Abstract
Background
Although pulsed dye laser (PDL) is the treatment of choice for port-wine stains (PWS), clinical resistance to PDL has been observed in 20–30% of cases. Several alternative treatment modalities have been introduced; however, there is still a lack of definite recommendations regarding the optimal treatment for difficult-to-treat PWS.
Objective
We aimed to systematically review and analyze the comparative effectiveness among treatments for problematic PWS.
Methods & Materials
We systematically searched for comparative studies assessing treatments for patients with difficult-to-treat PWS through relevant biomedical databases until August 2022. A Network Meta-Analysis (NMA) was conducted to estimate the odds ratio (OR) for all pairwise comparisons. The primary outcome is the improvement of lesions of more than 25%.
Results
Of the 2498 studies identified, six treatments from five studies were available for NMA. Compared with 585 nm short-pulsed dye laser (SPDL), intense pulsed light (IPL) was the most effective in clearing lesions (OR 11.81, 95% CI 2.15 to 64.89, very low confidence rating), followed by 585 nm long-pulsed dye laser (LPDL) (OR 9.95, 95% CI 1.75 to 56.62, very low confidence rating). The 1064 nm NdYAG, 532 nm NdYAG, and LPDL >585 nm exhibited potential superiority over SPDL 585 nm, although statistical significance was not observed.
Conclusions
IPL and 585 nm LPDL are likely to be more effective than 585 nm SPDL for treating difficult-to-treat PWS. Well-designed clinical trials are warranted to confirm our findings.
Introduction
Port-wine stains (PWS) are capillary malformations characterized by increased abnormally dilated blood vessels in the dermis. Many potential etiologies of PWS were proposed, such as neuronal dysregulation (Citation1,Citation2), genetic alterations, specifically the GNAQ gene (Citation3), and overexpression of vascular endothelial growth factors (VEGF) and their components (Citation4–6). PWS affects 0.3–2.8 percent of newborns (Citation7,Citation8). PWS can progress in vessel diameter, darkening, and thickening of the lesions with time. The 585 nm short pulsed dye laser (SPDL) is the treatment of choice for PWS, especially in relatively small abnormal vessels in pediatric patients (Citation9,Citation10). However, 20–30% had clinical resistance to PDL (Citation11–14). Although the definitions and etiologies of resistant and recalcitrant PWS were not clearly defined, some studies described recalcitrant as having incomplete or failed clearance after 8–15 prior pulsed dye laser (PDL) treatments, while resistant was defined as showing no more improvement or being unresponsive to PDL treatment (Citation15–18). Hypertrophic PWS usually had poor responsiveness to treatment and was also included in this problematic group. Different types of ecstatic vessels, too small or too big of vessel diameter, deeper vessels, a high melanin content, thickening of the lesions, re-innervation of vascular components, and the formation of fibrous tissue as a result of previous treatments are all possible causes (Citation11,Citation19–21). PWS commonly affects the head and neck areas (Citation22), leading to elevated appearance concerns and a need for treatment. Individuals with challenging facial PWS are at a higher risk of psychological burdens, particularly related to facial disfigurement (Citation23,Citation24).
The dynamic changes of the lesions over time and the limitations of various treatment modalities lead to unpredictable and variable treatment responses, challenging the treatment of advanced PWS. There are several treatment modalities used for difficult-to-treat PWS, including PDL, intense pulsed light (IPL), 532 nm potassium titanyl phosphate (KTP), 1064 nm neodymium-doped yttrium aluminum garnet (NdYAG), 755 nm alexandrite, 800–983 nm diode laser, and photodynamic therapy (PDT) (Citation25). Until now, no treatment guidelines or definite conclusions exist for these problematic patients. Therefore, we performed a Systematic Review (SR) and Network Meta-Analysis (NMA) to evaluate the comparative effectiveness among available treatments for problematic or difficult-to-treat PWS.
Methods
This SR and NMA were reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement extension for NMA (Citation26). The study protocol was registered in PROSPERO (CRD42022353677).
