Abstract
Objectives
Evaluate relationships between changes in dermatologic assessments and quality of life (QoL) measures; quantify dermatologic symptom severity impacts on QoL in patients with psoriatic arthritis (PsA) treated with tofacitinib.
Methods
Data were from two phase III studies; patients received tofacitinib 5 or 10 mg twice daily (BID), adalimumab 40 mg every other week, or placebo advancing to tofacitinib 5 or 10 mg BID at Month 3. Repeated measures longitudinal models assessed relationships between dermatologic assessments (predictors) Itch Severity Item (ISI), Physician’s Global Assessment of Psoriasis (PGA-PsO), and Patient’s Global Joint and Skin Assessment-Visual Analog Scale-Psoriasis question (PGJS-VAS-PsO), and QoL measures (outcomes) Dermatology Life Quality Index (DLQI) and Short Form-36 Health Survey Version 2 (SF-36v2). Models included one predictor and one outcome.
Results
Direct, approximately linear relationships existed between predictors and outcomes. ISI/PGA-PsO/PGJS-VAS-PsO improvements from baseline of ≥3/≥2/≥40-mm VAS corresponded with clinically meaningful DLQI improvements; improvements from baseline of ≥4/≥3/≥40-mm VAS generally corresponded with clinically meaningful improvements across component scores and all SF-36v2 domains.
Conclusions
Substantial links exist between dermatologic symptoms and QoL in patients with PsA, potentially informing patient-centered care and research. Rheumatologists should be aware of dermatologic manifestations and QoL impacts in patients with PsA.
ClinicalTrials.gov
NCT01877668; NCT01882439
Introduction
Psoriatic arthritis (PsA) is a chronic, progressive, inflammatory disease that is characterized by enthesitis, dactylitis, axial disease, and skin manifestations (Citation1,Citation2). It occurs in approximately 30% of patients with psoriasis (PsO) (Citation3), with global prevalence ranging from 0.01 to 0.19% (Citation4).
PsA can have substantial impacts on patient quality of life (QoL), work productivity, daily activities, and emotional wellbeing (Citation5). While it most commonly manifests as peripheral joint disease, it can also involve axial joints, as well as the skin and nails (Citation1,Citation3), with itching having been reported as the most bothersome dermatologic symptom of PsA (Citation6). Compared with patients with joint symptoms alone, those with both joint and active skin disease have been found to experience a more severe overall disease state, including higher levels of pain, greater numbers of concomitant conditions, and worse patient-reported outcomes (PROs) (Citation7). Administrative claims data from the US indicate that patients with PsA have significantly greater healthcare utilization and incur higher healthcare costs than patients with PsO and those without PsA or PsO diagnoses (Citation8,Citation9). A cross-sectional study of patients with PsA and dermatologic symptoms indicated that severity of skin involvement was associated with impacts on QoL; dermatologic symptoms contributed to feelings of shyness, embarrassment, shame, and guilt, and many patients reported severe negative impacts on social situations and relationships with others (Citation5). Therefore, effective treatment of dermatologic symptoms may be a rational strategy to improve QoL in patients with active PsA.
Tofacitinib is an orally bioavailable small molecule that inhibits by blocking the adenosine triphosphate binding site of the Janus kinase (JAK) protein (Citation10). In cellular settings where JAKs signal in pairs, tofacitinib preferentially inhibits signaling by heterodimeric cytokine receptors associated with JAK1 and/or JAK3, and has functional selectivity over JAK2 (Citation11). Tofacitinib was approved by the US Food and Drug Administration (FDA) for the treatment of PsA in 2017. In 2021, the US prescribing information for tofacitinib was revised, and a new boxed warning for major adverse cardiovascular events (MACE), and updated boxed warnings regarding mortality, malignancies, and thrombosis, with corresponding updates to applicable warnings and precautions, were included (Citation12). These updates followed the FDA’s completed review of the ORAL Surveillance trial, a post-marketing required safety study in patients with rheumatoid arthritis aged ≥50 years old with at least one additional cardiovascular risk factor evaluating the safety of tofacitinib at two doses (5 and 10 mg twice daily [BID]) compared with tumor necrosis factor inhibitor (TNFi) therapy. Initial co-primary endpoint results showed that patients treated with tofacitinib had a higher rate of MACE and malignancies relative to TNFi, regardless of tofacitinib dose received (Citation13).
