 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objectives
To undertake a comparison of Cal/BDP cream versus foam for the treatment of plaque psoriasis, with cross-trial population differences accounted for.
Materials and Methods
An anchored matching-adjusted indirect comparison was undertaken, using individual patient data for Cal/BDP cream and published aggregated data for Cal/BDP foam. Altogether, 11 outcomes were analyzed, including PGA success, mPASI75, DLQI-related outcomes and treatment satisfaction across numerous domains. For each outcome an odds ratio or mean difference was calculated to represent the relative efficacy of Cal/BDP cream versus foam. Methods were guided by NICE Decision Support Unit recommendations.
Results
After adjustment, baseline characteristics were balanced across treatment arms in each analysis. There were no statistically significant differences in PGA success, mPASI75 or DLQI outcomes between Cal/BDP cream and foam when they were compared after their recommended treatment durations (8 weeks for cream and 4 weeks for foam). For treatment satisfaction after 1 week of treatment, Cal/BDP cream was significantly superior to the Cal/BDP foam in all but one domain of the questionnaire.
Conclusions
Cal/BDP cream and Cal/BDP foam have equivalent efficacy and HRQoL (measured in DLQI) outcomes when used for the topical treatment of plaque psoriasis at their recommended treatment durations. A comparison of treatment satisfaction assessments after 1 week of treatment demonstrated that patients find Cal/BDP cream to be more convenient than foam.
Introduction
Psoriasis is an inflammatory, immune-mediated disease of the skin that can substantially impair a patient’s quality of life (Citation1). Approximately 80 to 90% of the total psoriasis population have chronic plaque psoriasis, which is characterized by thickened, erythematous, clearly demarcated skin lesions that can affect multiple areas of the body (Citation2,Citation3). Due to the chronic nature of the disease, long-term strategies must be devised to manage the condition. This includes the use of systemic treatments, such as biologic therapies, which are required for approximately 20% of the plaque psoriasis population with a moderate-to-severe form of the disease. The other 80% of the population typically has a localized form of the disease that can be treated with topical options (Citation2,Citation4).
A number of topical therapies are available for mild-to-moderate plaque psoriasis. This includes topical corticosteroids, vitamin D analogues, vitamin A derivatives (tazarotene), anthralin, and newer formulations of tar. In developed countries the two classes of topical treatment that is most commonly prescribed are vitamin D analogues and corticosteroids as they are generally preferred by patients, in particular, they are more cosmetically accepted than anthralin or tar preparations (Citation5). Additionally, vitamin D analogues and corticosteroids have different modes of action and, therefore, can be used in combination with clear evidence that these combinations are significantly superior to the use of vitamin D analogues or corticosteroids alone (Citation5,Citation6).
The choice of vehicle for treatment delivery can also have a substantial impact on the potency and effectiveness of topical therapy with options such as lotions, gels, creams and ointments available (Citation7). The first fixed-dose and licensed combination of a vitamin D analogue and corticosteroid was calcipotriene (50 µg/g) (CAL) and betamethasone dipropionate (0.5 mg/g, as betamethasone) (BDP), which was made available in both an ointment and a gel formulation. Subsequently, a foam formulation of Cal/BDP has been released. The findings from two double-blind multicentre randomized control trials (RCTs) found that the foam version of Cal/BDP was significantly more efficacious than both the ointment and gel formulations in terms of Physicians Global Assessment (PGA) and modified Psoriasis Area and Severity (mPASI) scores, with a comparable safety profile (Citation8,Citation9).
More recently, the first cream formulation of fixed-dose Cal/BDP combination has been developed and launched in Europe. Two phase III double-blind multicentre RCTs were undertaken to compare the cream and gel formulations. A pooled analysis of these trials indicated that Cal/BDP cream could be statistically superior to the gel formulation at all efficacy endpoints after eight weeks of treatment. This includes a significantly higher proportion of patients achieving PGA treatment success (43.2% vs 31.9%; p < .0001) and a greater reduction in mPASI score from baseline (−64.6% vs −56.4%, p < .0001) (Citation10).
