Abstract
Purpose
Evaluate the use of widefield radiation therapy (RT) in the management of extensive skin field cancerization (ESFC) with/without keratinocyte cancer (KC).
Methods
The National Dermatology Radiation Oncology Registry is a multidisciplinary collaboration (dermatologists and radiation oncologists). It captures disease description, prior therapies, radiation prescription, clinical effect, skin cosmesis scores, and toxicity data. This analysis included 12-month follow-up data on 89 treated fields from a subset of 83 patients.
Results
Clinical success (>90% field clearance) was 96% (ESFC, n = 63) and 88% (ESFC with KC, n = 26). Complete lesion response was seen in 96% of evaluable (n = 25) ESFC with KC. Recurrence (4/89 [5%]) and appearance of new lesions (10/89 [11%]) were minimal. Cosmetic outcome was excellent/good in 98% ESFC and 96% ESFC with KC. Grade 1–2 acute radiation dermatitis occurred in up to 80% of treated fields. The frequency of Grade 3 acute skin toxicities was low.
Conclusions
Registry data demonstrate the potential for widefield RT to treat patients with significant skin pathology who have exhausted other therapies and require durable, minimally invasive treatment options. At 12 months, observed clinical success rates were higher than those reported for topical interventions for ESFC. Ongoing follow-up is required to determine longer term outcomes.
Introduction
The initial concept of field cancerization was first defined in 1953 by Slaughter et al. in reference to oropharyngeal squamous cell carcinoma (SCC). It describes the expansion of mutated cells with an increased potential give rise to multiple cancers in that anatomical zone (Citation1). In skin, field cancerization involves the cluster of alterations observed in chronically photodamaged skin with several foci of keratinocyte cutaneous neoplasms. Areas affected by field cancerization have a high burden of both clinical and subclinical actinic damage and therefore are at a higher risk of developing multiple cutaneous lesions (Citation2). A number of tools have been validated that use precancerous actinic keratoses (AKs) as surrogates to grade field cancerization severity (Citation3,Citation4). This is clinically relevant as patients with multiple AKs, in addition to those with prior keratinocyte cancer (KCs), are at substantially increased risk of developing new cancers (Citation5). The concept of extensive skin field cancerization (ESFC) is a newer entity, for which there is no accepted standard definition. The current literature defines it as involving large areas, typically greater than 50cm2, marked by extensive UV damage, precancerous AKs, and keratinocyte cancers (KCs) including cutaneous SCC (cSCC) and/or basal cell carcinomas (BCC) (Citation6,Citation7). Patients with ESFC often exhibit widespread hyperkeratotic lesions that can be painful, pruritic, and cosmetically disfiguring, all of which can adversely impact quality of life (QoL). They routinely require repeated interventions to manage the hyperkeratosis and new KCs, which often leads to treatment fatigue which further negatively impacts QoL. Many of the existing field directed therapies have disappointing outcomes, with high recurrence rates in lesions that initially clear (21% for diclofenac and 46% for 5-flurouracil [5-FU]) and with overall reduction in AK numbers of around 50% at 12 months (Citation8).
Non-melanoma skin cancer (now called KC) is the fourth most common cancer globally, with an estimated 1.2 million cases reported in 2020 (Citation9–11). Estimates of the global prevalence of the two most common forms of KCs, BCC and cSCC, suggest that they increased by 77% and 310% respectively between 1990 and 2017 (Citation12). Despite this, their true prevalence and incidence remain underestimated primarily because many cancer registries do not require these cancers to be reported (Citation13–15). Adding to the burden of skin cancer, evidence shows that patients with more than one KC are likely to develop further KCs and have an increased risk of developing cSCC and/or melanoma (Citation16,Citation17).
A variety of treatment approaches to KCs is available, including surgery (simple excision or Mohs’ surgery), topical chemotherapy or immunotherapy, cryotherapy, curettage and electrodessication, photodynamic therapy, or radiation therapy (RT) (Citation18). The most commonly employed treatment is standard excision or Mohs’ micrographic surgery, which are associated with five-year recurrence rates of 5.5% and 2.9%, respectively (Citation19).