The efficacy and safety of tofacitinib 5 and 10 mg BID have been demonstrated in phase III, randomized, controlled studies of patients with active PsA with an inadequate response to either conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) or TNFi therapy, and were investigated in an open-label, long-term extension study (Citation14–16). A post-hoc analysis demonstrated that treatment with tofacitinib in patients with PsA was associated with clinically meaningful improvements in dermatologic endpoints, such as Itch Severity Item (ISI), Physician’s Global Assessment of Psoriasis (PGA-PsO), Patient’s Global Joint and Skin Assessment-Visual Analog Scale-PsO question (PGJS-VAS-PsO), and Dermatology Life Quality Index (DLQI) (Citation17); however, the relationships between these outcomes were not quantified.
This post-hoc analysis evaluated relationships between changes from baseline in dermatologic endpoints (ISI, PGA-PsO, and PGJS-VAS-PsO) and QoL measures (DLQI and Short Form-36 Health Survey Version 2 [SF-36v2]). We also sought to quantify the improvements in dermatologic outcomes required to change dermatology QoL (based on categorization of DLQI scores), as well as the incremental improvements in dermatologic outcomes required to achieve clinically meaningful improvements in general QoL (based on SF-36v2) in patients with PsA.
Materials and methods
Patients
This analysis used pooled data from two phase III, multicenter, placebo-controlled, double-blind, randomized studies of tofacitinib in patients with active PsA: OPAL Broaden (NCT01877668; N = 422; 12 months’ duration) and OPAL Beyond (NCT01882439; N = 394; 6 months’ duration) (Citation14,Citation15).
Patients enrolled in OPAL Broaden had an inadequate response to ≥1 csDMARD and were TNFi-naïve, while patients enrolled in OPAL Beyond had an inadequate response to ≥1 TNFi.
Patients were randomized to tofacitinib 5 mg BID, tofacitinib 10 mg BID, adalimumab 40 mg subcutaneous injection once every 2 weeks (OPAL Broaden only), or placebo; those receiving placebo advanced to tofacitinib 5 or 10 mg BID at Month 3 in a blinded manner. Patients in both studies received a stable dose of a single csDMARD (methotrexate, sulfasalazine, or leflunomide).
Studies were conducted in accordance with the Declaration of Helsinki and the International Council for Harmonisation Good Clinical Practice Guidelines, along with applicable local country regulations and laws. The study protocols were approved by the Institutional Review Boards and/or Independent Ethics Committee at each center. All patients provided written informed consent.
Assessments of dermatologic symptoms
ISI, PGA-PsO, and PGJS-VAS-PsO were used to assess dermatologic symptoms. ISI is a measure of the severity of itch (pruritus), assessed as a single-item, horizontal numeric rating scale (Citation18). Patients were asked to rate their itching due to PsO over the past 24 h on a numeric rating scale of 0 (‘no itching’) to 10 (‘worst possible itching’). PGA-PsO was assessed by physicians on a 5-point severity scale (0–4), reflecting global consideration of erythema, induration, and scaling, which were scored separately across all psoriatic lesions, with average erythema, induration, and scaling over the whole body, as defined by morphologic descriptors, rated separately (Citation19). PGJS-VAS-PsO was used to assess patients’ perceptions of disease using a 100-mm VAS; patients were asked to respond on a scale of ‘excellent’ to ‘poor’ to the question, ‘In all the ways your PSORIASIS affects you, how would you rate the way you felt over the past week?’ (Citation20).
Assessments of QoL
DLQI and SF-36v2 were used as dermatology-specific and generic measures of QoL, respectively. DLQI is a series of ten questions (Citation21), with scores having been previously categorized as reflecting no effect (0–1), a small effect (2–5), a moderate effect (6–10), a very large effect (11–20), or an extremely large effect (21–30) on a patient’s life, respectively (Citation22). For general inflammatory skin conditions, a change in DLQI score of at least 4 points is considered clinically meaningful (Citation23,Citation24); we thus applied this threshold to assessments of clinically meaningful changes in DLQI.
