Abstract
Objective
To describe real-world baseline characteristics and patient-reported outcomes (PROs) at 6-month and 12-month follow-up visits among patients with psoriasis who initiated and maintained secukinumab, stratified by prior exposure to biologics.
Methods
This real-world study included patients enrolled in the CorEvitas (formerly Corrona) Psoriasis Registry who initiated and maintained secukinumab through 6-month and/or 12-month follow-up. Demographics, clinical characteristics, and PROs were collected. PROs included Dermatology Life Quality Index (DLQI); itch, skin pain, fatigue, and EuroQol visual analog scales; and Work Productivity and Activity Impairment. Mean (SD) differences between baseline and follow-up visits were calculated for all outcomes.
Results
Overall, 652 patients had a 6-month follow-up visit, 460 (70.6%) were biologic experienced and 192 (29.4%) were biologic naive. Biologic-experienced and biologic-naive patients reported mean (SD) improvements in all PROs measured at 6-month follow-up. Similar improvements were seen among patients with a 12-month follow-up visit (n = 390) and both 6-month and 12-month follow-up visits (n = 326).
Conclusions
Biologic-experienced and biologic-naive patients with psoriasis who initiated and maintained secukinumab treatment reported improvements in PROs at 6-month and/or 12-month follow-up visits. These findings suggest that secukinumab is a potential biologic for psoriasis at any point along the patient treatment journey.
Introduction
Psoriasis is a chronic, systemic, inflammatory disease of the skin, affecting up to 2–4% of the population in the United States (Citation1,Citation2). Common psoriasis clinical manifestations include inflammation, itching, scaling, pain, and cracking of the skin (Citation3,Citation4). Patients with psoriasis experience reduced health-related quality of life (QOL) due to the physical burden associated with the signs and symptoms of the disease (Citation5,Citation6). Additionally, individuals with psoriasis may often experience diminished work productivity (Citation7); estimated productivity loss due to psoriasis-related presenteeism is $4.4 billion annually, and approximately 16.7% of unemployed patients with psoriasis were no longer working because of their disease (Citation8,Citation9).
Treatment guidelines for the management of psoriasis have been developed by the American Academy of Dermatology and National Psoriasis Foundation and include recommendations for biologic therapies alone or in combination with topical or other systemic medications for patients with moderate to severe psoriasis (Citation10). Biologics have been shown to have higher efficacy and effectiveness compared with conventional systemic medications or phototherapy (Citation11,Citation12). Tumor necrosis factor-α inhibitors (TNFis) were the first US Food and Drug Administration (FDA)-approved biologics for the treatment of psoriasis; thus, they have traditionally been used as first-line therapy. However, as biologics with alternative mechanisms of action were developed and demonstrated significant efficacy and favorable safety profiles for treating psoriasis symptoms (Citation13–15), it has become unclear which treatment is most effective. Given that numerous biologics have been approved by the FDA for the treatment of psoriasis, a consensus is lacking among dermatologists regarding which biologics are appropriate as a first-line therapy.
Among the FDA-approved biologics, secukinumab is a fully human monoclonal antibody that selectively targets interleukin 17 A and was approved for the treatment of active moderate to severe psoriasis in 2015. Secukinumab has demonstrated significant efficacy in improving the physical manifestations of psoriasis, with a favorable safety profile in clinical studies (Citation14,Citation16–19). While the safety and effectiveness of secukinumab have been reported in real-world studies in patients with psoriasis (Citation20–24), these analyses included mostly patients who had received prior biologic therapy, and follow-up was generally limited to 6 months (Citation25). Limited data are available on the use of secukinumab in patients who are biologic naive and followed up beyond 6 months of treatment (Citation26,Citation27).
Patient-reported outcomes (PROs) are important tools used in clinical trials and real-world studies to capture a comprehensive insight into the patient disease experience and are useful for assessing disease impact, QOL, and response to therapy (Citation28–30). Furthermore, PROs can also have a significant impact on the decision-making process for dermatologists when choosing between treatment options for patients with psoriasis (Citation31). This study aimed to describe baseline patient characteristics and changes in PROs at 6 and 12 months after treatment initiation for patients who maintained secukinumab use, stratified by prior biologic status.