Traditional RT has produced cure rates for KCs of 79–100% (Citation20,Citation21). Wide field RT is particularly useful in patients who have ESFC that has not been controlled by topical therapies combined with surgical or ablative techniques (Citation22,Citation23). RT for individual cancers is used as an adjunct or alternative to surgery for patients with high-risk lesions or when lesions are located in areas where surgery would be difficult to perform, or may result in significant functional or cosmetic morbidity such as the ear, periorbital area, mouth, scalp, or nose (Citation21). The recent introduction of Volumetric Modulated Arc Therapy (VMAT) or widefield RT allows more homogenous treatment of large skin fields including large and/or irregular curved surfaces, such as the skin of upper and lower limbs, head, and neck, delivering a more homogeneous radiation dose distribution while minimizing exposure to underlying non-involved tissue (Citation23,Citation24).
The National Dermatology Radiation Oncology Registry (NDROR) is a multidisciplinary collaboration between dermatologists, radiation oncologists (RO), and other skin cancer specialists. It was established to collect data on efficacy, cosmetic appearance, toxicity and QoL for patients undergoing widefield RT. This report describes the 12-month efficacy, toxicity, and cosmesis outcomes for patients with ESFC with or without KC treated with a widefield RT approach (Citation22). QoL data and longer term efficacy and toxicity will be reported in the future.
Materials and methods
Patient cohort
Patients were independently assessed for potential suitability for RT by the referring dermatologist or skin practitioner and by an RO. Factors considered included severity and extent of disease, skin cancer history (including histopathology), and experience with prior therapies. Patients over the age of 18 years undergoing RT for ESFC with or without KC at participating GenesisCare radiation oncology centers in Australia were invited to participate in the NDROR. All patients provided written informed consent prior to registry enrollment and treatment commencement. Patients were reviewed at least weekly by the RO and nursing staff during the treatment course. Observational follow-up visits post RT were conducted at 3, 6, and 12 months, with annual visits to occur up to a total follow-up period of five years. Clinical photography was performed at baseline, mid-treatment, end of treatment, and at all follow-up visits. After source data verification and quality assurance, this analysis describes a subset of 83 patients whose ESFC was either ≥50cm2 or comprised a smaller whole anatomic unit (such as the nose), had received treatment with widefield RT, and had a minimum of 12-months follow-up.
RT treatment protocol
Radiation therapy has long been employed in the management of skin cancer. For wide field and multiple skin lesions, electrons traditionally been the modality of choice because of high surface dose, rapid dose falloff, and its finite range. However, electron treatments are limited by scattering of electrons at oblique surfaces, dose heterogeneity across the target volume, and laborious treatment setup (Citation25). Nicolini et al. demonstrated VMAT to be superior to electrons in the treatment of lower extremities for the management of Kaposi’s sarcoma (Citation26). VMAT plans demonstrated better dose conformity and homogeneity for multiple lesions across the oblique treatment surface. This ability to conform radiation dose across multiple lesions and sculpt dose away from healthy tissue makes widefield RT a desirable treatment modality to treat multiple lesions across a field. IN addition, widefield RT has the unique ability to simultaneously treat KC and ESFC.
The widefield RT treatment protocol for ESFC has been published previously (Citation22).
Briefly, the referring practitioner identified the condition(s) and area(s) to be treated. The RO subsequently defined the treatment area or field including any area of in-field invasive disease requiring a higher radiation dose as delivered by a simultaneous integrated boost (SIB). The decision to extend treatment to the region with ESFC surrounding the clinically diagnosed cancer reflects collaborative consultation and consideration of other available treatment options. A clinical target volume (CTV) and planning target volume (PTV) were defined according to our previously published technique (Citation22). The recommended dose was 45 Gray (Gy) in 25 daily 1.8 Gy fractions delivered over five weeks to the area with field cancerization, and at least 55 Gy in 25 fractions to localized areas of invasive cancer using a SIB. Given that the data were collected in an observational registry, there was no centralized plan for RT quality assurance. In lieu of this, review of cases was available via a national, peer-review meeting and patient-specific quality assurance was undertaken for treatment plans according to the policy of the treating department.