Data sources and research strategy
We searched PubMed/MEDLINE, Scopus, EMBASE, and the Cochrane Library (clinical trials) for relevant literature from their inception to August 2022. We used a search strategy to find studies that compared available treatment modalities in difficult-to-treat PWS patients (hypertrophic, nodular, resistant, or recalcitrant) (Supplementary Tables 1–4). The authors (PP and SJ) also look at references from previous SR or Meta-Analysis (MA) studies that have already been done on the same topic.
Study selection and outcomes
Two investigators (SJ and VV) independently screened the titles and abstracts retrieved from database queries. Any disagreements about which to include or exclude were discussed and reviewed with the third investigator (PP).
We chose to include a randomized or placebo-controlled trial (RCT), a non-randomized experimental study, or a comparative observational study (such as a cohort or case-control study) based on the following criteria: (1) Patients of all ages with problematic or difficult-to-treat PWS; (2) All available treatment modalities for difficult-to-treat PWS, including both laser and non-laser treatments; and (3) A reported clearance scale of 25% lightening. In most studies concerning PWS, the term “ineffective” is used to describe scenarios where there is no clearance or clearance below 20 or 25% (Citation27–32). Achieving a clearance of more than 25% is deemed to be minimally clinically significant for individuals with resistant or recalcitrant PWS, and it has the potential to positively impact the quality of life, especially for the remaining facial area following prior treatment.
We excluded reviews (e.g. narrative reviews, SRs, and MAs), clinical practice guidelines, non-human studies, non-comparative studies (e.g. case reports, case series), non-English language publications, and no available full-text studies.
Data extraction and risk of bias assessment
Two investigators (SJ and VV) independently extracted the data, including study characteristics, patient characteristics, PWS characteristics, interventions, and outcomes. Any discrepancies were discussed and resolved with the third investigator (PP).
Two investigators (SJ and KT) independently assessed the risk of bias using the Cochrane revised Risk of Bias in randomized trials (RoB 2) (Citation33) and Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) tools for RCTs and observational studies (Citation34), respectively. The risk of bias for each of the five domains in RoB 2 was assessed using the algorithm proposed by the RoB 2 Development Group (Citation33). If all domains received low ratings, the studies were deemed to have a low risk of bias. On the other hand, if any domain received a high rating, the studies were classified as having a high risk of bias. The remaining studies were rated as some concerns. The seven domains of ROBINS-I were assessed and assigned ratings of low, moderate, serious, or critical risk of bias using the signaling questions provided in the ROBINS-I guidance documents. The overall risk of bias judgment for ROBINS-I was determined by identifying the highest level of risk of bias across all domains. Any discrepancies were resolved through discussion with the other investigators (PP and VV).
Data synthesis and analysis
All parts of data synthesis and analysis were done by PP and SJ, and the results were discussed with the team. Pairwise meta-analysis was utilized to estimate pooled odds ratio (OR) with a 95% confidence interval (CI) of direct comparison under the random-effects model (DerSimonian and Laird). The Cochrane Q test and I2 statistics were performed to identify the heterogeneity in each pairwise comparison. Random-effects NMA was performed to combine direct and indirect evidence of all treatment modalities (Citation35). Treatment effect estimates were presented as OR and 95% CI. For examining the agreement between the direct and indirect effects, the inconsistency model was employed to evaluate global consistency. Loop-specific consistency was used to identify the inconsistency within each triangular or quadratic loop. The inconsistency within the network of treatments was examined using the node-splitting approach. All treatment modalities were then ranked according to their surface area under the cumulative ranking (SUCRA) value and presented in rankograms. Publication bias was evaluated and illustrated using a comparison-adjusted funnel plot.