The results described above indicate that the foam and cream formulations may have superior efficacy when compared with the gel formulation, though a lack of head-to-head trial data prevents a certain conclusion. Given the importance of Cal/BDP for the topical treatment of plaque psoriasis it is informative to assess the relative efficacy of the two options. However, no published direct comparison of the foam and cream options is currently available. Typically, in the absence of direct treatment comparisons, indirect treatment comparisons can be made. Therefore, the objective of this study was to undertake an indirect comparison of Cal/BDP cream versus Cal/BDP foam.
Network meta-analyses are a common method for indirect comparisons, but they are subject to important limitations. In particular, differences in baseline characteristics of the patient’s in the study may bias the outputs if they cannot be adequately adjusted for (Citation11). This is particularly relevant for this study given the differences in the populations for the pivotal Cal/BDP foam and Cal/BDP cream trials. Notably, the Cal/BDP cream trials enrolled patients with mild to moderate psoriasis at baseline, whereas the Cal/BDP foam trials included all types of psoriasis severity (i.e. mild to severe).
In cases where individual patient data (IPD) are available for all trials of interest then it is possible to adjust for heterogeneity using techniques such as regression adjustment or propensity matching (Citation11). For this study, the authors had access to IPD for the two phase III trials for Cal/BDP cream but such data were unavailable for trials related to Cal/BDP foam. In such situations, it is possible to undertake a matching-adjusted indirect comparison (MAIC), which can be used to combine IPD for one treatment with aggregated summary data for another, with such summary data commonly available in primary publications for pivotal trials. Ideally, such an analysis should be anchored on a common comparator that is present in the trials of both treatments being compared (Citation12). MAICs that are unanchored are limited in their robustness due to the strong assumptions that are required.
A small number of published MAICs examined the relative efficacy of treatments for psoriasis. In particular, Papp et al. (Citation13) conducted a MAIC of Cal/BDP cream versus Cal/BDP foam. This existing MAIC utilizes the IPD from the Cal/BDP foam trials and the published aggregated outcomes from Cal/BDP cream trials (Citation10) in order to make anchored and unanchored comparisons. Bewley and colleagues have also previously completed an indirect comparison of Cal/BDP foam with apremilast, methotrexate, acitretin or fumaric acid esters for the treatment of plaque psoriasis (Citation14). Additionally, multiple MAICs have previously been undertaken to compare individual biologic plaque psoriasis therapies with one another (Citation15–17).
Overall, due to the data that were available to the study authors and the heterogeneity in the relevant trial populations, it was judged that a MAIC would be the most appropriate approach for comparing Cal/BDP cream with Cal/BDP foam.
Materials and methods
The data
For Cal/BDP cream treatment, the IPD were pooled from MC2-01-C2 (NCT03308799) to MC2-01-C7 (NCT03802344). Both trials were phase III investigator-blinded studies conducted in the USA and Europe, respectively. The population was adults with mild to moderate psoriasis according to Physicians Global Assessment (PGA). Patients were randomized to Cal/BDP cream, Cal/BDP gel or cream vehicle, once daily for eight weeks with an additional two weeks follow-up. The primary outcome was the number of patients achieving treatment success (defined overleaf) in PGA score and percentage change in Modified Psoriasis Area and Severity Index (mPASI) score at week 8 for MC2-01-C2 and MC2-01-C7, respectively.
For Cal/BDP foam, a comprehensive targeted literature review was undertaken to identify all relevant evidence. Only comparative RCT studies were included. The population of interest was adults with psoriasis of any severity. The search excluded studies in patients who had previously been treated with biologics, or where the results were reported for a mixed population only (without separate reporting of an eligible population). Eight trials of Cal/BDP foam were identified, reported upon in 39 different forms (conference abstracts, journal articles, study register entries, etc.). Of these, two trials were selected for use in the MAIC analyses; the PSO-ABLE trial (NCT02132936) and PSO-INSIGHTFUL (NCT02310646). Crucially, these two trials share a common comparator with the Cal/BDP cream trials, Cal/BDP gel. The presence of this common comparator allowed for an anchored MAIC to be undertaken, as recommended by the NICE Decision Support Unit (Citation12). The remaining six trials did not include Cal/BDP gel as a comparator and therefore were not used in the MAIC.