Prior experience with widefield RT for ESFC of limbs showed patients commonly developed an acute inflammatory process with edema of the limb, which could be uncomfortable for some patients and was sometimes sufficiently severe as to affect dosimetry and requiring replanning. This was frequent for treatment of antero-lateral legs below the knees. A planned break in treatment was therefore allowed for treatment of the upper or lower limb at the end of the first two weeks of treatment, resuming treatment if symptoms had improved.
Outcomes
At each timepoint, the RO assessed the treated fields for response to therapy. Treatment efficacy was measured using clinical assessment of the percentage clearance of visible actinic keratoses and resolution of KCs (if present) within the treated field (Citation27). Treatment success for field cancerization was defined as >90% clearance of the treated field, which exceeds the standard of ≥75% that has been used to define treatment success in clinical trials for topical field therapies (Citation28,Citation29). Treatment failure for field therapy was defined as either not ever achieving >90% clearance of the treated field, or a loss of improvement (<90% clearance) after successful field clearance. For fields with a KC treated with widefield RT (±SIB), treatment success was defined as complete response of the lesion by three months post-treatment, failure was defined as lesion persistence at three months post-treatment, and recurrence was defined as reappearance of the lesion following complete response. New lesions were defined as those appearing within the treated field where none were documented prior to undergoing RT and/or lesions of a different pathology from baseline. New lesions were typically diagnosed clinically or by histopathology if suspicious of new KC.
Skin toxicities were assessed using the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 (Citation30). Safety data were collected at mid-treatment, end of treatment, and at all follow-up visits. Cosmetic appearance was assessed using the 4-point Lovett scale (excellent, good, fair, and poor) (Citation31), at end of treatment, 3, 6, and 12 months. This scale is based on the degree of telangiectasia, pigment change, or fibrosis, wherein ‘excellent cosmesis’ is defined as no telangiectasia, pigment change, or fibrosis; ‘good cosmesis’ as mild telangiectasia or slight pigment change; ‘fair cosmesis’ as severe telangiectasia, or pigment change or mild-to-moderate fibrosis; and ‘poor cosmesis’ as severe fibrosis or skin contracture.
Ethical considerations
The NDROR was prospectively registered with the Australian New Zealand Clinical Trials Registry (ACTRN12618000627257), received ethics approval from Bellberry Ltd (approval number: 2017-04-288-A-3), was conducted in accordance with ethical principles founded in the Declaration of Helsinki, and all participants provided written informed consent.
Results
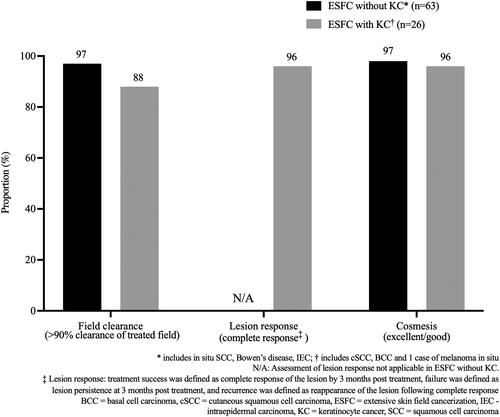
Between July 2018 and December 2019, 83 eligible patients (65 [78%] male) aged 46–96 years (median 75 years) received widefield RT to a total of 89 fields (≥50cm2 or whole anatomic unit such as the nose). The majority (63/89, 71%) of treatment sites had ESFC without KC (). Among fields of ESFC with KC (26/89; 29%, cSCC was the most frequently identified histopathology (16/89; 18%), followed by BCC (9/89; 10%). One of the fields was melanoma in situ (1/89; 1%). The anatomical zones treated () reflected typical UV exposure patterns, with head and neck being most frequent (43/89; 48%), followed by upper limb and hand (23/89; 26%), and lower limb (20/89; 23%). The majority of patients had undergone prior treatment for their ESFC with or without KC, and 82% of fields had received between one and four prior types of therapy (). The most frequently utilized treatments included topical 5-FU, cryotherapy, and surgical excision.
Table 1. Summary of baseline characteristics.
Radiation therapy treatments administered
Of the 89 treatment fields, 67 (75%) were treated with VMAT, 21 (24%) with VMAT plus SIB, and 1 (1%) field received Intensity Modulated Radiation Therapy (IMRT), which was considered equivalent to VMAT for this registry. Data are presented separately for ESFC alone and for ESFC with KC.