Methods
Data source
The CorEvitas Psoriasis Registry is a large, independent, prospective, observational cohort of patients with psoriasis that was launched in April 2015 in collaboration with the National Psoriasis Foundation (Citation32). Patients were enrolled in the registry if they met the following inclusion criteria: psoriasis diagnosed by a dermatologist, aged ≥18 years, and initiated or switched to a US FDA-approved systemic therapy for the treatment of psoriasis at enrollment or within the previous 12 months of the date of enrollment. As of December 10 2020, patients were recruited by 527 practicing dermatologists in 252 private and academic clinical sites across 46 US states and Canadian provinces.
Data were collected using questionnaires completed by patients and their treating dermatologists during routine clinical office visits at registry enrollment and follow-up visits occurring at approximately 6-month intervals. Data were collected in accordance with ethical principles that have their origins in the Declaration of Helsinki and are consistent with Good Clinical Practice. All participating investigators were required to obtain full board approval for conducting research involving human subjects. Sponsor approval and continuing review was obtained through a central internal institutional review board (IRB; IntegReview, Protocol number is Corrona-PSO-500). For academic investigative sites that did not receive a waiver to use the central IRB, approval was obtained from the respective governing IRBs and documentation of approval was submitted to the Sponsor prior to initiating any study procedures.
Study design and patient population
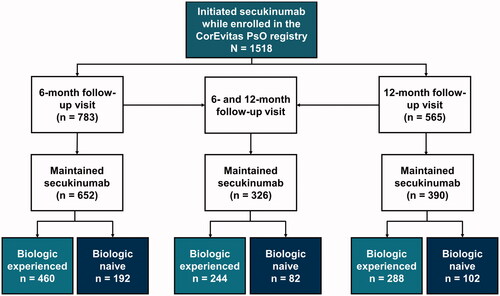
This analysis included US patients with psoriasis enrolled in the CorEvitas Psoriasis Registry and used data collected between April 15 2015, and December 10 2020. Patients were included in the analysis if they initiated secukinumab at or after enrollment in the registry (baseline) and maintained continuous secukinumab treatment up to a 6-month and/or 12-month follow-up visit (). Three subpopulations of patients were analyzed: (1) patients who had a follow-up visit within 6 months of their baseline visit (window, 5–9 months); (2) patients who had a follow-up visit within 12 months of their baseline visit (window, 11–15 months) if they did not have a 6-month visit, or those who had a visit within 6 months (window, 5–9 months) of their initial 6-month visit (10–18 months window from baseline visit); and (3) patients who had both a 6- and 12-month follow-up visit. Patients were stratified by their prior exposure to biologics: biologic experienced vs biologic naive. Biologic-experienced patients had a history of prior use of ≥1 biologic medication for the treatment of psoriasis at the time of secukinumab initiation. Biologic-naive patients were defined as having no history of biologic medication use for the treatment of psoriasis at the time of secukinumab initiation.
Variables
Primary outcome variables
Primary outcome variables were PROs collected at both baseline and follow-up visits and included the following: (1) patient-reported itch, skin pain, and fatigue (visual analog scale [VAS]: 0-100; higher scores indicating worse status); (2) categorical Dermatology Life Quality Index (DLQI) to assess skin impact on QOL: 0–1 (psoriasis had no effect on patient’s life), 2–5 (small effect), 6–10 (moderate effect), 11–20 (very large effect), or 21–30 (extremely large effect); (3) Work Productivity and Activity Impairment (WPAI) questionnaire: percent impairment in each of the 4 WPAI domains (absenteeism while working [work time missed], presenteeism while working [reduced on-the-job effectiveness], impairment while working, and overall activity impairment); (4) patient global assessment of disease activity (PtGA; VAS: 0–100), and (5) the EQ-VAS: overall patient health state today (VAS: 0–100; higher scores indicating better QOL).
Other variables
Patient characteristics collected at the baseline visit included the following: patient demographics (age, sex, race/ethnicity, and smoking status), clinical characteristics (duration of psoriasis, age of onset of psoriasis, comorbid psoriatic arthritis [PsA], PsA disease duration, Psoriasis Area and Severity Index [PASI], percent affected body surface area (BSA), body weight (kg) and body mass index (BMI, kg/m2), physician-reported history of comorbidities), and treatment history (number of prior biologics).