Subgroup analysis was performed by types of PWS, resistant or hypertrophy. Initially, a sensitivity analysis was planned by excluding studies with a sample size of less than the 10th percentile to verify the robustness of the primary outcome. However, due to the limited number of studies, this could not be appropriately conducted. Nonetheless, a leave-one-out sensitivity analysis (excluding one study at each analysis) was performed to examine the robustness of the estimated primary results. All analyses were performed using STATA version 17 (StataCorp, Lakeway, TX). Statistical significance was set at a p-value of less than 0.05.
Grading the strength of evidence
The strength of evidence was evaluated independently by two investigators (PP and SJ) using the Confidence in Network Meta-Analysis (CINeMA) approach (Citation36). The confidence in the estimated treatment effect from the NMA was rated as high, moderate, low, or very low based on the within-study risk of bias, reporting bias, indirectness, imprecision, heterogeneity, and incoherence. The confidence rating for RCTs would start at high, whereas the rating would start at a moderate level for non-randomized and observational studies. Two steps would drop the rating for each major concern identified and one step for each of some concerns. However, this rule may be altered as appropriate if the domains of CINeMA with concerns were interconnected.
Results
Characteristics and quality of included studies
A total of 2498 studies were identified. After an initial screening, 2420 studies with irrelevant titles and abstracts were excluded. Seventy-eight studies were retrieved for further eligibility assessment. Finally, the qualitative synthesis included eight eligible comparative studies with 308 difficult-to-treat PWS (Supplementary Figure 1) (Citation19,Citation37–43). The reasons for exclusion of 70 studies from this review are shown in Supplementary Table 5. There were two RCTs (Citation42,Citation43), four prospective cohort studies (Citation38–41), and two retrospective cohort studies (Citation19,Citation37). The characteristics of the included studies are described in . The proportion of females (53.7%) was slightly higher than males. The age range was 2–78 years. The included studies examined the effect of treatment on two types of PWS, resistant and hypertrophic. The definition of treatment resistant for each study are shown in Supplementary Table 6. The majority of PWS were located in the head and neck region (70.4%).
Table 1. Characteristics of the included studies.
Five studies examined the effectiveness of different types of energy-based device, such as SPDL, long-pulsed dye laser (LPDL), and IPL. Supplementary Table 7 shows the details on each intervention arm, including parameter setting, treatment duration, and assessment period for each included study. The reported side effects of the available modalities in our study were scarring, permanent hyperpigmentation, and permanent hypopigmentation . Scarring was observed in 1.9% of patients treated with >585 nm LPDL (2 out of 108 patients), 2.9% of patients treated with 585 nm LPDL (1 out of 35 patients), 10% of patients treated with bleomycin therapy (1 out of 10 patients), and 28.6% of patients treated with alexandrite laser (6 out of 21 patients). Additionally, permanent hyperpigmentation occurred in 10% of patients treated with IPL (3 out of 30 patients) and in 30% of patients treated with bleomycin therapy (3 out of 10 patients). Furthermore, permanent hypopigmentation was observed in 3.3% of patients treated with IPL (1 out of 30 patients) (Supplementary Table 8).
An RCT by Carlsen et al. (Citation42) was rated as having low risk, whereas another one by Horbach et al. (Citation43) was rated with some concerns based on the RoB2 tool (Supplementary Table 9 and 10). The majority of non-randomized studies were evaluated using the ROBINS-I and determined to have a moderate risk of bias, with the exception of the study conducted by Chang et al. which was assigned a serious risk rating (Supplementary Table 11).
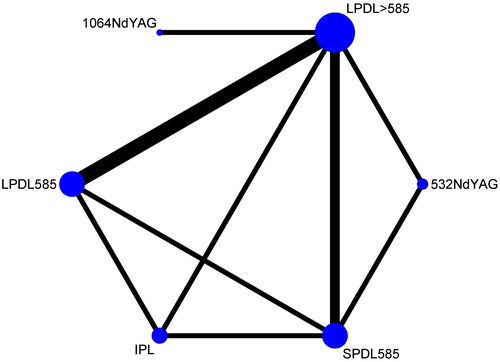
The six available modalities from five non-randomized studies (259 patients) (Citation37–41) were included in the network comparison (585 nm SPDL, 585 nm and >585 nm LPDL, incoherent polychromatic filtered flashlamps IPL, 532 nm, and 1064 nm NdYAG laser. The 755 nm alexandrite laser, along with two enhanced methods for drug delivery, namely electrotherapy combining bleomycin sclerotherapy and the non-laser thermomechanical system (Tixel device) combining rapamycin (RPM), had not been compared to other modalities in this NMA. The network diagrams illustrated all comparisons among the six available treatment modalities ().