PSO-ABLE was a phase III investigator-blinded study conducted in France, the UK and the USA (Citation9,Citation18). The population was adults with mild to severe psoriasis according to PGA. Patients were randomized 4:4:1:1 to once-daily Cal/BDP foam, Cal/BDP gel, foam vehicle or gel vehicle for up to 12 weeks with an additional two weeks of follow-up. The primary outcome was PGA treatment success with Cal/BDP foam assessed at week four and Cal/BDP gel assessed at week eight.
PSO-INSIGHTFUL was an open-label crossover study conducted in Canada and Germany (Citation19). The population was adults with mild to severe psoriasis according to PGA. Following a four-week washout period, patients were randomized 1:1 to once-daily Cal/BDP foam for one week, followed by once-daily Cal/BDP gel for one week or vice versa. The primary outcome was treatment preference. A one-week treatment cycle was considered sufficient to assess the usability of each product while limiting the impact of treatment efficacy on preference.
Outcome measures
Eleven outcomes were chosen to assess efficacy, health-related quality of life (HRQoL) and treatment preferences.
Efficacy:
The proportion of patients achieving PGA treatment success, is defined as a PGA score of zero (clear) or one (almost clear) and with a minimum two-point decrease in PGA score from baseline.
The proportion of patients achieving a 75% reduction in the mPASI.
Health-related quality of life:
(3) The proportion of patients achieving Dermatology Life Quality Index (DLQI) satisfaction, is defined as a DLQI score of zero or one.
(4) The proportion of patients achieving a minimal clinically important difference (MCID) in DLQI score, is defined as a reduction in DLQI score of greater than or equal to five.
(5) The improvement in DLQI score from baseline.
Treatment satisfaction:
(6) Ease of application.
(7) Not greasy v1.
(8) Not greasy v2.
(9) Felt moisturizing.
(10) Easily incorporated into a daily routine.
(11) Overall satisfaction.
Efficacy and HRQoL outcomes were assessed at the end of the recommended treatment duration (week eight for Cal/BDP cream and Cal/BDP gel, and week four for Cal/BDP foam) (Citation20,Citation21). Efficacy and HRQoL outcomes were assessed in PSO-ABLE and were reported in Paul et al. (Citation9) and Griffiths et al. (Citation18), respectively.
Treatment satisfaction was assessed at week one for both treatments in PSO-INSIGHTFUL with outcomes reported in Hong et al. (Citation19). Treatment satisfaction was measured with the Psoriasis Treatment Convenience Scale (PTCS) in the Cal/BDP cream trials and the Topical Product Usability Questionnaire (TPUQ) in PSO-INSIGHTFUL. However, the two questionnaires share similar questions and matching of questions was undertaken as outlined in .
Table 1. PTCS questions matched TPUQ questions.
PTCS means were converted to TPUQ means as:
and the corresponding standard deviation as:
Statistical methods
MAICs were conducted according to the methods described in Signorovitch et al. (Citation11) and NICE TSD 18 (Citation12). All analyses were conducted using R version 4.1.0 or later (Citation22) and the MAIC package (Citation23). Owing to different pools of available data for each category of outcomes, the matching adjustment was undertaken separately for each of them. This enabled the sample size for each analysis to be maximized, as subjects were not required to have a complete record of all outcomes of interest in order to be analyzed. Further, the DLQI outcomes were split into two analysis sets so that the subset of patients ineligible for an MCID could be included in the analysis of the other two DLQI outcomes. There were four analysis sets for matching adjustment, overall.