Data analysis
Separate analyses were performed in each patient cohort (6-month follow-up, 12-month follow-up, and 6-month and 12-month follow-up) and were stratified by prior biologic treatment status. Patient demographics and clinical characteristics at baseline were assessed using descriptive statistics. Frequency counts and percentages were described for ordinal and categorical variables; means and SDs were used for continuous variables. The mean (SD) change in continuous variables between the baseline visit and appropriate follow-up visit was calculated as the difference in outcome measure at follow-up from baseline, such that a negative change indicates that the score decreased over time. Change in categorical outcomes was expressed as the frequency count and percentages of patients in each category at baseline and subsequent follow-up visits.
Results
Baseline patient characteristics
A total of 1518 patients initiated secukinumab, 652 and 390 of whom maintained secukinumab and completed a 6-month and a 12-month follow-up visit, respectively ( and ). Of those patients who had a 6-month follow-up visit, 460 (70.6%) were biologic experienced and 192 (29.4%) were biologic naive; similar proportions were seen in patients with 12-month visits (288 [73.8%] were biologic experienced and 102 [26.2%] were biologic naive).
Table 1. Baseline characteristics of all biologic-experienced and biologic-naive patients who initiated secukinumab and maintained treatment at 6-month and 12-month follow-up visits.
Overall, among all patients with available 6-month follow-up visit data, the mean age of patients at baseline was 51.3 years and 52.8% were male (). The majority of these patients were White (79.0%), obese (body mass index ≥30 kg/m2; 50.2%), and never smoked (52.0%). More than 20% of biologic-experienced patients had received ≥3 prior biologics. Of the biologic-experienced and biologic-naive patients who remained on secukinumab and had a 6-month follow-up visit, the mean age at psoriasis diagnosis was approximately 33 and 37 years, respectively (). Additionally, approximately 60% and 35% of biologic-experienced and biologic-naive patients, respectively, had comorbid PsA. At baseline, biologic-experienced and biologic-naive patients reported mean (SD) PASI scores of 7.3 (6.9) and 8.2 (7.8), respectively, and mean (SD) BSA of 13.1% (15.2%) and 15.2% (17.5%), respectively. Similar demographics and clinical characteristics were seen in patients who remained on secukinumab through the 12-month follow-up.
Table 2. Baseline clinical characteristics and PRO scores of all biologic-experienced and biologic-naive patients who initiated secukinumab and maintained treatment at 6-month and 12-month follow-up visits.
Improvements in PRO measures at 6-month or 12-month follow-up visits
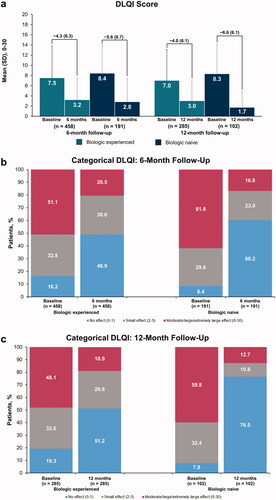
Both biologic-experienced and biologic-naive patients demonstrated improvements in PROs at the 6-month follow-up visits. DLQI scores for biologic-experienced and biologic-naive patients improved by a mean (SD) of 4.3 (6.3) and 5.6 (6.7), respectively, at 6 months (). Similar mean (SD) improvements were observed in biologic-experienced and biologic-naive patients with 12-month follow-up visits (4.0 [6.1] and 6.6 [6.1], respectively). The proportion of biologic-experienced and biologic-naive patients who achieved DLQI scores of 0 to 1 (no effect) increased from baseline (16.2% and 8.4%, respectively) to 6-month follow-up visit (48.9% and 60.2%, respectively) (). Similar improvements in DLQI scores were reported by patients who maintained secukinumab through a 12-month follow-up; the proportion of biologic-experienced patients with DLQI scores of 0 to1 shifted from 19.3% at baseline to 51.2% at follow-up, while the proportion of biologic-naive patients with DLQI scores of 0 to1 shifted from 7.8% to 76.5% at 12 months ().
