Abstract
Objectives
Fumaric acid esters (FAEs) are a well-established treatment option for long-term therapy of moderate to severe plaque psoriasis. This study examines effectiveness of FAEs for the treatment of plaque psoriasis in real-world practice at 12 months and if patient characteristics affect the odds of clinical response.
Methods
A descriptive, multivariable logistic regression analysis was conducted in a cohort drawn from the German registry PsoBest. Baseline patient characteristics were assessed as potential treatment effect modifiers.
Results
444 patients (mean age 47.0 years, 39.0% female) were eligible for response analysis using nonresponder imputation at month 12. Of these, 39.6% achieved clinical response, i.e. Psoriasis Area and Severity Index (PASI) ≤ 3 or skin clearance. In logistic regression analysis (R2 = 0.114), only baseline PASI was a significant factor: patients with PASI < 10 had a 4 times higher odds (p ≤ .001, OR 4.088), patients with PASI of 10–20 a twofold higher odds of response (p ≤ .044, OR 1.961) compared to those with PASI > 20. Neither sex, age, body weight, disease duration, comorbidity nor pretreatment had an impact on the odds of response (p > .05).
Conclusions
FAEs showed a favorable response at 12 months, largely independent of patient characteristics.
Introduction
Psoriasis is an immune-mediated, chronic inflammatory skin condition affecting around 2–3% of the world’s population (Citation1,Citation2). Approximately 30% of patients suffering from plaque psoriasis have moderate to severe disease and require systemic therapy (Citation3,Citation4). Fumaric acid esters (FAEs) are the standard first-line treatment for moderate to severe psoriasis in Germany representing the most widely used systemic therapy (Citation5). A proprietary mixture of different salts of ethyl hydrogen fumarate and dimethyl fumarate was approved in 1994 by German authorities as first systemic FAE formulation. The prodrug dimethyl fumarate (DMF) and its active metabolite monomethyl fumarate (MMF) have since been identified as the active ingredients of FAEs (Citation6). Although the mode of action of DMF has not yet been fully elucidated, its impact on multiple pathways and cell types seems to contribute to its immunomodulatory effects (Citation7). MMF inhibits neutrophilic granulocytes (Citation6–10) and modulates the Th17-driven inflammatory response by suppressing the secretion of interleukin 23 (Citation11). Further, DMF is a strong inducer of the antioxidative NRF2 pathway, which is thought to mediate the neuroprotective effects of DMF in multiple sclerosis (Citation12,Citation13).
Possibly due to the immunomodulatory mode of action and the need for initial uptitration, FAEs elicit a somewhat delayed clinical response, emerging after around six to eight weeks (Citation14,Citation15). Maximum effectiveness is usually seen after 6–12 months (Citation16–18).
While gastrointestinal intolerability or flushing can pose a challenge to patients particularly in the first weeks of treatment, these side effects tend to improve over time. During long-term treatment of psoriasis, FAEs have an acceptable safety profile and are usually well tolerated and effective (Citation16,Citation17,Citation19). Since the safety profile for FAEs is well established, it is not within the scope of this analysis.
In clinical practice, patient characteristics including disease duration, body weight or comorbidity may readily impact safety or therapeutic response of pharmacological treatments (Citation20–22). Despite the vast experience with FAEs in Germany for treating psoriasis, information on patient demographics and baseline characteristics that have the potential to modify clinical response in real-world practice is scarce.
Based upon data derived from the German Psoriasis Registry PsoBest, this multivariable logistic regression analysis seeks to assess effectiveness at 12 months and elucidate baseline factors that affect the odds of achieving therapeutic response to FAEs. To our knowledge, this is the first analysis of its kind.
Materials and methods
Patient registry
The German Psoriasis Registry PsoBest observes patients with moderate to severe plaque psoriasis with or without psoriatic arthritis and assesses data on long-term effectiveness, safety, patient benefit and treatment regimens. All patients need to provide written informed consent to participate in the PsoBest registry before enrollment. Adult patients with moderate to severe psoriasis treated in dermatological practices or clinics throughout Germany are included when they receive any systemic (antipsoriatic) drug (including biologics) for the first time (i.e. they have to be naïve for the inclusion medication). If a patient’s systemic medication is changed after inclusion in the registry, the patient continues to be observed in PsoBest. Patients are followed up for ten years. Before the initiation of the registry, ethic approval was obtained (NCT01848028). Data are collected in PsoBest at predefined intervals corresponding to baseline, 3-, 6- and 12-months following treatment initiation and every six months thereafter.