ESFC alone (n = 63) was prescribed a fractionated dose (25 fractions [100%] for a period of five weeks) of 45 Gy (35/63, [55%]), 50 Gy (20/63, [32%]), or 55 Gy (7/63, [11%]), and one radiosensitive patient was prescribed a lower dose of 27 Gy in 15 fractions over five weeks. A standardized treatment protocol was implemented after the commencement of the registry which largely accounts for prescription variation. Treatment breaks were prescribed in 21 of 63 (33%) of ESFC without KC treatments, most of which were for the upper (n = 13) or lower limb (n = 7), and one scalp. Overall, the median delivered dose for field cancerization was 50 Gy (range 16.2 − 55 Gy) delivered in an average of 24 fractions (range 9–25) over a mean 6.6 weeks (range 3–11 weeks). A total of 8 of 63 (13%) ESFC fields did not receive a complete treatment course, 7 were incomplete due to acute toxicity, and in 1 case the patient declined further treatment (). On average, these ESFC patients received 78.5% of their prescribed dose (range 60-96%).
Table 2. Summary of outcomes in patients that did not complete treatment.
Fields with KC (n = 26) were most frequently prescribed a dose comprising 45 Gy to the field with a simultaneous boost dose to the lesion of 455-60Gy in an average of 25 fractions (range 20–30) over a mean of 5.7 weeks (range 4–7). Fifteen (15) of 16 SCCs were prescribed widefield RT plus SIB at a median dose of 50 Gy in 25 fractions (100%), with the remaining SCC prescribed a dose of 50 Gy in 20 fractions. All 16 treatment plans reflect tumoricidal doses. Excluding the one instance of a delivered dose of 2.2 Gy in 10 fractions, the median delivered dose was 50 Gy (range 41.4 − 60 Gy), with a mean fractionation of 25 (range 20–30) over an average of 6.5 weeks (range 5–10). The RT prescription (dose or fraction) was not changed in 22 of 26 (85%) prescriptions. In fields with KC, 54% (14/26) of treatments were conducted without a break and 31% (8/26) were conducted with a planned break. Four (15%) RT prescriptions were stopped prematurely due to acute treatment toxicity in fields with KC ().
Changes to RT plans occurred in 9% (8/89) of prescribed treatments as a result of replanning due to alteration in patient contour (n = 4), RO modifications to prescription (n = 2), and equipment/technical related (n = 2).
Treatment outcome
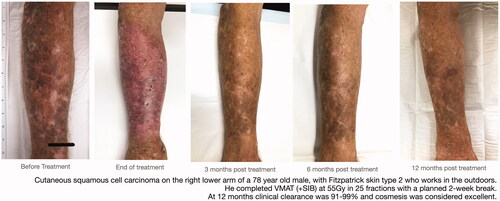
At 12 months post RT, 97% (61/63) of field treatments for ESFC without KC were deemed successful (). For ESFC fields with KC (n = 26), efficacy was measured as both field clearance and lesion response with success thresholds at >90% clearance and complete response, respectively. At 12 months post RT, 88% (23/26) of RT treated fields were assessed as having achieved >90% field clearance (). KC assessment at 12 months showed clinical resolution of invasive disease in 96% (24/25) with one lesion being not assessable at 12 months due to surgery and skin flap in an adjacent area. provides representative images before, during, and after treatment. As can be seen in this case, an acute skin reaction is typically observable by the end of treatment; however, this largely resolves by 3 months with continued improvement at 6 and 12 months.
Despite an incomplete treatment course in 15% (13/89) of fields, it was observed that 100% of these fields achieved treatment success, and cosmesis outcomes were excellent or good in 92% of cases, regardless of pathology type (). Recurrence was observed in 5% (4/89) of treated fields. The occurrence of new lesions in field were identified in 11% (10/89) of treated fields. Treatment of these recurrences and new lesions in field included surgical excision, cryotherapy, and observation. The response to the treatment will be monitored in longer term follow-up.