Figure 2. Improvements in mean DLQI scores (a) and in categorical DLQI scores from baseline to 6-month (b) or 12-month (c) follow-up visit among biologic-experienced and biologic-naive patients with psoriasis who initiated and maintained secukinumaba. DLQI: Dermatology Life Quality Index. aLabels across baseline and follow-up visits represent mean (SD) differences.
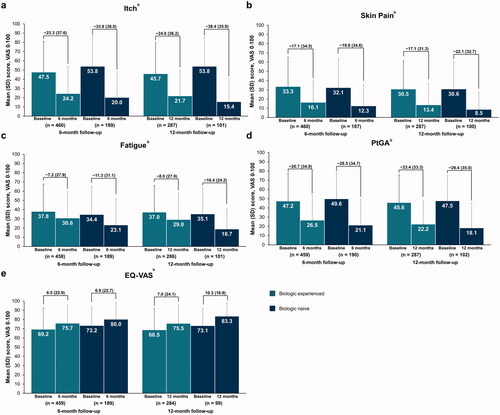
Patients initiating and maintaining secukinumab through 6 or 12 months reported improvements in psoriasis symptoms. Compared with baseline, biologic-experienced and biologic-naive patients reported reduced mean (SD) itch (−23.3 [37.6] and −33.8 [38.0]), skin pain (−17.1 [34.5] and −19.8 [34.6]), and fatigue (−7.2 [27.9] and −11.3 [31.1]) VAS scores at 6-month follow-up, respectively; similar improvements were observed in patients with a 12-month follow-up visit (). General measures of QOL assessed with PtGA and EQ-VAS scores also improved in both biologic-experienced and biologic-naive patients through 12 months ().
Figure 3. Improvements in mean patient-reported outcome measures of itch (a), skin pain (b), fatigue (c), PtGA (d), and EQ-VAS (e) from baseline to 6- or 12-month follow-up visit in patients with psoriasis who initiated and maintained secukinumaba. PRO: patient-reported outcome; PtGA: patient global assessment of disease activity; VAS: visual analog scale. aLabels across baseline and follow-up visits represent mean (SD) differences. bFor overall itch, pain, and fatigue, a decrease in mean VAS score denotes an improvement from baseline; for EQ-VAS, an increase in mean VAS score denotes an improvement from baseline.
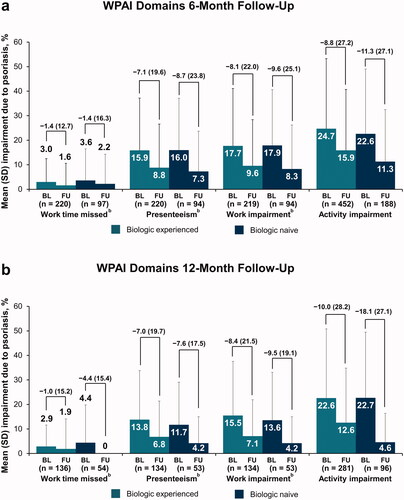
Improvements in WPAI scores were also seen with secukinumab treatment, irrespective of prior biologic use. Improvements in presenteeism, work impairment, and activity impairment were seen in patients with a 6-month or 12-month follow-up, in both cohorts (). Biologic-experienced patients reported similar improvements in work time missed with secukinumab treatment through 6 or 12 months; biologic-naive patients reported comparable reductions in work time missed at 6 months, with similar improvement reported at 12 months.
Figure 4. Improvement in WPAI domains from baseline to the 6-month (a) or 12-month (b) follow-up visit in patients with psoriasis who initiated and maintained secukinumaba. BL: baseline; FU: follow-up; WPAI: Work Productivity and Activity Impairment. aLabels across baseline and follow-up visits represent mean (SD) differences. bWork time missed, presenteeism, and work impairment values are based on patients with psoriasis who were employed.