Patients and data
These analyses comprise PsoBest patients enrolled through 31 December 2015 who started FAEs treatment (proprietary mixture of different salts of ethyl hydrogen fumarate and dimethyl fumarate) at registry entry (prospectively observed treatment time) with a minimum follow-up time of 12 months after enrollment. This dataset was previously utilized to characterize patients treated with FAEs in routine care (Citation23). Patients were allowed to switch their treatment within the first 12 months, but then were referred to as responders or nonresponders depending on the reason for stopping or switching treatment. Patients had to have a valid baseline Psoriasis Area and Severity Index (PASI) > 3 and follow-up measurement at month 12. To account for variation of visit schedules in clinical practice, any visit between months 10–14 was accepted. Data are assessed using standardized case report forms (CRFs).
The variables age (years), sex, body weight (kg), body mass index (BMI, kg/m2), waist–hip ratio, duration of illness (years), positive family history, PASI (range 0–72), body surface area (BSA, 0–100%), Dermatology Life Quality Index (DLQI, range 0 = no impairment to 30 = extremely high impairment) (Citation24), nail psoriasis, psoriatic arthritis, selected comorbidities and prior treatments were considered in these analyses.
Data on comorbidity are collected in PsoBest using prelisted diseases or disease clusters. Additional comorbidities may be recorded using free text. Cardiovascular disease (prelisted in the CRF) captures peripheral artery occlusive disease, heart failure, coronary heart disease, thrombosis, hypertension and cerebrovascular disease. Metabolic disease includes diabetes mellitus type I and type II, disorders of lipid metabolism and hyperuricemia. Psychiatric or addictive disease includes depression, sleep apnea syndrome (following the classification of system organ class of Medical Dictionary for Regulatory Activities (MedDRA) at time of CRF development) and alcohol abuse. Liver disease encompasses liver cirrhosis, hepatic steatosis and chronic liver damage. Further details on the registry have been published before (Citation25). The evaluation was carried out according to the Declaration of Helsinki, the EU directive on data protection and the guidelines of good clinical practice.
Treatment stop can be reported at any time including the reason for discontinuation (skin clearance, adverse events rated as side effects, onset of contraindication and free text field).
Data analyses
Analyses on baseline characteristics were performed using standard descriptive statistical measures: absolute and relative frequencies for categorical variables and mean, standard deviation, median, minimum and maximum for continuous variables. Daily dose per exposure time (mg/day exposed) has been calculated as the quotient of the cumulative dose during exposure and the number of days of exposure. Response was defined as reaching an absolute PASI ≤ 3 at month 12. Absolute rather than relative PASI was selected as response criterion since patients do not undergo a medication washout in registries. As a consequence, 80.0% of patients had a PASI < 20 and 30.9% a PASI < 10 at baseline. Patients with missing data on clinical response or who stopped treatment during the first 12 months due to any reason other than ‘discontinuation due to skin clearance’ were referred to as nonresponders. Primary response analysis using nonresponder imputation (NRI) was conducted in all patients eligible for baseline analysis (n = 444, ). A sensitivity analysis was run using observed cases in all patients with data available on model parameters at 12 months (n = 321 patients) (patients fully observed, i.e. observed cases (OC)).
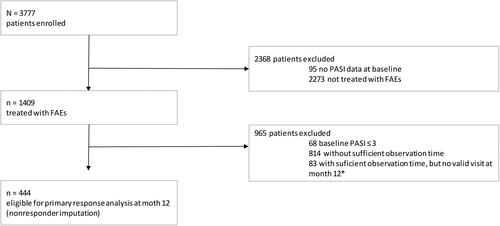
Figure 1. Patient flow. The analysis is based on a cohort drawn from the German Psoriasis Registry PsoBest including 444 patients who had received fumaric acid esters (FAEs) treatment and met a minimal observation time of 12 months in the registry. *10–14 months after inclusion.