Toxicity: acute to month 12
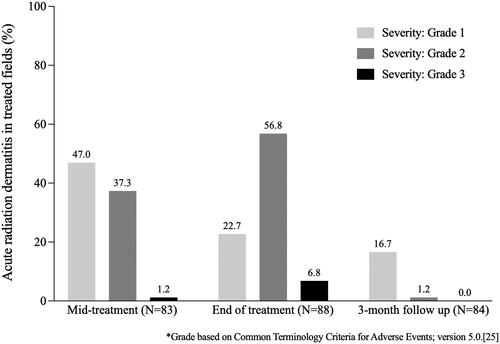
Acute radiation dermatitis when treating the skin is an expected consequence of RT. Most patients developed radiation dermatitis during treatment, and this was largely resolved at three months post RT (). The severity of acute radiation dermatitis peaked at the end of treatment, with Grade 2 reactions occurring in 57% (50/88) treated fields, while Grade 3 reactions accounted for only a small proportion (8%, 7/88) and were clustered at the mid- and end-of-treatment timepoints.
Nine percent (10 of 89) treatments were discontinued before receiving the prescribed dose. The primary reason for early discontinuation of RT was acute treatment toxicity (). This registry did not collect concomitant medications and no data is available for the use of analgesics. However, as per standard practice, analgesia was provided as appropriate to the level of pain reported and the patient’s individual clinical circumstances. Anecdotally, pain was managed with simple analgesics such as paracetamol. However, patients who develop an acute inflammatory response associated with limb edema sometimes required opiates such as oxycodone and/or low dose systemic corticosteroids.
Table 3. Summary of acute toxicities at time of stopping treatment.
Treatment emergent acute skin toxicities are summarized by frequency and severity in . Apart from acute radiation dermatitis, the most frequently observed skin reactions were erythema, dryness, and pain of skin.
Table 4. Acute skin toxicities at treatment sites, data shown are toxicities that had ≥25% incidence of Grade 1 events at any timepoint.
There was a very low frequency of Grade 3 acute skin toxicities at treatment sites, with two or less events at any single treatment site and timepoint: dryness (one event at each of mid-treatment and 12 month visits), oozing/crusting (one event at each of mid-treatment and end of treatment visits), pain of skin (two events at end of treatment visit), pruritus (two events at end of treatment visit and one event at 12 month visit), papulation (one event at each of mid-treatment and end of treatment visits), and non-healing ulceration (one event at each of end of treatment, 6 month, and 12 month visits). Long-term toxicity data continue to be collected via the registry. Acute edema in the lower limb subgroup was mostly Grade 1 in severity and occurred most commonly during treatment. No Grade 3 leg edema events were reported.
Cosmetic outcomes at month 12
At month 12 post-treatment, the majority (62/63, 98%) of treated ESFC without KC fields were assessed as having achieved excellent (49/63, 78%) or good (13/63, 21%) cosmesis on the Lovett scale. The assessment was noted as fair in 2% (1/63) of fields. Similarly, fields with KC showed excellent (17/26, 65%) or good (7/26, 27%) cosmesis on the Lovett scale. The assessment was noted as fair in 1 (4%) field and was noted not applicable/not assessable in the remaining 1 field.
Discussion
The use of widefield RT for the treatment of patients with ESFC with or without KC represents a significant technical advance from prior radiotherapeutic approaches in terms of radiation dose distribution and reproducibility. While the need for longer term follow-up is recognized, this report demonstrates its high level of efficacy and tolerability at 12 months in a cohort of patients with ESFC with or without KC with disease recurrence after prior intervention. Widefield RT allows delivery of a precise radiation dose to precancerous skin regions and simultaneously delivers tumoricidal dose to identified in-field cancers.
Treatment success in this patient cohort was defined as >90% field clearance for ESFC without KC, and >90% field clearance plus complete resolution of KC for ESFC with KC. The majority achieved this goal 12 months after treatment; field clearance was 97% for ESFC without KC and 88% for ESFC with KC. Treatment success with topically applied therapies has been typically defined as achieving 75% field clearance at 12 months, often on a per lesion basis, and with a less severely affected population (Citation8,Citation28). Recurrence with topical therapies is common at the 12 month follow-up point, ranging from 40% for imiquimod (Citation8), 50% for 5-FU[29] to 60% for diclofenac (Citation32). As a reflection of this problem, 82% of fields in this cohort had received at least one and up to four prior types of therapy before receiving RT.