The SF-36v2 questionnaire examines eight general health domains: physical functioning (PF), role limitations due to physical health (RP), bodily pain (BP), general health perceptions (GH), vitality (VT), social functioning (SF), role limitations due to emotional problems (RE), and mental health (MH). These can also be summarized as Physical and Mental Component Summary (PCS and MCS, respectively) scores. Thresholds for clinically meaningful changes in SF-36v2 component and domain scores in this analysis were based on the 1998 US general population sample normative dataset (Citation25): PF, 3.5; RP, 3.2; BP, 4.5; GH, 5.7; VT, 5.5; SF, 5.0; RE, 3.8; MH, 5.5; PCS score, 3.1; MCS score, 3.8.
Data analysis
All available data from all patients across all treatment groups in OPAL Broaden and OPAL Beyond, from baseline to study end, were included in this post-hoc analysis.
A repeated measures longitudinal model (Citation26) assessed relationships between ISI, PGA-PsO, and PGJS-VAS-PsO scores, as predictors, and DLQI, SF-36v2 domain scores, and SF-36v2 component summary scores, as outcomes. Each longitudinal model included one predictor and one outcome.
For SF-36v2 component scores, norm-based scores were used, whereby a score of 50 and a standard deviation of 10 reflected normative scores for the US general population; higher scores indicated less impairment (Citation25). Norm-based scores were also used for SF-36v2 domains in the analyses. Higher scores indicated a better QoL, a difference of 10 points corresponded to the difference of 1 standard deviation, while a difference of 5 points corresponded to a standard deviation of 0.5.
Models were implemented using predictors as both continuous and categorical variables. For models implemented using predictors as continuous variables, DLQI score or SF-36v2 domains (including component summary scores) were used as dependent variables. These models impose a linear relationship between predictors and outcomes. Predictors were also used as categorical variables to assess the appropriateness of the linear approximation of relationships between predictors and outcomes. When predictors were used as categorical variables, this model did not impose any functional relationship between predictors and outcomes.
Results
Patients
For this post-hoc analysis, data for 816 patients (422 patients from OPAL Broaden; 394 patients from OPAL Beyond) were available. Their baseline demographics and disease characteristics have been reported previously (Citation14,Citation15).
Relationships between ISI/PGA-PsO/PGJS-VAS-PsO and DLQI/SF-36v2
ISI, PGA-PsO, and PGJS-VAS-PsO scores all had approximately linear relationships with DLQI, SF-36v2 PCS score, and SF-32v2 MCS score (), and each of the 8 SF-36v2 domains (Supplementary Figures 1–3). Results from models in which predictors were used as categorical variables supported the linearity assumption for relationships between predictors and outcomes (Supplementary Figures 1–3).
Figure 1. Relationships between ISI and (A) DLQI, (B) SF-36v2 PCS score, and (C) SF-36v2 MCS score (pooled data from OPAL Broaden and OPAL Beyond). SF-36v2 analyses, N = 816; DLQI analyses, N = 815. Relationships were assessed with the ISI predictor used as a continuous (black lines) and categorical (gray lines) variable. DLQI: Dermatology Life Quality Index; ISI: Itch Severity Item; MCS: Mental Component Summary; PCS: Physical Component Summary; SF-36v2: Short Form-36 Health Survey Version 2.
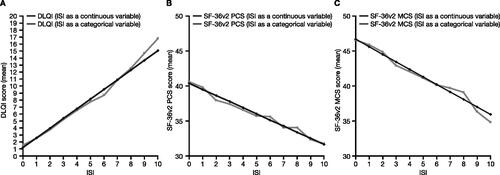
Figure 2. Relationships between PGA-PsO and (A) DLQI, (B) SF-36v2 PCS score, and (C) SF-36v2 MCS score (pooled data from OPAL Broaden and OPAL Beyond). SF-36v2 analyses, N = 816; DLQI analyses, N = 815. Relationships were assessed with the PGA-PsO predictor used as a continuous (black lines) and categorical (gray lines) variable. DLQI: Dermatology Life Quality Index; MCS: Mental Component Summary; PCS: Physical Component Summary; PGA-PsO: Physician’s Assessment of Psoriasis; SF-36v2: Short Form-36 Health Survey Version 2.