A multivariable logistic regression model of several baseline parameters (age, sex, body weight, duration of illness, PASI (categorized), cardiovascular, metabolic, liver, psychiatric or addiction disease, prior treatments (categorized)) was performed to evaluate any potential relation with therapeutic response at month 12 (PASI ≤ 3 or a treatment stop due to skin clearance) based on maximum likelihood estimation. Parameter selection was informed by expert opinion. All independent variables were continuous or dummy-coded (if categorical) and analyzed for intervariable correlation using Pearson’s r. For each group formed by categorial parameters, the group size was proven to be greater than 25. For the variable selection procedure, all variables in a block were included in a single step; backward variable selection was performed to check for improved model fit. To check whether the regression model is significant overall, a chi-squared test was performed. Significance of treatment effect modifiers was evaluated using Wald test. Nagelkerkes R2 and strength of effect according to Cohen were calculated to assess the model quality in the context of logistic regression. Significance level was set α = 0.05. Since this was an exploratory analysis, no a priori power or sample size calculation was performed.
Data management and analyses were performed using SPSS version 25 (IBM, Armonk, NY). Missing data on potential treatment effect modifiers were not imputed. Consequently, the number of cases available for the regression model was limited to the number of patients fully observed for model parameters (n = 286).
Results
Baseline demographics and disease characteristics
As of the cutoff date (December 31, 2015), 3777 patients registered to PsoBest were available for data analyses. Of these, 1409 had received FAEs treatment when first entering PsoBest. A total of 444 patients met the specific study criteria and were included in this analysis. The majority of patients excluded from the analysis (n = 814) were not considered due to insufficient observation time with an enrollment date later than 12 months before the data cutoff (). Details of patient characteristics and demographics are provided in . Taken together, patients included in this analysis showed a moderate to severe disease activity and suffered from a high impairment of health-related quality of life.
Table 1. Baseline demographics and disease characteristics.
Dose of and response to FAEs
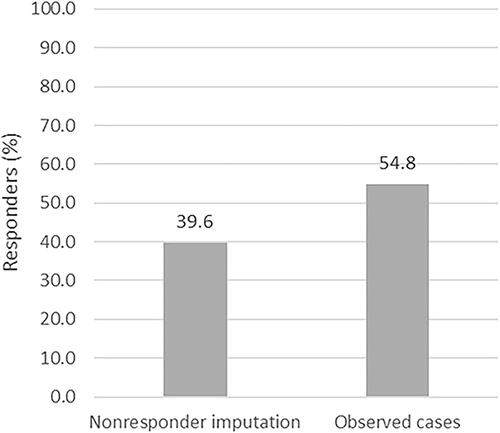
Considering patients with missing data as nonresponders, 176 of 444 patients (39.6%) achieved a response, defined here as an absolute PASI ≤ 3 or a treatment stop due to skin clearance (), indicating only minimal residual disease activity. Analyzing the data at 12 months as OC yielded slightly higher results with 176 of 321 patients (54.8%) reaching PASI ≤ 3 or a treatment stop due to skin clearance (). Baseline PASI improved by at least 75% (PASI 75) in 37.6% (NRI) and 52.0% (OC) of the patients, respectively (data not shown). Mean (SD) absolute PASI change within one year was 4.4 (3.0) for patients with baseline PASI > 3 and < 10, 10.1 (4.8) for patients with baseline PASI 10–20 and 19.2 (8.1) for patients with baseline PASI > 20. Relative PASI reduction was 35.2%, 30.2% and 27.4%, respectively. The mean daily dose of FAEs per exposure time between responders and nonresponders was significantly different, with 274.3 (205.4) mg/day and 230.5 (173.2) mg/day, respectively (p ≤ .03). The time of exposure between both groups, however, differed not significantly (p > .05).
Figure 2. Therapeutic effectiveness of fumaric acid esters (FAEs) at 12 months. Therapeutic response to FAEs treatment was defined as Psoriasis Area and Severity Index (PASI) ≤ 3 at 12 months or a treatment stop due to skin clearance. Nonresponder imputation (n = 444) and observed cases (n = 321) is shown.