The complete response rate for KCs was 96% which aligns with historical RT efficacy rates and is comparable to excision efficacy rates.
These data indicate a highly promising outcome with durability to 12 months after treatment. The low recurrence rate (5%) at 12 months compares favorably to other therapies (Citation8), and analysis of longer term data on this patient cohort, as well as the rest of the NDROR is awaited to determine the durability of effect and ultimate disease control. Particular effort will be made in the long-term follow-up and observation of outcomes in fields that require further treatment due to recurrence and/or new lesions. An additional question will be whether field RT reduces the incidence of future in-field KCs, given the high probability of their development in patients with this extent of actinic damage, precancerous lesions and/or invasive cancers. Comprehensive and validated scoring tools are needed to objectively quantify the extent and severity of pretreatment ESFC and to evaluate long-term post-treatment outcomes.
As many of the most severely sun-damaged regions involve the head and neck region, cosmetic outcomes are especially important. Cosmesis at 12 months post widefield RT was assessed in this series as good to excellent in almost all treated fields (98% ESFC alone, 96% ESFC with KC).
The frequent occurrence of acute radiation dermatitis is seen an undesirable outcome when treating deeply located tumors, such as breast cancer. However, widefield RT treatment targeting the skin will invariably induce this reaction. Acute radiation dermatitis was expected, and the majority of events were Grade 1–2 and peaked at the end of treatment. Toxicities and adverse effects are noted with topical treatments in field disease, including severe pain at rates of 16% with 5-FU to 62% with photodynamic therapy (Citation28). The observed withdrawal rate of 9% due to acute toxicity was similar to that reported with topical field treatments, such as imiquimod at 6% (Citation33).
Despite the encouraging safety profile observed in this analysis, toxicity data associated with widefield RT treatment of ESFC, particularly the treatment of limbs, requires ongoing evaluation. Close clinical observation and management of acute radiation dermatitis, skin pain, edema and any skin wounds are essential. It should be noted that chronic adverse skin effects from traditional radiotherapy usually become clinically manifest by 6–12 months following treatment. However, a conventionally fractionated regimen as used with widefield RT should minimize the risk of late toxicities including telangiectasia and fibrosis.
The current dataset, and subsequent longer term reporting from the NDROR, should ideally be supported with high quality clinical evidence, particularly regarding comparison to standard of care, and active planning is ongoing for a randomized controlled trial. The data presented here suggest that incorporating a planned break may have helped to improve the acute toxicity profile, thereby increasing the likelihood of a patient completing the full prescribed course. Furthermore, these planned breaks appeared not to negatively impact disease control, despite extending the overall treatment time.
The limitations of this report include some incomplete data, which is typical of a data registry All efforts were made to include eligible registry participants. However, while data verification was undertaken to minimize missing and incomplete data, it was not independently monitored. A further limitation is that the reported patients do not have a control group. Some variation of prescription is expected for patient factors such as anticipated tolerability of treatment due to age or comorbidity, anatomical location of the field, and disease factors including presence of gross invasive disease requiring a boost dose. This was borne out in our data.
The population reflected patients treated at GenesisCare clinics in Australia; however, this cohort may not be widely representative, specifically, as they may have been patients with more advanced disease, or more difficult to control with other measures. The follow-up period of 12 months gives a reasonable indication of efficacy and permits comparison with other ESFC treatment modalities; however, longer term data will be informative to adequately observe duration of effect and emergence of possible longer term skin toxicities.
The registry continues to enroll new patients and to follow existing patients for the detection of long-term effects. Although late adverse effects are not expected, it is important that this is recorded within the registry. Longer term follow-up is also important to fully understand the durability of efficacy provided by widefield RT in treating ESFC.
In conclusion, this report describes 12-month post widefield RT outcomes for patients, with advanced ESFC, with and without KC, who had failed other therapies . The data yield promising insights into the early experience of modern radiotherapeutic treatment for patients with skin cancer and field cancerization. The data show field clearance responses of 96% and 88% for patients with ESFC without KC or with KC, respectively, with durability to 12 months post-treatment. These observed clinical success rates are higher than those reported for topical interventions (Citation28). Complete response of invasive disease was achieved in 96% of ESFC with KC at 12 months. In collaboration with referrers, widefield RT for ESFC can be considered as a treatment option for patients with advanced disease who have exhausted other treatment options and who have a high risk of future cancer in the wider area surrounding the cancer. The nature of these criteria support that its use will be of most value in elderly patients. Ongoing follow-up will be required to determine longer term outcomes.