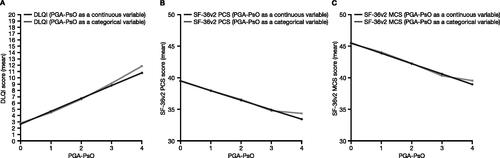
Figure 3. Relationships between PGJS-VAS-PsO and (A) DLQI, (B) SF-36v2 PCS score, and (C) SF-36v2 MCS score (pooled data from OPAL Broaden and OPAL Beyond). SF-36v2 analyses, N = 816; DLQI analyses, N = 815. Relationships were assessed with the PGJS-VAS-PsO predictor used as a continuous (black lines) and categorical (gray lines) variable. DLQI: Dermatology Life Quality Index; MCS: Mental Component Summary; PCS: Physical Component Summary; PGJS-VAS-PsO: Patient’s Global Joint and Skin Assessment-Visual Analog Scale-Psoriasis question; SF-36v2: Short Form-36 Health Survey Version 2.
Quantification of relationships based on categorization of DLQI score
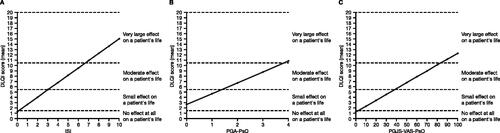
Strong relationships were observed between ISI, PGA-PsO, PGJS-VAS-PsO, and DLQI, illustrating a direct link between severity of itch, severity and patient perception of dermatologic symptoms, and dermatology QoL, respectively, and allowing the quantification of this association, based on categorization of DLQI score () (Citation22). ISI scores of 0–3, PGA-PsO scores of 0–1, and PGJS-VAS-PsO scores of 0–30 mm corresponded with DLQI scores categorized as having no or a small effect on a patient’s life; ISI scores of 4–6, PGA-PsO scores of 2–3, and PGJS-VAS-PsO scores of 40–80 mm corresponded with DLQI scores categorized as having a moderate effect on a patient’s life; and ISI scores of 7–10, PGA-PsO scores of 4, and PGJS-VAS-PsO scores of 90–100 mm corresponded with DLQI scores categorized as having a very large effect on a patient’s life ().
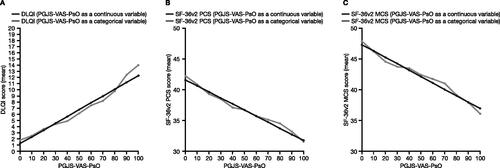
Figure 4. Relationships of (A) ISI, (B) PGA-PsO, and (C) PGJS-VAS-PsO with DLQI categories (pooled data from OPAL Broaden and OPAL Beyond). DLQI analyses, N = 815. Midpoints between adjacent severity categories were used as cutoffs for interpretation of the relationships of ISI, PGA-PsO, and PGJS-VAS-PsO vs. DLQI. DLQI: Dermatology Life Quality Index; ISI: Itch Severity Item; PGA-PsO: Physician’s Assessment of Psoriasis; PGJS-VAS-PsO: Patient’s Global Joint and Skin Assessment-Visual Analog Scale-Psoriasis question.
Quantification of relationships, based on clinically meaningful improvements in DLQI and SF-36v2
Improvements of ≥3 points in ISI, ≥2 points in PGA-PsO, and ≥40 mm VAS in PGJS-VAS-PsO, respectively, were associated with clinically meaningful improvements in DLQI score (). Improvements of ≥4 points in ISI, ≥3 points in PGA-PsO, and ≥40 mm VAS in PGJS-VAS-PsO were associated with clinically meaningful improvements in the SF-36v2 PCS and MCS components, and clinically meaningful improvements across several SF-36v2 domains ().
Table 1. Relationship between incremental improvements in ISI/PGA-PsO/PGJS-VAS-PsO and changes in DLQI scores (pooled data from OPAL Broaden and OPAL Beyond).
Table 2. Relationship between incremental improvements in ISI/PGA-PsO/PGJS-VAS-PsO and changes in SF-36v2 component and domain scores.
Discussion
This post-hoc analysis used data from patients with PsA who received a background csDMARD in the OPAL Broaden and OPAL Beyond studies, and demonstrated approximately linear relationships between dermatologic endpoints (ISI, PGA-PsO, and PGJS-VAS-PsO) and QoL (DLQI and all SF-36v2 component and domain scores). We quantified the relationship between severity of dermatologic outcomes and dermatology QoL in terms of the impact of a given change on a patient’s life (based on categorization of DLQI score), as well as the relationships between improvements in dermatologic outcomes and improvements in dermatology or general QoL. Scores of 7–10/4/90–100 mm VAS in ISI/PGA-PsO/PGJS-VAS-PsO, respectively, corresponded with DLQI scores categorized as having a very large effect on a patient’s life. Improvements from baseline of ≥3-point/≥2-point/≥40-mm VAS in ISI/PGA-PsO/PGJS-VAS-PsO, respectively, were associated with clinically meaningful improvements in DLQI. Improvements from baseline of ≥4-point/≥3-point/≥40-mm VAS in ISI/PGA-PsO/PGJS-VAS-PsO, respectively, were generally associated with clinically meaningful improvements across all SF-36v2 component and domain scores. In view of the well-recognized mental health sequelae that are commonly observed in patients with PsA, it is important to note that improvements were observed in both SF-36v2 physical and mental health domains.