Step one: Calculate weights
Individual patients from the pooled Cal/BDP cream trials were reweighted such that the average baseline characteristics matched the baseline characteristics reported in the Cal/BDP foam trial. Weights were calculated using a logistic propensity score model, with all matching variables included as covariates. Matched-on baseline characteristics consisted of all baseline variables that were available and consistently defined in both trials. Patients from the pooled Cal/BDP cream trials who were missing any of the matched characteristics or relevant outcomes were excluded. The distribution of the weights was inspected to check for the presence of large numbers of non-zero weights, indicating differences in trial populations, and for extreme weights that may skew results. The effective sample size (ESS) for the pooled Cal/BDP cream trials was calculated using the weights. A small ESS may indicate little overlap between the two trial populations, which may result in unstable estimates (Citation12).
Different weights were calculated using PSO-ABLE and PSO-INSIGHTFUL. Furthermore, for PSO-ABLE, different weights were calculated for efficacy outcomes, satisfaction/change in DLQI and MCID in DLQI. This was due to differences in the sample size in the pooled Cal/BDP cream IPD and was intended to maximize the available data.
Step two: Weight adjusted outcomes
The weighted IPD was used to calculate adjusted outcomes in the pooled Cal/BDP cream trials. Weighted odds ratios (ORs) and weighted mean differences (MD) were estimated to compare Cal/BDP cream versus Cal/BDP gel for dichotomous and continuous outcomes respectively. The adjusted outcomes were calculated using a weighted logistic regression (for ORs) or weighted linear regression (for MDs) including the treatment arm as the only covariate. This approach is equivalent to calculating adjusted outcomes using a weighted average but allows standard errors for the adjusted outcomes to be calculated using a robust sandwich estimator. Hence, a sandwich estimator was used to provide estimates of standard error accounting for the fact that weights are estimated rather than fixed or known (Citation12). These standard errors were used to calculate confidence intervals for the adjusted outcomes.
Step three: Treatment comparisons
The treatment effects of Cal/BDP cream and Cal/BDP foam were estimated using standard ITC methodology (Citation24). The outcomes of the reweighted Cal/BDP cream population from step two were compared with those reported in the Cal/BDP foam trial, to estimate ORs and MDs for Cal/BDP cream versus Cal/BDP foam, using Cal/BDP gel as a common comparator. For comparative purposes, the same analysis was performed using unweighted data from the pooled Cal/BDP cream trials (i.e. unadjusted outcomes).
Results
Baseline characteristics before and after matching, for each analysis, are presented in . After matching, baseline characteristics were balanced across the two arms. As a result of matching, the Cal/BDP cream effective sample size (ESS, a concept described in (Citation12)) decreased to 925 for efficacy outcomes, 946 for the DLQI satisfaction and improvement from baseline, 658 for MCID in DLQI and 680 for treatment satisfaction. In all cases, the distributions of the weights were deemed appropriate with no overly influential individuals. Histograms of weights can be found in the supplementary material.
Table 2. Baseline characteristics before and after matching.
The results of all analyses are presented in with forest plots shown in . Overall, the matching adjustment had a minor impact on the results compared to a naïve comparison. When measured by PGA success or mPASI75, Cal/BDP cream at week 8 was numerically lower than Cal/BDP foam at week 4 but this effect was not statistically significant. When measured by DLQI (satisfaction, MCID or improvement from baseline), patients treated with Cal/BDP cream improved more than Cal/BDP foam, but this effect was again not statistically significant. In terms of treatment satisfaction compared at week 1, Cal/BDP cream was superior to Cal/BDP foam for all domains except for ‘easily incorporated into daily routine’ where CAL/BDP cream scored higher than CAL/BDP foam but the effect did not reach statistical significance.
Figure 1. Forest plot of Cal/BDP cream at week 8 vs. Cal/BDP foam at week 4 for PGA success, mPASI75, DLQI satisfaction and MCID in DLQI score. Relative treatment effects are presented as odds ratios (OR) with 95% confidence intervals (CI). A value greater than 1 favors Cal/BDP cream.