PROs for patients with both 6-month and 12-month follow-up visits
Of the 326 patients who maintained secukinumab through both follow-up visits, 244 (74.8%) were biologic experienced and 82 (25.2%) were biologic naive (). Patients were similar to the other cohorts with respect to demographics and clinical characteristics (Supplemental Table 1). Patients reported improvements in DLQI scores after 6 months, which were sustained through the 12-month follow-up visit, irrespective of prior biologic use (Supplemental Figure 1(A)). The proportion of biologic-experienced and biologic-naive patients who reported DLQI scores of 0 to 1 (no effect) increased from baseline (18.7% and 7.4%) to the 6-month follow-up visit (55.2% and 72.8%) and was comparable through 12 months of secukinumab treatment (51.9% and 79.0%), respectively (Supplemental Figure 1(B,C)). Patients reported improved PROs for itch, skin pain, fatigue, PtGA, and EQ-VAS at both 6 and 12 months, irrespective of prior biologic experience (Supplemental Figure 2). Biologic-experienced and biologic-naive patients reported improved WPAI outcomes at 6 months, with similar improvements seen among those who maintained secukinumab through 12 months (Supplemental Figure 3).
Discussion
This analysis is one of the first in the United States to evaluate PRO measures for a large, biologic-naive population from secukinumab initiation through 12 months of maintained use and demonstrates the long-term effectiveness of secukinumab from the patient perspective. Patients who initiated and maintained continuous secukinumab treatment reported improvements in PROs, including DLQI, itch, skin pain, fatigue, PtGA, EQ-VAS, and WPAI, at 6-month and/or 12-month follow-up visits, irrespective of prior biologic use.
Previous real-world analyses from the CorEvitas (formerly Corrona) Psoriasis Registry demonstrated that continuous secukinumab treatment resulted in significant improvements in PROs at 6-month follow-up (Citation25). However, these analyses were limited because the patient cohort included few biologic-naive patients, which precluded stratification by prior biologic experience. Patients in the previous analysis of all patients with psoriasis, who were primarily biologic experienced, achieved mean (SD) improvements of −30.8 (38.8), −23.2 (33.85), and −8.8 (31.7) in itch, pain, and fatigue VAS scores, respectively, and reported significant reductions in the WPAI domains of overall work hours affected, impairment while working, and daily activities impaired. Our study substantiates and expands on those results by showing that biologic-naive patients demonstrated comparable improvements at both 6-month and 12-month follow-up visits over all PROs included. This is among the first studies to focus on biologic-naive patients in either clinical trials or real-world settings and demonstrates that secukinumab may be an effective first-line therapy for patients with moderate to severe psoriasis.
Throughout this analysis, biologic-naive patients experienced improvements in outcome measures comparable to those who were biologic experienced. While this finding is consistent with that of several real-world studies measuring changes in DLQI upon secukinumab treatment (Citation21,Citation22,Citation26), limited analyses are available in which patients were stratified by biologic experience. To our knowledge, this is among the first real-world analyses to explore improvements in DLQI in addition to a diverse range of PROs within a biologic-naive population treated with secukinumab. The majority of real-world studies focus on DLQI to assess skin impact on QOL, with less emphasis on the signs and symptoms of psoriasis (e.g. itch, skin pain, and fatigue). Our study addresses these knowledge gaps by providing detailed assessments of the effectiveness of secukinumab across many PROs in patients with different biologic experiences.
The results of our analysis suggest that secukinumab may be suitable as a first-line treatment for psoriasis. While TNFis are traditionally used as first-line therapies, recent evidence has suggested that newer classes of biologics with alternative mechanisms of action, such as secukinumab, may lead to more favorable outcomes compared with TNFis. The phase 3 FIXTURE clinical trial directly compared QOL measures between secukinumab and etanercept and found that 57% of patients treated with secukinumab achieved DLQI scores of 0 to 1 by week 12 compared with 35% of patients treated with etanercept (p < .001) (Citation30). Additional studies comparing the long-term effectiveness of secukinumab and TNFis are warranted.