Treatment effect modifiers of response to FAEs
A multivariable logistic regression analysis was carried out to determine baseline characteristics that could potentially affect the odds of treatment response to FAEs at 12 months. The following baseline variables were selected and analyzed: duration of illness, PASI (analyzed as strata PASI < 10, PASI 10–20 and PASI > 20), sex, age, body weight, pretreatment (analyzed as hierarchic strata: no systemic, only nonbiologic, biologic pretreatment), preexisting cardiovascular disease, psychiatric or addictive disease, metabolic disease and liver disease (binary). Data from 286 patients of the current dataset containing sufficient information on FAEs response at 12 months and on model parameters were used for the regression model. Correlation analysis confirmed that all variables were sufficiently independent with Pearson’s r indicative of only weak correlations (Supplementary Table S1). Consequently, all selected variables were considered eligible and included in the model. Omnibus test of model coefficients resulted in a significant model (p ≤ .008), but only a model fit of Nagelkerkes R2 = 0.114 could be reached, i.e. 11.4% of the variance in the data analyzed could be explained by the model used. According to Cohen, this is a small strength of effect (f = 0.359) (Citation26). Backward variable selection did not lead to an improved model fit.
The overall regression analysis revealed that baseline PASI was the sole factor that significantly affected the odds of treatment response. Here, baseline PASI > 20 was set as reference category. Patients with a baseline PASI < 10 had 4 times higher odds to reach a response (p ≤ .001, odds ratio (OR) 4.088, 95% confidence interval (CI) [1.973, 8.469]) compared to those with severe psoriasis (PASI > 20). Similarly, baseline PASI 10–20 had a significantly higher odds of response compared to PASI > 20 (p ≤ .044, OR 1.961, 95% CI [1.018, 3.779]), although the effect was weaker ().
Table 2. Treatment effect modifiers of response to fumaric acid esters (FAEs).
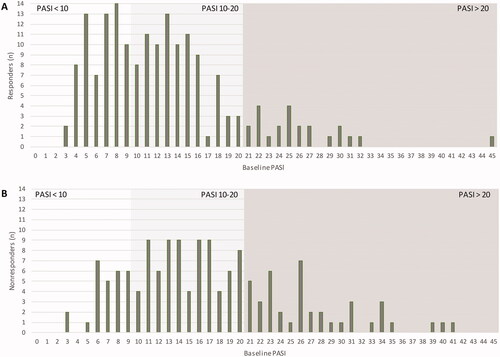
A bar graph depicting the distribution of baseline PASI of responders and nonresponders is provided in . Interestingly, both groups encompass patients with PASI < 10 as well as patients suffering from very severe disease with PASI > 20. However, while baseline PASI < 10 was frequently found among responders, it was rarely seen in nonresponders. In contrast hereto, PASI of >20 at baseline were infrequent in responders ().
Figure 3. Distribution of Psoriasis Area and Severity Index (PASI) baseline scores in responders (A) and nonresponders (B) at 12 months. Patients eligible for response analysis had a minimum PASI > 3 at baseline. Therapeutic response to FAEs treatment was defined as PASI ≤ 3 at 12 months or a treatment stop due to skin clearance. Responders (n = 176), nonresponders (n = 145). Presented data are rounded.
Importantly, the regression analysis could not identify another variable as significant treatment effect modifier. Neither sex, nor age, body weight or disease duration came close to reaching statistical significance. Similarly, no preexisting comorbid condition including cardiovascular disease, metabolic disease, liver disease, psychiatric or addictive disease or systemic pretreatment had any significant impact on the odds of achieving clinical response at month 12 ().