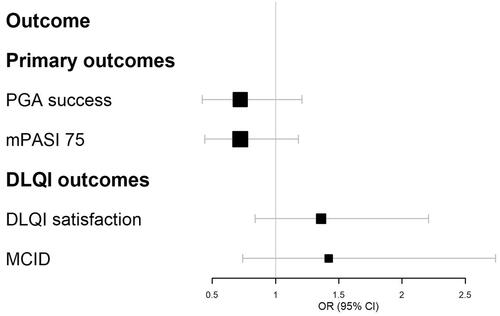
Figure 2. Forest plot of Cal/BDP cream at week 8 vs. Cal/BDP foam at week 4 for DLQI improvement from baseline and Cal/BDP cream vs. Cal/BDP foam treatment satisfaction outcomes at week 1. Relative treatment effects presented as mean differences (MD) with 95% confidence intervals (CI). A value greater than 0 favors Cal/BDP cream. DLQI improvement was initially evaluated as ‘change from baseline’ but has been converted to a positive number to aid interpretation.
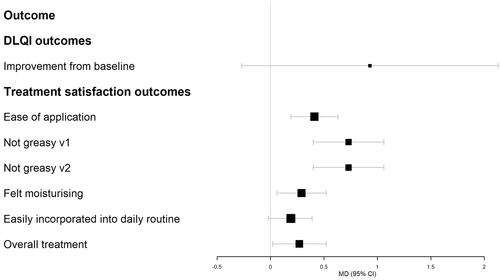
Table 3. Results.
Discussion
Summary of findings and clinical implications
In the absence of a head-to-head randomized control trial of Cal/BDP cream and Cal/BDP foam, an anchored MAIC was undertaken to assess Cal/BDP cream versus Cal/BDP foam for efficacy and HRQoL when used for their recommended treatment duration (8 weeks and 4 weeks for cream and foam respectively). Treatment satisfaction after 1 week of treatment was also assessed. After adjusting for differences in baseline characteristics, there are no significant differences in the efficacy outcomes for these fixed-dose Cal/BDP combinations. Therefore, it can be concluded that, when these treatments are used for their recommended treatment duration, they have similar efficacy. Likewise, there were no statistically significant differences in DLQI outcomes thereby indicating a similar impact of the two treatments on patient HRQoL. In five out of six treatment satisfaction domains, Cal/BDP cream was statistically superior to Cal/BDP foam. Therefore, patients appear to find Cal/BDP cream to be more convenient after 1 week of treatment.
Ease of use and convenience of application are, among others, important factors in improving patient adherence to topical treatments in psoriasis (Citation25). An improvement in the vehicle has the potential to result in significant clinical and patient benefits (Citation26). Topical vehicles formulated to provide minimal residue and fast absorption, allowing the patient to dress shortly after applying the drug and not staining clothing, are expected to promote satisfaction and consequently promote adherence (Citation27). The similar efficacy but higher patient satisfaction obtained in this study with Cal/BDP cream suggests that the improved cosmetic characteristics of this new aqueous formulation are better accepted than those of the greasier and less easy to apply Cal/BDP foam formulation. This enhanced acceptability could translate into greater adherence and, in the end, greater effectiveness of topical treatment.
Papp et al. published a MAIC of Cal/BDP foam versus Cal/BDP cream based on IPD from Cal/BDP foam trials (Citation13). The study focused exclusively on efficacy outcomes (mPASI and PGA) and included both anchored and unanchored analyses. The anchored analyses utilized the same data sources as the current study and reached the same conclusions in terms of the comparative efficacy at the recommended treatment durations (i.e. no statistically significant difference). Therefore, the similarity of the results validates the outputs of the anchored MAICs.