Patients’ prior biologic experience is an important clinical characteristic, as patients with psoriasis often need to switch biologics due to lack of efficacy. The PROSE study demonstrated that by week 52 of secukinumab use, 75.3% of patients naive to any systemic therapy achieved DLQI scores 0–1 compared with 73.3% of patients with prior systemic experience and 62.0% of patients with prior biologic experience (Citation28). Furthermore, several real-world studies have demonstrated that patients naive to systemic therapies or biologics respond better to treatment with biologics compared with experienced patients (Citation33–35); however, these analyses focused on the severity of clinical symptoms, such as PASI, and limited evidence is available on changes in patient QOL or PROs in response to treatment. Additionally, a real-world study using electronic medical records to assess patient satisfaction found that the proportion of patients satisfied with their secukinumab treatment after 18 months was higher in biologic-naive patients (36.6%) than in biologic-experienced (21.7%) or systemic-experienced (25.8%) patients (Citation23). Our study corroborates the results demonstrated in clinical trials, with proportions of biologic-experienced and biologic-naive patients achieving DLQI scores of 0 to 1 after secukinumab use through 12 months of 51.2% and 76.5%, respectively, and suggests that biologic-naive patients would experience improved QOL from primary treatment with secukinumab.
This study does have some limitations to consider. Participation in the CorEvitas Psoriasis Registry by dermatologists is voluntary, and the patient cohort is regularly treated by dermatologists; therefore, the results may not be generalizable to all patients with psoriasis seen elsewhere in general practice. Nevertheless, the patients included in this study were more representative of US patients in routine clinical settings compared with clinical trials. This study only included patients who maintained continuous secukinumab use at follow-up visits; thus, the outcomes may not be representative of all patients initiating secukinumab. Patients who are on continuous therapy may be those who have more favorable response to treatment, or vice versa. These results are purely descriptive, and no statistical tests were performed to formally compare the outcomes between biologic-experienced and biologic-naive patients. These findings should not be used to assess the relative effectiveness of other FDA-approved biologics
Conclusions
In this real-world, US-based study of patients with psoriasis, both biologic-experienced and biologic-naive patients who initiated and maintained treatment with secukinumab demonstrated improvements in PROs at the 6-month and/or 12-month follow-up visit. These results suggest that secukinumab is effective in patients who remain on treatment, regardless of prior exposure to biologics. The findings presented here suggest that improvements in PROs at 6 months can be sustained through 12 months in those patients who remain on therapy and that secukinumab may be an effective first-line biologic therapy for psoriasis.
Medical writing
Medical writing support was provided by Samantha O’Dwyer, PhD, of Health Interactions, Inc, and was funded by Novartis Pharmaceuticals Corporation. This manuscript was developed in accordance with Good Publication Practice (GPP3) guidelines. The authors had full control of the content and made the final decision on all aspects of this publication.
Supplemental Material
Download PDF (252 KB)Disclosure statement
BS is an honoraria consultant for AbbVie, Almirall, Amgen, Arcutis, Arena, Aristea, Asana, Boehringer Ingelheim, Immunic Therapeutics, Bristol Myers Squibb, Connect Biopharma, Dermavant, EPI Health, Equillium, Evelo Biosciences, Janssen, Leo, Eli Lilly, Maruho, Meiji Seika Pharma, Mindera Health, Novartis, Pfizer, UCB Pharma, Sun Pharma, Regeneron, Sanofi-Genzyme, Ventyxbio, and vTv Therapeutics; a shareholder of Connect Biopharma and Mindera Health; received speakers fees from AbbVie, Eli Lilly, Janssen, Regeneron, and Sanofi-Genzyme; served as an investigator for Dermavant, AbbVie, CorEvitas, LLC (formally known as Corrona, LLC) Psoriasis Registry, Dermira, Cara, and Novartis; receives consulting fees as a co-scientific director for the CorEvitas Psoriasis Registry; and receives honoraria as the Editor-in-Chief for the Journal of Psoriasis and Psoriatic Arthritis. DP and EL are employees of Novartis Pharmaceuticals Corporation. RM, MM-C, and NG are employees of CorEvitas, LLC (formerly known as Corrona, LLC). ML is an employee of Mount Sinai and receives research funds from AbbVie, Amgen, Arcutis, Avotres, Boehringer Ingelheim, Cara Therapeutics, Dermavant Sciences, Eli Lilly, Incyte, Janssen Research & Development, LLC, Ortho Dermatologics, Regeneron, and UCB, Inc., and is a consultant for Aditum Bio, Almirall, AltruBio Inc., AnaptysBio, Arcutis, Inc., Arena Pharmaceuticals, Aristea Therapeutics, Arrive Technologies, Avotres Therapeutics, BiomX, Brickell Biotech, Boehringer Ingelheim, Bristol Myers Squibb, Cara Therapeutics, Castle Biosciences, CorEvitas, LLC (formerly known as Corrona, LLC), Dermavant Sciences, Dr. Reddy’s Laboratories, Evelo Biosciences, Evommune, Inc., Facilitation of International Dermatology Education, Forte Biosciences, Foundation for Research and Education in Dermatology, Helsinn Therapeutics, Hexima Ltd., LEO Pharma, Meiji Seika Pharma, Mindera, Pfizer, Seanergy, and Verrica.
