110.
- Barbarot S, Auziere S, Gadkari A, et al. Epidemiology of atopic dermatitis in adults: results from an international survey. Allergy. 2018;73(6):1284–1293.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139(3):583–590.
- Simpson EL, Bieber T, Eckert L, et al. Patient burden of moderate to severe atopic dermatitis (AD): insights from a phase 2b clinical trial of dupilumab in adults. J Am Acad Dermatol. 2016;74(3):491–498.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135(1):56–66.
- Sibbald C, Drucker AM. Patient burden of atopic dermatitis. Dermatol Clin. 2017;35(3):303–316.
- LePoidevin LM, Lee DE, Shi VY. A comparison of international management guidelines for atopic dermatitis. Pediatr Dermatol. 2019;36(1):36–65.
- Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32(5):657–682.
- Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32(6):850–878.
- Bruin-Weller M, Pink AE, Patrizi A, et al. Disease burden and treatment history among adults with atopic dermatitis receiving systemic therapy: baseline characteristics of participants on the EUROSTAD prospective observational study. J Dermatolog Treat. 2021;32(2):164–173.
- Bruin-Weller M, Pink AE, Patrizi A, et al. EUROSTAD prospective observational study: baseline characteristics, atopic dermatitis severity, and patient-reported outcomes. J Am Acad Dermatol. 2019;81(4):AB58.
- Rehal B, Armstrong AW, Armstrong A. Health outcome measures in atopic dermatitis: a systematic review of trends in disease severity and quality-of-life instruments 1985-2010. PLOS One. 2011;6(4):e17520.
- Hanifin JM, Thurston M, Omoto M, et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. 2001;10(1):11–18.
- Yosipovitch G, Reaney M, Mastey V, et al. Peak pruritus numerical rating scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol. 2019;181(4):761–769.
- Basra MK, Fenech R, Gatt RM, et al. The dermatology life quality index 1994-2007: a comprehensive review of validation data and clinical results. Br J Dermatol. 2008;159(5):997–1035.
- Schram ME, Spuls PI, Leeflang MM, et al. EASI, (objective) SCORAD and POEM for atopic eczema: responsiveness and minimal clinically important difference. Allergy. 2012;67(1):99–106.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370.
- Rabin R, Gudex C, Selai C, et al. From translation to version management: a history and review of methods for the cultural adaptation of the EuroQol five-dimensional questionnaire. Value Health. 2014;17(1):70–76.
- de Bruin-Weller M, Gadkari A, Auziere S, et al. The patient-reported disease burden in adults with atopic dermatitis: a cross-sectional study in Europe and Canada. J Eur Acad Dermatol Venereol. 2020;34(5):1026–1036.
- Zuberbier T, Lötvall J, Simoens S, et al. Economic burden of inadequate management of allergic diseases in the European Union: a GA(2) LEN review. Allergy. 2014;69(10):1275–1279.
- Eckert L, Gupta S, Gadkari A, et al. Burden of illness in adults with atopic dermatitis: analysis of national health and wellness survey data from France, Germany, Italy, Spain, and the United Kingdom. J Am Acad Dermatol. 2019;81(1):187–195.
- Katoh N, Saeki H, Kataoka Y, et al. Atopic dermatitis disease registry in Japanese adult patients with moderate to severe atopic dermatitis (ADDRESS-J): baseline characteristics, treatment history and disease burden. J Dermatol. 2019;46(4):290–300.
- Wei W, Ghorayeb E, Andria M, et al. A real-world study evaluating adeQUacy of existing systemic treatments for patients with moderate-to-severe atopic dermatitis (QUEST-AD): baseline treatment patterns and unmet needs assessment. Ann Allergy Asthma Immunol. 2019;123(4):381–388.e2.
- Akinlade B, Guttman-Yassky E, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials. Br J Dermatol. 2019;181(3):459–473.
- Silverberg JI, Guttman-Yassky E, Gadkari A, et al. Real-world persistence with dupilumab among adults with atopic dermatitis. Ann Allergy Asthma Immunol. 2021;126(1):40–45.
- Spekhorst LS, Ariëns LFM, Schaft J, et al. Two-year drug survival of dupilumab in a large cohort of difficult-to-treat adult atopic dermatitis patients compared to cyclosporine A and methotrexate: results from the BioDay registry. Allergy. 2020;75(9):2376–2379.
- Armstrong AW, Huang A, Wang L, et al. Real-world utilization patterns of systemic immunosuppressants among US adult patients with atopic dermatitis. PLOS One. 2019;14(1):e0210517.
- Law Ping Man S, Bouzillé G, Beneton N, et al. Drug survival and postdrug survival of first-line immunosuppressive treatments for atopic dermatitis: comparison between methotrexate and cyclosporine. J Eur Acad Dermatol Venereol. 2018;32(8):1327–1335.