The results of the unanchored analyses differ markedly from those of the anchored analyses with Cal/BDP foam more efficacious than Cal/BDP cream in five of the six analyses conducted (Citation13). However, the unanchored MAICs are associated with substantial limitations. Firstly, unanchored MAICs do not utilize a common comparator and, therefore, are considered less robust than anchored comparisons (Citation12). Furthermore, the grouping of trials by region (Europe, United States) brought about different criteria for selecting trials for matching and the PSO-ABLE trial, the sole source of Cal/BDP foam data for the anchored analysis, was not included in the unanchored MAICs. Instead, the analyses were informed by two alternative Phase III trials (PSO-FAST, PSO-LONG) and two Phase II trials (LEO90100-35, −07). The PSO-LONG trial was an open-label trial which may have resulted in superficially high efficacy and, therefore, should not be compared with the RCT data since this introduces a bias in favor of Cal/BDP foam. The LEO90100-07 study is limited by the small sample size. Finally, many of the unanchored analyses selectively used only one of the phase III CAL/BDP cream trial data sets (NCT03308799) to inform Cal/BDP cream outcomes, rather than pooling aggregate data from the two phases III trials with a very similar design. Since efficacy results vary between the two phase III trials, it is likely that this omission is another biasing factor in favor of Cal/BDP foam in the unanchored MAICs.
Strengths
A MAIC approach was chosen to adjust for cross-trial population differences. All analyses were based on methodological guidance from the NICE Decision Support Unit (Citation12), including the strong recommendation to undertake an anchored analysis when possible.
The large pool of IPD for Cal/BDP cream, from two RCTs, was integral to the robustness of this analysis. The pooled trial data amounted to 1,101 patients across the Cal/BDP cream and active comparator arms. Since the weighting process results in a smaller EES than the original sample size it was important that the original dataset was large enough to withstand this reduction and maintain statistical power. Whilst there was no pre-determined suitable value, the relatively large ESS found in all analyses indicates sufficient overlap between trial populations and supports robust findings.
An array of clinical outcomes were assessed as part of this study and it is believed that this provides a multi-dimensional evaluation of the relative efficacy of the cream and foam versions of Cal/BDP fixed-dose combination. An analysis based on just one of these outcomes may miss certain nuances that are now evident, such as the potential preference that patients may have for Cal/BDP cream due to greater convenience despite similar efficacy outcomes.
Limitations
The purpose of a MAIC is to provide an indirect comparison of two treatments where adjustments are made for differences in patient populations. However, as with any indirect comparison, the MAIC method cannot adjust for differences in patient characteristics that were not observed, or not reported. Related to this point, data from the Cal/BDP foam studies were not clearly reported for all studies incorporated in the MAICs. For example, in the proportion of patients achieving an MCID, DLQI may be determined from a subset of patients with a baseline DLQI score of at least five, but this requirement was not explicitly defined in the publication by Griffiths et al. (Citation18). To complete the analysis of this outcome it was assumed that this requirement had been applied in the existing analysis.
Complete case analysis was used for the pooled IPD, whereby only patients with no missing data on the variables of interest were included. This approach was taken since data were assumed to be missing completely at random and because the proportion of missing data was low (less than 10% in all analyses). Under these assumptions, the impact of missing data is negligible. However, where these assumptions are not met, complete case analysis may yield biased and inefficient estimates of effect size.
For dichotomous outcomes, the underlying number of patients with or without an event was not reported in the Cal/BDP foam studies. These values were required to produce estimates of the relative efficacy and corresponding confidence intervals. Hence, the event counts were estimated using the absolute outcomes (proportions) and the corresponding number of patients at a given time point. Since the reported absolute outcomes were rounded this resulted in decimal values for the number of patients.
In the Cal/BDP foam study used for the treatment satisfaction analysis, the baseline characteristics reported were predominantly categorized. For example, BMI was reported as <25 kg/m2, 25–30 kg/m2 and >30 kg/m2. As a result, it was necessary to generate corresponding categorical variables in the IPD from the Cal/BDP cream trials. While matching was deemed acceptable using the categorical variables, using the mean values for continuous variables is preferred.