Author contributions
Conceived the concept of this work and designed the study: LS
Involved in the conduct of the study and contributed to data collection: AP, DC, BW, CB, SS, RS, PO’B, LS
Contributed to data analysis and interpretation of the results: AP, WC, DC, BW, CB, SS, RS, PO’B, LS, PF
Manuscript writing and revision for intellectual content: AP, WC, DC, BW, CB, SS, RS, PO’B, LS, PF
Approved the final version of the article: AP, WC, DC, BW, CB, SS, RS, PO’B, LS, PF
Guarantor of the article: AP
Acknowledgements
The authors wish to thank the patients who participated in this research, the co-investigators, nurses, study coordinators and administrative support staff at each of the study sites. Special thanks to Patrick Foley for Data Management and Janet Wilson for Project Management, both are paid employees of GenesisCare Pty Ltd. The authors acknowledge the input of McCloud Consulting, Sydney, NSW, Australia for statistical analyses and Hazel Palmer MSc, ISMPP CMPP™ of Scriptix Pty Ltd for editorial assistance in the preparation of this manuscript, both funded by GenesisCare Pty Ltd.
Disclosure statement
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: AP, WC, DC, BW, and PO’B are employees of GenesisCare. PF reports no conflicts of interest in relation to GenesisCare. SS, RS and LS report receipt of personal fees for consultancy work from GenesisCare outside of the submitted work. CB reports the receipt of personal fees for consultancy work and other from GenesisCare outside of the submitted work. LS reports being a paid advisor for the NDROR. LS, SS and CB report being unpaid members of an advisory panel that consults on the guidelines for the NDROR.
Data availability statement
Research data are stored in an institutional repository and will be shared upon reasonable request and with permission of GenesisCare Pty Ltd by emailing the corresponding author.
Additional information
Funding
References
- Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6(5):963–968.
- Willenbrink TJ, Ruiz ES, Cornejo CM, et al. Field cancerization: definition, epidemiology, risk factors, and outcomes. J Am Acad Dermatol. 2020;83(3):709–717.
- Acar A, Karaarslan I. Comparison of actinic keratosis and severity index with physician global assessment and total lesion count and the ability to predict skin cancer. Dermatol Pract Concept. 2022;12(1):e2022031.
- Baker C, James A, Supranowicz M, et al. Method of assessing skin cancerisation and keratoses (MASCK™): development and photographic validation in multiple anatomical sites of a novel assessment tool intended for clinical evaluation of patients with extensive skin field cancerisation. Clin Exp Dermatol. 2022.DOI:10.1111/ced.15136.
- Madani S, Marwaha S, Dusendang JR, et al. Ten-Year follow-up of persons with Sun-Damaged skin associated with subsequent development of cutaneous squamous cell carcinoma. JAMA Dermatol. 2021;157(5):559–565.
- Dréno B, Cerio R, Dirschka T, et al. A novel actinic keratosis field assessment scale for grading actinic keratosis disease severity. Acta Derm Venereol. 2017;97(9):1108–1113.
- Soyer HP, Prow TW, Jemec GBE, editors. Actinic keratosis (current problems in dermatology). Basel, Switzerland: Karger; 2014.
- Sinclair R, Baker C, Spelman L, et al. A review of actinic keratosis, skin field cancerisation and the efficacy of topical therapies. Australas J Dermatol. 2021;62(2):119–123.
- Ferlay J, Ervik M, Lam F, et al. Global cancer observatory: cancer today. Lyon, France: International Agency for Research on Cancer; 2020.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Urban K, Mehrmal S, Uppal P, et al. The global burden of skin cancer: a longitudinal analysis from the global burden of disease study, 1990–2017. JAAD Int. 2021;2:98–108.
- Perera E, Gnaneswaran N, Staines C, et al. Incidence and prevalence of non-melanoma skin cancer in Australia: a systematic review. Australas J Dermatol. 2015;56(4):258–267.