Discussion
In this study, we report a multivariable logistic regression analysis that investigates whether patient characteristics at baseline can influence the odds of achieving clinical response to FAEs in routine practice after 12 months. The only significant treatment effect modifier we could identify was baseline PASI. This is supported by the trend of numerically higher relative PASI reductions in the subgroups with lower baseline PASI. The analysis is based on a cohort drawn from the German Psoriasis Registry PsoBest including 444 patients who had received FAEs treatment and had a minimal observation time of 12 months in the registry. Clinical response was achieved by 39.6% (NRI) and 54.8% (OC) of patients at 12 months. Similarly, PASI 75 was achieved by 37.6% (NRI) and 52.0% (OC), respectively. The effectiveness seen in our analysis compares favorably to efficacy data reported in clinical trials with PASI 75 response of around 50.0% (as observed analysis) and 33.7% using NRI (Citation27). Relatively high discontinuation rates and shorter treatment duration usually seen in clinical trials may account for the slightly higher effectiveness seen in our cohort.
The average dose and corresponding exposure time reported here were similar in responders and nonresponders.
Our analysis revealed that the likelihood of treatment response to FAEs at 12 months is not significantly different among patients of either sex, varying age and body weight, with or without systemic pretreatment, duration of illness or comorbidity.
Increased body weight is frequently associated with reduced response to biologic therapy (Citation28,Citation29). Unlike biologics, FAEs allow for an individualized dose titration. This may explain why our analysis did not identify body weight as a significant effect modifier of response.
Comorbidity is relevant to the management of psoriasis as it may have a detrimental effect on antipsoriatic treatments, possibly decreasing therapeutic efficacy (Citation29,Citation30) or enhancing toxicity (Citation21). Of note, our analysis did not identify any preexisting comorbidity including cardiovascular, liver, metabolic and psychiatric or addictive disease as treatment effect modifiers, supporting findings from a previous study on FAEs in patients with psoriasis and comorbidities (Citation18). DMF is a NRF2 agonist exerting broad antioxidative, cytoprotective and antiinflammatory responses (Citation7) that have been found to mitigate inflammation and tissue dysfunction in humans (Citation31,Citation32) and animal models of neurodegeneration, cardiovascular, metabolic and hepatic disease (Citation33–40). It can be speculated that these effects may help to overcome a potential inflammatory crosstalk between psoriasis and the comorbid condition (Citation41) thereby maintaining treatment response of FAEs.
In concert with the safety concerns with using other conventional systemics such as ciclosporin or methotrexate in patients with several of these comorbidities (Citation21,Citation42), our data suggest that FAEs should be considered as a treatment option in patients suffering from these conditions.
Our multivariable logistic regression analysis shows that only disease severity at baseline significantly affects the odds of treatment response to FAEs at 12 months. Patients with a baseline PASI < 10 had 4 times higher odds to reach a response (OR 4.088, 95% CI [1.973, 8.469]) compared to those with severe psoriasis (PASI > 20). Similarly, baseline PASI 10–20 had a significantly higher odds of response compared to PASI > 20 (OR 1.961, 95% CI [1.018, 3.779]), although the effect was smaller compared to baseline PASI < 10.
Arguably, from a clinical point of view, this finding might be somewhat expected, as the odds of reaching a PASI ≤ 3 could be perceived to be markedly higher in patients with a baseline PASI around 10 as compared to patients with a baseline PASI > 20. Although this correlation surely is not limited to conventional therapies, current guidelines reflect this issue recommending to consider biologics as first line systemic therapy in patients with an expected inadequate response to conventional therapies (e.g. with a baseline PASI > 20).
Intriguingly, some studies suggest that treatment response to FAEs is independent of disease severity: A retrospective noninterventional study found a similar degree of PASI reduction in patients with PASI of 10–20 compared with those suffering from PASI > 20 or those with scores < 10 (Citation17). The small sample size of patients with reported PASI values and the retrospective nature of this study, enrolling only patients who tolerated FAEs and were undergoing long-term treatment, however, need to be taken into account when evaluating these data.
Our analysis has several limitations. Firstly, only a share of patients treated with FAEs in PsoBest qualified for regression analysis, resulting in a relatively small sample size of the model. We presume, however, that this does not constitute a relevant risk in terms of a potential selection bias, since selection of patients is mainly driven by the date of entry in the registry and treatment start. Hence, the selection of patients should not modify treatment success. As this analysis is based on real-world data, introduction of bias inherent with a nonrandomized and uncontrolled study design cannot be excluded.