Our data were generated from the two different patient populations enrolled in OPAL Broaden and OPAL Beyond. In OPAL Broaden, patients had an inadequate response to ≥1 csDMARD and were TNFi-naïve (Citation15), while patients in OPAL Beyond had an inadequate response to ≥1 TNFi (Citation14). In a previous post-hoc analysis of relationships between PsA disease activity and PROs in these two trials, greater changes from baseline in PsA disease activity measures were reported for patients enrolled in OPAL Beyond than for those enrolled in OPAL Broaden (Citation27). However, a markedly close relationship between disease activity measures and PRO outcomes was seen among patients from both trials (Citation27). Similarly, our results illustrate relationships between dermatologic symptoms and QoL, regardless of patient population or treatment history.
Overall, our findings align with previous studies suggesting that improvement in dermatologic symptoms occur simultaneously with improvements in QoL in patients with active PsA. The efficacy of tofacitinib on dermatologic symptoms of PsA and improvements in QoL has been demonstrated (Citation17). Additionally, previous post-hoc analyses of OPAL Broaden (Citation28) and OPAL Beyond (Citation29) studies of tofacitinib reported improvements across PtGA, Pain, and PGJS measures that corresponded with improvements in SF-36v2 domain scores and component summary scores in patients with PsA. Similarly, analysis of two phase III studies of ixekizumab in patients with PsA indicated that improvements in both joint and skin manifestations were necessary to achieve optimal patient QoL levels (Citation30). The impact of improving dermatologic symptoms on patient QoL extends beyond patients with PsA. For example, patients with atopic dermatitis treated with the JAK1 inhibitor abrocitinib in clinical trials, who experienced improvements in dermatologic signs and symptoms, reported concomitant improvements in QoL (Citation31), indicating that understanding the relationship between dermatologic symptoms and patient QoL may have relevance across therapies and disease states. However, analyses aiming to quantify this relationship to the extent we have presented here are lacking. Our results emphasize the importance of effectively managing dermatologic symptoms in patients with active PsA, and the resulting positive impact on their QoL. The adoption of a treat-to-target approach in the management of PsA has not been widespread in clinical practice (Citation32). However, where opportunity permits treatment change or dose adjustment that can be titrated to response, the findings of this study point to the desirable magnitude of improvement in dermatologic outcomes required in order to achieve a therapeutic goal of meaningful QoL benefit.
Our data also highlight the value of PROs to expand our understanding of the patient perspective and to provide robust data that complement measures of efficacy and safety. A previous post-hoc analysis reported linear relationships between measures of PsA disease activity and PROs, where 1-point differences in PsA Disease Activity Score and minimal disease activity were associated with clinically meaningful changes in a number of PROs, including pain and fatigue (Citation27). The authors noted that these associations provided interpretable and quantifiable metrics between measures of PsA disease activity and PROs, similar to the results we present here, demonstrating a quantifiable link between dermatologic symptoms of PsA and patient QoL.
Limitations of this analysis include its post-hoc nature and the fact that the data are specific to the patient population studied in the OPAL Broaden and OPAL Beyond studies. Furthermore, the population of this post-hoc analysis was heterogeneous, due to data being pooled from two different patient populations. It is also plausible that improvements in musculoskeletal symptoms contributed to QoL outcomes in addition to dermatologic symptoms; our analysis did not account for the impact of musculoskeletal symptoms. Caution should therefore be exercised when generalizing results to the wider patient population with PsA and other related immunologic diseases. As with empirical-based findings in general, we encourage other researchers to confirm our findings in other studies.