It is a limitation that many of the phase III trials of Cal/BDP foam did not share a common comparator with the Cal/BDP cream trials. There was just one foam trial that allowed for an anchored comparison of efficacy outcomes, whilst the remaining foam data could not be utilized. It is clear, from a timely comparison of our results with those presented by Papp et al. (Citation13), that the selection of data for use in indirect treatment comparisons is extremely influential on the results.
Lastly, the treatment satisfaction MAIC was based on data recorded after one week of therapy for each treatment and did not consider the length of treatment. However, a previous study by Stein Gold et al. did measure treatment satisfaction with Cal/BDP cream over 8 weeks of treatment, based on the PTCS, and found that treatment satisfaction did not diminish over this time period (Citation28).
Conclusion
This MAIC indicates that Cal/BDP cream and Cal/BDP foam have equivalent efficacy and HRQoL outcomes when used for the topical treatment of plaque psoriasis at their respective recommended treatment durations but based on treatment satisfaction assessments after 1 week of treatment, there is evidence that patients find Cal/BDP cream to be more convenient than foam.
Author contributions
All authors contributed to the statistical analysis plan, including the selection of outcomes of interest and decisions made around methodological assumptions. Erin Barker and Hannah Baker were responsible for undertaking the analysis. All authors contributed to the manuscript draft.
Supplemental Material
Download Zip (39.8 KB)Disclosure statement
Anthony Bewley has received ad hoc consultancy/travel grants from: Abbvie, Almirall, Bieirsdorf, Eli Lilly, Galderma, Janssen, Leo Pharma, Novartis, Sanofi, UCB. Erin Barker, Hannah Baker, Will Green and Brooke Avery are employees of York Health Economics Consortium, who were commissioned by Almirall and MC2 Therapeutics to provide consultancy and undertake the analysis. Jordi Galván and Aina Pi-Blanque are employed by Almirall S.A., Spain. Morten Praestegaard and Paw Trebbien are employed by MC2 Therapeutics A/S, Denmark.
Data availability statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting individual patient data are not available.
Additional information
Funding
References
- Langley R, Krueger G, Griffiths C. Psoriasis: epidemiology, clinical features, and quality of life. Annals Rheumatic Dis. 2005;64(suppl_2):ii18–ii23.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–1072.
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370(9583):263–271.
- SG LF, Arndt KA, LeBoit PE, et al. Topical therapies for psoriasis: improving management strategies and patient adherence. Semin Cutan Med Surg. 2016;35:S36–S44.
- Mason AR, Mason J, Cork M, et al. Topical treatments for chronic plaque psoriasis. Cochrane Database Syst Rev. 2009;15;(2):CD005028.
- Kragballe K, Noerrelund K, Lui H, et al. Efficacy of once‐daily treatment regimens with calcipotriol/betamethasone dipropionate ointment and calcipotriol ointment in psoriasis vulgaris. Br J Dermatol. 2004;150(6):1167–1173.
- Segaert S, Calzavara-Pinton P, de la Cueva P, et al. Long-term topical management of psoriasis: the road ahead. J Dermatolog Treat. 2022;33(1):111–120.
- Koo J, Tyring S, Werschler WP, et al. Superior efficacy of calcipotriene and betamethasone dipropionate aerosol foam versus ointment in patients with psoriasis vulgaris–a randomized phase II study. J Dermatolog Treat. 2016;27(2):120–127.
- Paul C, Stein Gold L, Cambazard F, et al. Calcipotriol plus betamethasone dipropionate aerosol foam provides superior efficacy vs. gel in patients with psoriasis vulgaris: randomized, controlled PSO‐ABLE study. J Eur Acad Dermatol Venereol. 2017;31(1):119–126.
- Pinter A, Green L, Selmer J, et al. A pooled analysis of randomized, controlled, phase 3 trials investigating the efficacy and safety of a novel, fixed dose calcipotriene and betamethasone dipropionate cream for the topical treatment of plaque psoriasis. J Eur Acad Dermatol Venereol. 2022;36(2):228–236.