Our model can assist in the clinical decision-making process, further individual patient characteristics not covered by this model, however, need to be taken into account when selecting the appropriate treatment in clinical practice.
In summary, our results showed that FAEs have a comparable likelihood of treatment success in a broad range of patients of both sex, varying age and body weight. Preexisting comorbidity, disease duration and prior therapy did not affect the likelihood of clinical response. Patients with PASI < 10 or between 10 and 20 at baseline had a significantly higher odds of treatment success than those with PASI > 20.
Supplemental Material
Download MS Excel (14.3 KB)Acknowledgements
The authors thank the Scientific Communication Team of the IVDP, in particular Merle Twesten and Mario Gehoff, for copy editing.
Disclosure statement
Kristian Reich has served as advisor and/or paid speaker for and/or participated in clinical trials sponsored by Abbvie, Almirall, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Forward Pharma, Gilead, Galderma, Janssen-Cilag, Kyowa Kirin, Leo, Lilly, Medac, Novartis, Ocean Pharma, Pfizer, Sanofi, UCB; Professor Reich is co-founder of Moonlake Immunotherapeutics.
Ulrich Mrowietz has been an advisor and/or received speakers’ honoraria and/or received grants and/or participated in clinical trials of the following companies: AbbVie, Aditxt, Almirall Hermal, Amgen, Aristea, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Dr. Reddy’s, Eli Lilly, Foamix, Formycon, Immunic, Janssen-Cilag, LEO Pharma, Medac, Novartis, Phi-Stone, Pierre Fabre, Sanofi-Aventis, UCB.
Christina Sorbe declared no conflict of interest.
Ralph von Kiedrowski has been an investigator, consultant, advisor or speaker for AbbVie, ALK Scherax, Almirall Hermal, Beiersdorf Dermo Medical, Biofrontera, Biogen, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, DermaPharm, Foamix, Gilead, Hexal, Janssen-Cilag, LEO Pharma, Eli Lilly, Meda, Medac, Menlo, Merck Sharp Dohme, Novartis, Dr. R. Pfleger, Pfizer, Regeneron, Sanofi, Stallergenes Greer, Stiefel GSK, Tigercut, UCB.
Sebastian Diemert is an employee of Almirall Hermal.
Lisa Schaeffer declared no conflict of interest.
Natalia Kirsten has served as advisor and/or paid speaker for and/or participated in clinical trials sponsored by Abbvie, Amgen, Boehringer Ingelheim, Celgene, Janssen-Cilag, LEO Pharma, Eli Lilly, Novartis, Pfizer, Sanofi, UCB, Uuru.
Nesrine Ben-Anaya declared no conflict of interest.
Matthias Augustin has served as advisor and/or paid speaker for and/or participated in research projects sponsored by Abbott/AbbVie, ALK Scherax, Almirall, Amgen, Beiersdorf, Biogen Idec, BMS, Boehringer Ingelheim, Celgene, Centocor, Dermira, Eli Lilly, Forward Pharma, Fresenius, Galderma, GSK, Hexal, Incyte, Janssen-Cilag, LEO Pharma, Lilly, Medac, Menlo, Merck, MSD, Mylon, Novartis, Pfizer, Regeneron, Sandoz, Sanofi-Aventis, Stallergenes, Stiefel, Teva, TK, Trevi, UCB and Xenoport.
Data availability statement
All data generated or analyzed during this study are included in the published article.
Additional information
Funding
References
- Parisi R, Symmons DPM, Griffiths CEM, et al. Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) Project Team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377–385.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361(5):496–509.
- Blome C, Gosau R, Radtke MA, et al. Patient-relevant treatment goals in psoriasis. Arch Dermatol Res. 2016;308(2):69–78.
- Nast A, Amelunxen L, Augustin M, et al. S3 guideline for the treatment of psoriasis vulgaris, update – short version part 1 – systemic treatment. J Dtsch Dermatol Ges. 2018;16(5):645–669.
- Augustin M. Einsatz von Systemtherapeutika und Biologika in der leitliniengerechten Therapie der mittelschweren bis schweren Psoriasis vulgaris. PsoNet Magazin. 2017;4(Suppl):1–28.