Figure 1. Networks of all treatment comparisons (5 studies, 15 treatment pairs, 259 difficult-to-treat PWS). the size of nodes corresponds to the number of treatment trials. Treatment pairs with direct evidence are connected with solid black lines. The thickness of lines corresponds to the number of participants within each comparison.
Ability to achieve more than 25 percent lightening of PWS
In comparison to SPDL 585 nm, IPL demonstrated significant effectiveness in improving the lesion (OR 11.81, 95% CI 2.15 to 64.89), followed by LPDL 585 nm (OR 9.95, 95% CI 1.75 to 56.62). The 1064 nm NdYAG, 532 nm NdYAG, and LPDL >585 nm showed a tendency to be superior to SPDL 585 nm, although statistical significance was not observed. The pooled effect estimates from direct and indirect evidence for each treatment method were summarized in the league table (). All treatment modalities were ranked based on their SUCRA ().
Figure 2. The surface under the cumulative ranking curve (SUCRA) of each available treatment modality for clinical improvements.
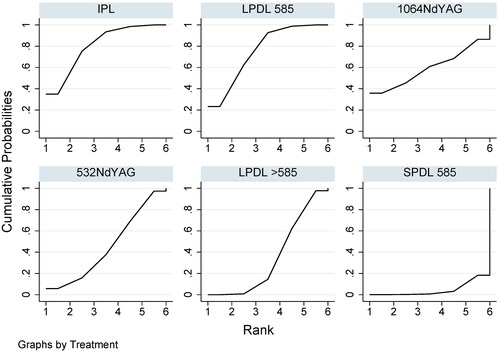
Table 2. League table of odds ratio (OR) for clinical improvements among all available pairwise comparisons.
4.36
Subgroup analysis
Of the five studies included in this NMA, three focused on resistant PWS and two focused on hypertrophic PWS. The subgroup analysis results show that the treatment ranking from the main analysis was preserved in both subgroups (Supplementary Table 12 and Supplementary Figures 2 and 3). In other words, the types of PWS, whether hypertrophic or resistant, had minimal impact on the treatment effects.
Sensitivity analysis
Leave-one-out sensitivity analysis confirmed the robustness of the primary analysis with similar treatment ranking (Supplementary Table 13).
Transitivity, heterogeneity, inconsistency, reporting bias, and strength of evidence assessment
The included patient domain, the intervention definitions, and the evaluated outcome were similar across studies and likely to fulfill the transitivity assumption of this NMA. Heterogeneity was identified in several pairs of treatment comparisons. For this NMA, there was no evidence of inconsistency according to the node-splitting (Supplementary Table 14), and the loop-specific approach (Supplementary Table 15). The global inconsistency test also showed non-significance results (p = 0.530). As there was only a small number of studies available for inclusion in this SR and NMA, reporting bias was regarded as some concerns. The CINeMA evaluation of the primary outcome is shown in Supplementary Table 16. All pairwise comparisons had major concerns for within-study bias as all included studies were non-randomized studies with moderate risk of bias. The domains with a high proportion of pairwise comparisons that raised major concerns were imprecision and heterogeneity. Even though all pairwise had no concerns for incoherence and indirectness, the confidence rating of all estimated treatment effects was graded at very low confidence.