- Signorovitch JE, Sikirica V, Erder MH, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15(6):940–947.
- Phillippo DM, Ades A, Dias S, et al. NICE DSU technical support document 18: methods for population-adjusted indirect comparisons in submissions to NICE. Report by the Decision Support Unit; 2016.
- Papp KA, Thoning H, Gerdes S, et al. Matching-adjusted indirect comparison of efficacy outcomes in trials of calcipotriol plus betamethasone dipropionate foam and cream formulations for the treatment of plaque psoriasis. J Dermatolog Treat. 2022:1–9.
- Bewley A, Shear N, Calzavara‐Pinton P, et al. Calcipotriol plus betamethasone dipropionate aerosol foam vs. apremilast, methotrexate, acitretin or fumaric acid esters for the treatment of plaque psoriasis: a matching‐adjusted indirect comparison. J Eur Acad Dermatol Venereol. 2019;33(6):1107–1115.
- Hampton P, Borg E, Hansen JB, et al. Efficacy of brodalumab and guselkumab in patients with moderate-to-Severe plaque psoriasis who are inadequate responders to ustekinumab: a matching adjusted indirect comparison. PTT. 2021;ume 11:123–131.
- Signorovitch JE, Wu EQ, Yu AP, et al. Comparative effectiveness without head-to-head trials. Pharmacoeconomics. 2010;28(10):935–945.
- Warren R, Brnabic A, Saure D, et al. Matching‐adjusted indirect comparison of efficacy in patients with moderate‐to‐severe plaque psoriasis treated with ixekizumab vs. secukinumab. Br J Dermatol. 2018;178(5):1064–1071.
- Griffiths CE, Gold LS, Cambazard F, et al. Greater improvement in quality of life outcomes in patients using fixed-combination calcipotriol plus betamethasone dipropionate aerosol foam versus gel: results from the PSO-ABLE study. Eur J Dermatol. 2018;28(3):356–363.
- Hong CH, Papp K, Lophaven K, et al. Patients with psoriasis have different preferences for topical therapy, highlighting the importance of individualized treatment approaches: randomized phase IIIb PSO‐INSIGHTFUL study. J Eur Acad Dermatol Venereol. 2017;31(11):1876–1883.
- Electronic Medicines Compendium (EMC). Wynzora 50 micrograms/g + 0.5 mg/g cream ∼ Summary of product characteristics. [cited 2022 March 17]. Available from: https://www.medicines.org.uk/emc/product/13200/smpc.
- Electronic Medicines Compendium (EMC). Enstilar Cutaneous Foam ∼ Summary of product characteristics. [cited 2021 March 8]. Available from: https://www.medicines.org.uk/emc/medicine/31833.
- Team RC. R: a language and environment for statistical computing. 2020, R Foundation for Statistical Computing; 2020. Vienna, Austria. Available from: https://www.R-project.org.
- Young R. maic: Matching-Adjusted Indirect Comparison. R package version 0.1.3; 2021.
- Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–691.
- Bewley A, Page B. Maximizing patient adherence for optimal outcomes in psoriasis. J Eur Acad Dermatol Venereol. 2011;25:9–14.
- Iversen L, Dauden E, Segaert S, et al. Reformulations of well‐known active ingredients in the topical treatment of psoriasis vulgaris can improve clinical outcomes for patients. J Eur Acad Dermatol Venereol. 2017;31(8):1271–1284.
- Vasconcelos V, Teixeira A, Almeida V, et al. Patient preferences for attributes of topical anti-psoriatic medicines. J Dermatolog Treat. 2019;30(7):659–663.
- Stein Gold L, Green L, Dhawan S, et al. A phase 3, randomized trial demonstrating the improved efficacy and patient acceptability of fixed dose calcipotriene and betamethasone dipropionate cream. J Drugs Dermatol. 2021;20(4):420–425.