- Mrowietz U, Morrison PJ, Suhrkamp I, et al. The pharmacokinetics of fumaric acid esters reveal their in vivo effects. Trends Pharmacol Sci. 2018;39(1):1–12.
- Brück J, Dringen R, Amasuno A, et al. A review of the mechanisms of action of dimethylfumarate in the treatment of psoriasis. Exp Dermatol. 2018;27(6):611–624.
- Hanson J, Gille A, Offermanns S. Role of HCA2 (GPR109A) in nicotinic acid and fumaric acid ester-induced effects on the skin. Pharmacol Ther. 2012;136(1):1–7.
- Müller S, Behnen M, Bieber K, et al. Dimethylfumarate impairs neutrophil functions. J Invest Dermatol. 2016;136(1):117–126.
- Tang H, Lu JY-L, Zheng X, et al. The psoriasis drug monomethylfumarate is a potent nicotinic acid receptor agonist. Biochem Biophys Res Commun. 2008;375(4):562–565.
- Ghoreschi K, Brück J, Kellerer C, et al. Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. J Exp Med. 2011;208(11):2291–2303.
- Brennan MS, Matos MF, Li B, et al. Dimethyl fumarate and monoethyl fumarate exhibit differential effects on KEAP1, NRF2 activation, and glutathione depletion in vitro. PLoS One 2015;10(3):e0120254.
- Ruggieri S, Tortorella C, Gasperini C. Pharmacology and clinical efficacy of dimethyl fumarate (BG-12) for treatment of relapsing-remitting multiple sclerosis. Ther Clin Risk Manag. 2014;10:229–239.
- Nast A, Boehncke W-H, Mrowietz U, et al. S3-Leitlinie zur Therapie der Psoriasis vulgaris Update 2011. J Dtsch Dermatol Ges. 2011;9(Suppl 2):S1–S104.
- Mrowietz U, Szepietowski JC, Loewe R, et al. Efficacy and safety of LAS41008 (dimethyl fumarate) in adults with moderate-to-severe chronic plaque psoriasis: a randomized, double-blind, Fumaderm® – and placebo-controlled trial (BRIDGE). Br J Dermatol. 2017;176(3):615–623.
- Mrowietz U, Barker J, Boehncke W-H, et al. Clinical use of dimethyl fumarate in moderate-to-severe plaque-type psoriasis: a European expert consensus. J Eur Acad Dermatol Venereol. 2018;32(Suppl 3):3–14.
- Reich K, Thaci D, Mrowietz U, et al. Efficacy and safety of fumaric acid esters in the long-term treatment of psoriasis–a retrospective study (FUTURE). J Dtsch Dermatol Ges. 2009;7(7):603–611.
- Thaçi D, Weisenseel P, Philipp S, et al. Efficacy and safety of fumaric acid esters in patients with psoriasis on medication for comorbid conditions – a retrospective evaluation (FACTS). J Dtsch Dermatol Ges. 2013;11(5):429–435.
- Dickel H, Bruckner T, Altmeyer P. Long-term real-life safety profile and effectiveness of fumaric acid esters in psoriasis patients: a single-centre, retrospective, observational study. J Eur Acad Dermatol Venereol. 2018;32(10):1710–1727.
- Naldi L, Addis A, Chimenti S, et al. Impact of body mass index and obesity on clinical response to systemic treatment for psoriasis. Evidence from the psocare project. Dermatology 2008;217(4):365–373.
- Montaudié H, Sbidian E, Paul C, et al. Methotrexate in psoriasis: a systematic review of treatment modalities, incidence, risk factors and monitoring of liver toxicity. J Eur Acad Dermatol Venereol. 2011;25(Suppl 2):12–18.
- Edson-Heredia E, Sterling KL, Alatorre CI, et al. Heterogeneity of response to biologic treatment: perspective for psoriasis. J Invest Dermatol. 2014;134(1):18–23.
- Mrowietz U, Sorbe C, Reich K, et al. Fumaric acid esters for the treatment of psoriasis in Germany: characterising patients in routine care. Eur J Dermatol. 2020;30(1):41–48.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216.