This analysis indicates that there are substantial and quantifiable links between dermatologic symptoms, such as itch, and QoL in patients with PsA. Importantly, improvements in dermatologic symptoms translated to clinically meaningful changes in measures of dermatology and general QoL. The data may help inform patient care and patient-centered research. Rheumatologists treating patients with PsA should be aware of itch and other dermatologic manifestations, which can have a significant impact on QoL in patients with PsA.
Supplemental Material
Download PDF (479.2 KB)Acknowledgments
This study was sponsored by Pfizer Inc. Medical writing support, under the guidance of the authors, was provided by Karen Thompson, PhD, CMC Connect, McCann Health Medical Communications, and was funded by Pfizer Inc, New York, NY, USA in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med. 2015;163:461–464). PC Taylor thanks the National Institute of Health Research for their funding of The NIHR Biomedical Research Centre in Musculoskeletal Disease at Oxford University Hospitals NHS Trust and the University of Oxford.
Disclosure statement
PCT has acted as a consultant for AbbVie, Biogen, Bristol-Myers Squibb, Celltrion, Eli Lilly, Fresenius, Galapagos, Gilead Sciences, GlaxoSmithKline, Janssen, Nordic Pharma, Pfizer Inc, Roche, Sanofi, and UCB; and has received research grants from Celgene and Galapagos. AGB, JCC, PY, and RG are employees of Pfizer Inc. AGB and PY are stockholders of Pfizer Inc. JFM is a consultant and/or investigator for AbbVie, Amgen, Biogen, Bristol-Myers Squibb, Dermavant, Eli Lilly, Janssen, Leo Pharma, Novartis, Pfizer Inc, Regeneron, Sanofi, Sun Pharma, and UCB. GY has acted as a consultant for Bellus Health Inc, Eli Lilly, Galderma, Kiniksa, Leo Pharma, Novartis, Pfizer Inc, Sanofi Regeneron, Sienna, and Trevi; and has received research grants from Galderma, Kiniksa, Leo Pharma, Novartis, Pfizer Inc, and Sanofi Regeneron.
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. PCT had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design: PCT, AGB, JCC, PY, RG, JFM, GY.
Acquisition of data: AGB, JCC, JFM.
Analysis of data: PCT, AGB, JCC, PY, JFM, GY.
Interpretation of data: PCT, AGB, JCC, PY, RG, JFM, GY.
Data availability statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Additional information
Funding
References
- Ogdie A, Weiss P. The epidemiology of psoriatic arthritis. Rheum Dis Clin North Am. 2015;41(4):545–568.
- Gladman DD, Antoni C, Mease P, et al. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64(2):ii14–7.
- Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med. 2017;376(10):957–970.
- Stolwijk C, van Onna M, Boonen A, et al. Global prevalence of spondyloarthritis: a systematic review and Meta-regression analysis. Arthritis Care Res (Hoboken). 2016;68(9):1320–1331.
- Merola JF, Shrom D, Eaton J, et al. Patient perspective on the burden of skin and joint symptoms of psoriatic arthritis: results of a multi-national patient survey. Rheumatol Ther. 2019;6(1):33–45.
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70(5):871–881.e30.
- de Vlam K, Merola JF, Birt JA, et al. Skin involvement in psoriatic arthritis worsens overall disease activity, patient-reported outcomes, and increases healthcare resource utilization: an observational, cross-sectional study. Rheumatol Ther. 2018;5(2):423–436.
- Merola JF, Dennis N, Chakravarty SD, et al. Healthcare utilization and costs among patients with psoriasis and psoriatic arthritis in the USA-a retrospective study of claims data from 2009 to 2020. Clin Rheumatol. 2021;40(10):4061–4070.
- Merola JF, Herrera V, Palmer JB. Direct healthcare costs and comorbidity burden among patients with psoriatic arthritis in the USA. Clin Rheumatol. 2018;37(10):2751–2761.
- European Medicines Agency: Science, Medicines, Health [Internet]. Assessment report: Xeljanz, International non-proprietary name: tofacitinib. Amsterdam, The Netherlands: European Medicines Agency; c1995–2022; 2017. Available from: https://www.ema.europa.eu/en/documents/assessment-report/xeljanz-epar-public-assessment-report_en.pdf
- European Medicines Agency: Science, Medicines, Health [Internet]. Xeljanz (tofacitinib citrate): summary of product characteristics. Amsterdam, The Netherlands: European Medicines Agency; c1995–2022; 2022. Available from: https://www.medicines.org.uk/emc/medicine/33167
- US Food and Drug Administration [Internet]. Xeljanz® (tofacitinib): highlights of prescribing information. Silver Spring (MD): US Food and Drug Administration; c2022; 2021. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/203214s028,208246s013,213082s003lbl.pdf
- Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386(4):316–326.