- AIHW. Australian cancer incidence and mortality (ACIM) books: non-melanoma skin cancer, all types. Canberra (Australia): Australian Institute of Health and Welfare; 2014. Available from: www.aihw.gov.au/acim-books
- Anselmo Lima C, Sampaio Lima M, Maria Da Silva A, et al. Do cancer registries play a role in determining the incidence of non-melanoma skin cancers? Eur J Dermatol. 2018;28(2):169–176.
- Czarnecki D, Sutton T, Czarnecki C, et al. A 10-year prospective study of patients with skin cancer. J Cutan Med. Surg. 2002;6(5):427–429.
- Wehner MR, Linos E, Parvataneni R, et al. Timing of subsequent new tumors in patients who present with basal cell carcinoma or cutaneous squamous cell carcinoma. JAMA Dermatol. 2015;151(4):382–388.
- Cancer Council Australia Keratinocyte Cancers Guideline Working Party. Clinical practice guidelines for keratinocyte cancer. [cited 2021 Apr 8]. Sydney (Australia): Cancer Council Australia; 2019. Available from: https://wiki.cancer.org.au/australia/Guidelines:Keratinocyte_carcinoma. Af, editor. [Version URL: https://wiki.cancer.org.au/australiawiki/index.php?oldid=213931
- Stuart SE, Schoen P, Jin C, et al. Tumor recurrence of keratinocyte carcinomas judged appropriate for Mohs micrographic surgery using appropriate use criteria. J Am Acad Dermatol. 2017;76(6):1131.e1–1138.e1.
- Cho M, Gordon L, Rembielak A, et al. Utility of radiotherapy for treatment of basal cell carcinoma: a review. Br J Dermatol. 2014;171(5):968–973.
- Likhacheva A, Awan M, Barker CA, et al. Definitive and postoperative radiation therapy for basal and squamous cell cancers of the skin: executive summary of an American society for radiation oncology clinical practice guideline. Pract Radiat Oncol. 2020;10(1):8–20.
- Potter A, Price M, Papworth D, et al. A technique for treating extended skin field cancerisation using volumetric modulated arc therapy. Int J Radiol Radiat Ther. 2019;6(4):111–119.
- Wong B, Christie D, Hellyer J, et al. Volumetric modulated arc therapy (VMAT) for extensive skin field cancerisation (ESFC) – exploring the limits of treatment volumes with a case series of backs. IJRRT. 2020;7(6):184–192.
- Santos ED, Green JA, Bhandari N, et al. Tangential volumetric modulated radiotherapy – a new technique for large scalp lesions with a case study in lentigo maligna. Int J Bioautomation. 2015;19(2):223–236.
- Lozano F, Perez N, Iglesias A, et al. Volumetric arc therapy for total scalp irradiation: case report for a recurrent basal cell carcinoma of the scalp. Ecancermedicalscience. 2017;11:737–737.
- Nicolini G, Abraham S, Fogliata A, et al. Critical appraisal of volumetric-modulated arc therapy compared with electrons for the radiotherapy of cutaneous Kaposi's sarcoma of lower extremities with bone sparing. Br J Radiol. 2013;86(1023):20120543.
- WHO handbook for reporting results of cancer treatment. 1979.
- Jansen MHE, Kessels J, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380(10):935–946.
- Pomerantz H, Hogan D, Eilers D, et al. Long-term efficacy of topical fluorouracil cream, 5%, for treating actinic keratosis: a randomized clinical trial. JAMA Dermatol. 2015;151(9):952–960.
- Institute NC. Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50. 2017
- Lovett RD, Perez CA, Shapiro SJ, et al. External irradiation of epithelial skin cancer. Int J Radiat Oncol Biol Phys. 1990;19(2):235–242.
- Dréno B, Amici JM, Basset-Seguin N, et al. Management of actinic keratosis: a practical report and treatment algorithm from AKTeam™ expert clinicians. J Eur Acad Dermatol Venereol. 2014;28(9):1141–1149.
- Salasche SJ, Levine N, Morrison L. Cycle therapy of actinic keratoses of the face and scalp with 5% topical imiquimod cream: an open-label trial. J Am Acad Dermatol. 2002;47(4):571–577.