Data availability statement
Data are available from CorEvitas, LLC through a commercial subscription agreement and are not publicly available. No additional data are available from the authors.
Additional information
Funding
References
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70(3):512–516.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 1: Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58(5):826–850.
- Sampogna F, Gisondi P, Melchi CF, et al. Prevalence of symptoms experienced by patients with different clinical types of psoriasis. Br J Dermatol. 2004;151(3):594–599.
- Globe D, Bayliss MS, Harrison DJ. The impact of itch symptoms in psoriasis: results from physician interviews and patient focus groups. Health Qual Life Outcomes. 2009;7:62.
- Bickers DR, Lim HW, Margolis D, Society for Investigative Dermatology, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55(3):490–500.
- Edson-Heredia E, Zhu B, Guo J, et al. Disease burden and quality of life in psoriasis patients with and without comorbid psoriatic arthritis: results from National Psoriasis Foundation panel surveys. Cutis. 2015;95(3):173–178.
- Fowler JF, Duh MS, Rovba L, et al. The impact of psoriasis on health care costs and patient work loss. J Am Acad Dermatol. 2008;59(5):772–780.
- Pearce DJ, Singh S, Balkrishnan R, et al. The negative impact of psoriasis on the workplace. J Dermatolog Treat. 2006;17(1):24–28.
- Vanderpuye-Orgle J, Zhao Y, Lu J, et al. Evaluating the economic burden of psoriasis in the United States. J Am Acad Dermatol. 2015;72(6):961–967.e5.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–1072.
- Armstrong AW, Puig L, Joshi A, et al. Comparison of biologics and oral treatments for plaque psoriasis: a meta-analysis. JAMA Dermatol. 2020;156(3):258–269.
- Armstrong AW, Foster SA, Comer BS, et al. Real-world health outcomes in adults with moderate-to-severe psoriasis in the United States: a population study using electronic health records to examine patient-perceived treatment effectiveness, medication use, and healthcare resource utilization. BMC Dermatol. 2018;18(1):4.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375(4):345–356.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338.
- Strober BE, Bissonnette R, Fiorentino D, et al. Comparative effectiveness of biologic agents for the treatment ofpsoriasis in a real-world setting: results from a large, prospective, observational study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]). J Am Acad Dermatol. 2016;74(5):851–861.e4.
- Bagel J, Blauvelt A, Nia J, et al. Secukinumab maintains superiority over ustekinumab in clearing skin and improving quality of life in patients with moderate to severe plaque psoriasis: 52-week results from a double-blind phase 3b trial (CLARITY). J Eur Acad Dermatol Venereol. 2021;35(1):135–142.
- Reich K, Sullivan J, Arenberger P, et al. Secukinumab shows high and sustained efficacy in nail psoriasis: 2.5-year results from the randomized placebo-controlled TRANSFIGURE study. Br J Dermatol. 2021;184(3):425–436.
- Thaçi D, Puig L, Reich K, et al. Secukinumab demonstrates sustained efficacy in clearing skin and improving patient-reported outcomes in patients with moderate-to-severe psoriasis through 2 years of treatment: Results from the CLEAR study. J Am Acad Dermatol. 2019;81(6):1405–1409.
- Gottlieb AB, Kubanov A, van Doorn M, et al. Sustained efficacy of secukinumab in patients with moderate-to-severe palmoplantar psoriasis: 2·5-year results from GESTURE, a randomized, double-blind, placebo-controlled trial. Br J Dermatol. 2020;182(4):889–899.