- Gladman D, Rigby W, Azevedo VF, et al. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med. 2017;377(16):1525–1536.
- Mease P, Hall S, FitzGerald O, et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med. 2017;377(16):1537–1550.
- Nash P, Coates LC, Fleishaker D, et al. Safety and efficacy of tofacitinib up to 48 months in patients with active psoriatic arthritis: final analysis of the OPAL balance long-term extension study. Lancet Rheumatol. 2021;3(4):E270–83.
- Merola JF, Papp KA, Nash P, et al. Tofacitinib in psoriatic arthritis patients: skin signs and symptoms and health-related quality of life from two randomized phase 3 studies. J Eur Acad Dermatol Venereol. 2020;34(12):2809–2820.
- Ständer S, Luger T, Cappelleri JC, et al. Validation of the Itch Severity Item as a measurement tool for pruritus in patients with psoriasis: results from a phase 3 tofacitinib program. Acta Derm Venereol. 2018;98(3):340–345.
- Langley RGB, Feldman SR, Nyirady J, et al. The 5-point Investigator's Global Assessment (IGA) scale: a modified tool for evaluating plaque psoriasis severity in clinical trials. J Dermatol Treat. 2015;26(1):23–31.
- Cauli A, Gladman DD, Mathieu A, et al. Patient global assessment in psoriatic arthritis: a multicenter GRAPPA and OMERACT study. J Rheumatol. 2011;38(5):898–903.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)-a simple practical measure for routine clinical use . Clin Exp Dermatol. 1994;19(3):210–216.
- Hongbo Y, Thomas CL, Harrison MA, et al. Translating the science of quality of life into practice: what do Dermatology Life Quality Index scores mean? J Invest Dermatol. 2005;125(4):659–664.
- Basra MKA, Salek MS, Camilleri L, et al. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology. 2015;230(1):27–33.
- Khilji FA, Gonzalez M, Finlay AY. Clinical meaning of change in Dermatology Life Quality Index scores [abstract]. Br J Dermatol. 2002;147(62):50.
- Ware JE, Kosinski M, Bjorner JB, et al. User’s manual for the SF-36v2 Health Survey. 2nd ed. Lincoln (RI): Quality Metric Incorporated; 2007.
- Cappelleri JC, Zou KH, Bushmakin AG, et al. Patient-reported outcomes: measurement, implementation and interpretation. Boca Raton (FL): Chapman & Hall/CRC Press; 2013.
- Coates LC, Bushmakin AG, FitzGerald O, et al. Relationships between psoriatic arthritis composite measures of disease activity with patient-reported outcomes in phase 3 studies of tofacitinib. Arthritis Res Ther. 2021;23(1):94.
- Strand V, de Vlam K, Covarrubias-Cobos JA, et al. Tofacitinib or adalimumab versus placebo: patient-reported outcomes from OPAL Broaden-a phase III study of active psoriatic arthritis in patients with an inadequate response to conventional synthetic disease-modifying antirheumatic drugs. RMD Open. 2019;5(1):e000806.
- Strand V, de Vlam K, Covarrubias-Cobos JA, et al. Effect of tofacitinib on patient-reported outcomes in patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors in the phase III, randomised controlled trial: OPAL Beyond. RMD Open. 2019;5(1):e000808.
- Kavanaugh A, Gottlieb A, Morita A, et al. The contribution of joint and skin improvements to the health-related quality of life of patients with psoriatic arthritis: a post hoc analysis of two randomised controlled studies. Ann Rheum Dis. 2019;78(9):1215–1219.
- Silverberg JI, Thyssen JP, Simpson EL, et al. Impact of oral abrocitinib monotherapy on patient-reported symptoms and quality of life in adolescents and adults with moderate-to-severe atopic dermatitis: a pooled analysis of patient-reported outcomes. Am J Clin Dermatol. 2021;22(4):541–554.
- Dures E, Shepperd S, Mukherjee S, et al. Treat-to-target in PsA: methods and necessity. RMD Open. 2020;6(1):e001083.