- Augustin M, Spehr C, Radtke MA, et al. German psoriasis registry PsoBest: objectives, methodology and baseline data. J Dtsch Dermatol Ges. 2014;12(1):48–57.
- Kelley K, Preacher KJ. On effect size. Psychol Methods. 2012;17(2):137–152.
- Sticherling M, Mrowietz U, Augustin M, et al. Secukinumab is superior to fumaric acid esters in treating patients with moderate-to-severe plaque psoriasis who are naive to systemic treatments: results from the randomized controlled PRIME trial. Br J Dermatol. 2017;177(4):1024–1032.
- Cassano N, Galluccio A, Simone C D, et al. Influence of body mass index, comorbidities and prior systemic therapies on the response of psoriasis to adalimumab: an exploratory analysis from the APHRODITE data. J Biol Regul Homeost Agents. 2008;22(4):233–237.
- Driessen RJB, Boezeman JB, van de Kerkhof PCM, et al. Three-year registry data on biological treatment for psoriasis: the influence of patient characteristics on treatment outcome. Br J Dermatol. 2009;160(3):670–675.
- Brewer L, Rogers S. Fumaric acid esters in the management of severe psoriasis. Clin Exp Dermatol. 2007;32(3):246–249.
- Boehncke S, Fichtlscherer S, Salgo R, et al. Systemic therapy of plaque-type psoriasis ameliorates endothelial cell function: results of a prospective longitudinal pilot trial. Arch Dermatol Res. 2011;303(6):381–388.
- Salmen A, Gold R. Mode of action and clinical studies with fumarates in multiple sclerosis. Exp Neurol. 2014;262(Pt A):52–56.
- Cuadrado A, Manda G, Hassan A, et al. Transcription factor NRF2 as a therapeutic target for chronic diseases: a systems medicine approach. Pharmacol Rev. 2018;70(2):348–383.
- Zhou S, Jin J, Bai T, et al. Potential drugs which activate nuclear factor E2-related factor 2 signaling to prevent diabetic cardiovascular complications: a focus on fumaric acid esters. Life Sci. 2015;134:56–62.
- Amin FM, Abdelaziz RR, Hamed MF, et al. Dimethyl fumarate ameliorates diabetes-associated vascular complications through ROS-TXNIP-NLRP3 inflammasome pathway. Life Sci. 2020;256:117887.
- Gerhardt S, König V, Doll M, et al. Dimethylfumarate protects against TNF-α-induced secretion of inflammatory cytokines in human endothelial cells. J Inflamm. 2015;12:49.
- Lin-Holderer J, Li L, Gruneberg D, et al. Fumaric acid esters promote neuronal survival upon ischemic stress through activation of the Nrf2 but not HIF-1 signaling pathway. Neuropharmacology 2016;105:228–240.
- Luo M, Sun Q, Zhao H, et al. The effects of dimethyl fumarate on atherosclerosis in the apolipoprotein E-deficient mouse model with streptozotocin-induced hyperglycemia mediated by the nuclear factor erythroid 2-Related factor 2/antioxidant response element (Nrf2/ARE) signaling pathway. Med Sci Monit. 2019;25:7966–7975.
- Ashrafian H, Czibik G, Bellahcene M, et al. Fumarate is cardioprotective via activation of the Nrf2 antioxidant pathway. Cell Metab. 2012;15(3):361–371.
- Ibrahim SG, El-Emam SZ, Mohamed EA, et al. Dimethyl fumarate and curcumin attenuate hepatic ischemia/reperfusion injury via Nrf2/HO-1 activation and anti-inflammatory properties. Int Immunopharmacol. 2020;80:106131.
- Ganzetti G, Campanati A, Molinelli E, et al. Psoriasis, non-alcoholic fatty liver disease, and cardiovascular disease: Three different diseases on a unique background. World J Cardiol. 2016;8(2):120–131.
- Gisondi P, Del Giglio M, Girolomoni G. Considerations for systemic treatment of psoriasis in obese patients. Am J Clin Dermatol. 2016;17(6):609–615.