- Augustin M, Jullien D, Martin A, et al. Real-world evidence of secukinumab in psoriasis treatment – a meta-analysis of 43 studies. J Eur Acad Dermatol Venereol. 2020;34(6):1174–1185.
- Nguyen HT, Pham NTU, Tran TNA, et al. Secukinumab demonstrated high effectiveness in Vietnamese patients with moderate-to-severe plaque psoriasis in a real-world clinical setting: 16 week results from an observational study. Dermatol Ther (Heidelb). 2021;11(5):1613–1621.
- Zeng JX, Luo Q, Wen J, et al. Real-world investigation of the efficacy and safety of secukinumab for psoriasis treatment in a Chinese population. Chin Med J (Engl). 2020;134(1):117–119.
- Armstrong AW, Patil D, Levi E, et al. Real-world satisfaction with secukinumab in clearing the skin of patients with plaque psoriasis through 24 months of follow-up: results from US dermatology electronic medical records. Dermatol Ther (Heidelb). 2021;11(5):1733–1749.
- Deodhar A, Mease PJ, McInnes IB, et al. Long-term safety of secukinumab in patients with moderate-to-severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis: integrated pooled clinical trial and post-marketing surveillance data. Arthritis Res Ther. 2019;21(1):111.
- Strober BE, Germino R, Guana A, et al. US real-world effectiveness of secukinumab for the treatment of psoriasis: 6-month analysis from the Corrona psoriasis registry. J Dermatolog Treat. 2020;31(4):333–341.
- Thaçi D, Körber A, von Kiedrowski R, et al. Secukinumab is effective in treatment of moderate-to-severe plaque psoriasis: real-life effectiveness and safety from the PROSPECT study. J Eur Acad Dermatol Venereol. 2020;34(2):310–318.
- Torres T, Balato A, Conrad C, et al. Secukinumab drug survival in patients with psoriasis: a multicenter, real-world, retrospective study. J Am Acad Dermatol. 2019;81(1):273–275.
- Augustin M, Dauden E, Mrowietz U, et al. Secukinumab treatment leads to normalization of quality of life and disease symptoms in psoriasis patients with or without prior systemic psoriasis therapy: the PROSE study results. J Eur Acad Dermatol Venereol. 2021;35(2):431–440.
- Strober B, Greenberg JD, Karki C, et al. Impact of psoriasis severity on patient-reported clinical symptoms, health-related quality of life and work productivity among US patients: real-world data from the Corrona psoriasis registry. BMJ Open. 2019;9(4):e027535.
- Strober B, Sigurgeirsson B, Popp G, et al. Secukinumab improves patient-reported psoriasis symptoms of itching, pain, and scaling: results of two phase 3, randomized, placebo-controlled clinical trials. Int J Dermatol. 2016;55(4):401–407.
- Feldman SR, Regnier SA, Chirilov A, et al. Patient-reported outcomes are important elements of psoriasis treatment decision making: a discrete choice experiment survey of dermatologists in the United States. J Am Acad Dermatol. 2019;80(6):1650–1657.
- Strober B, Karki C, Mason M, et al. Characterization of disease burden, comorbidities, and treatment use in a large, US-based cohort: Results from the Corrona psoriasis registry. J Am Acad Dermatol. 2018;78(2):323–332.
- van Lumig PP, van de Kerkhof PC, Boezeman JB, et al. Adalimumab therapy for psoriasis in real-world practice: efficacy, safety and results in biologic-naive vs. non-naive patients. J Eur Acad Dermatol Venereol. 2013;27(5):593–600.
- Galluzzo M, Talamonti M, De Simone C, et al. Secukinumab in moderate-to-severe plaque psoriasis: a multi-center, retrospective, real-life study up to 52 weeks observation. Expert Opin Biol Ther. 2018;18(7):727–735.
- Seneschal J, Lacour JP, Bewley A, et al. A multinational, prospective, observational study to estimate complete skin clearance in patients with moderate-to-severe plaque PSOriasis treated with BIOlogics in a REAL world setting (PSO-BIO-REAL). J Eur Acad Dermatol Venereol. 2020;34(11):2566–2573.