Discussion
To our knowledge, this is the first NMA to compare the effectiveness of available treatment modalities for difficult-to-treat PWS. Our study demonstrated that IPL and 585 nm LPDL were likely more effective modalities than 585 nm SPDL based on the SUCRA ranking. Whether the lesion is classified as resistance or hypertrophy is unlikely to significantly affect the comparative effectiveness of all treatment modalities examined in this study. This is due to the presence of multiple factors contributing to resistant PWS, with hypertrophy being merely one of them. The previous MA by Cinkara et al. demonstrated that PDL was effective for treatment-naïve capillary malformations of the head and neck region when compared with 532 nm NdYAG laser, IPL, and PDT (Citation44). However, unpredictable and variable results often happen in any situation.
From the principle of selective photothermolysis, the best treatment for PWS should be a laser set to damage the hemoglobin determined by wavelength, appropriate energy to heat red blood cells and vessel endothelium, and a pulse duration equal to or less than the thermal relaxation time of vessels with a diameter of about 20 µm (Citation45). The absorption coefficient of oxyhemoglobin also affects the response to treatment. The oxyhemoglobin absorption peaks at 418, 452, and 577 nm, whereas 577 nm induces a better penetration depth (0.5 mm) and less absorption by melanin. The 585 nm wavelength found similar vascular absorption but had deeper penetration (1.2 mm) (Citation46). Theoretically, longer wavelengths, a larger spot size, higher fluence, and a longer pulse duration tend to increase treatment efficacy (Citation31). In difficult-to-treat PWS, the deeper and more ectatic vessels may respond better to a longer pulse duration (more than 0.45 ms). The optimum pulse duration for most PWS, which have a diameter of 20–150 µm (Citation18,Citation47) should be set between 1 and 10 msec (Citation48,Citation49).
According to these principles, IPL and 585 nm LPDL should be effective for further clearing difficult-to-treat PWS because IPL emits incoherent broad-spectrum light, which can be determined in wavelength by cutoff filters to the appropriate absorption spectrum of vascular lesions (500–1100 nm) (Citation50,Citation51). The advantages of IPL are its capability for longer and variable pulse durations with variable fluence energy-induced damage to different depths and diameters of vessels (Citation52).The 585 nm LPDL could potentially cause greater damage to larger ectatic vessels than the 585 nm SPDL. Although 595 nm can penetrate deeper, the absorption coefficient of hemoglobin is lower than that of 585 nm (Citation46). The 1064 nm NdYAG and 755 nm alexandrite lasers can also penetrate 50–75% deeper but with lower hemoglobin absorption than PDL (Citation53–55). While the 532 nm NdYAG laser was highly absorbed by hemoglobin, it had lower tissue penetration due to its short wavelength (Citation56,Citation57). From our network comparison, wavelengths longer than 585 nm LPDL, 532 nm, and 1064 nm NdYAG lasers had not demonstrated greater efficacy than other modalities. Unfortunately, the Tixel-induced RPM, the combination of electrosclerotherapy and bleomycin sclerotherapy, and 755 nm alexandrite have not been linked to other modalities in network comparisons.
The comparative response to each treatment was often difficult. The tested area may not represent the whole lesion due to the heterogeneity of the lesions. Due to the time changes, the lesions changed and had variable treatment responses. The treatment in early childhood is more responsive than at a later age (Citation58). The vessels with a diameter of less than 20 µm or more than 150 µm had poor responses (Citation59). The red lesions usually represent medium-sized vessels, while pink tends to be smaller and purple is larger, indicating a poor response to treatment (Citation38). Facial PWS had a better response than non-facial PWS. The vessel’s depth, more than 400 µm, had a poor response (Citation59). The smaller lesions (less than 20 cm2) were significantly lighter than those larger (Citation60). Further studies should aim to examine the effect modification of the age of patients at treatment and the type, color, location, and size of the lesions.. It may be necessary to evaluate a larger sample size for each treatment modality. Additionally, the best way to increase the accuracy of comparisons in future studies is to encourage the use of both subjective and objective measurements of effectiveness..
Furthermore, it is crucial to closely monitor the side effects of each intervention, particularly scarring caused by alexandrite laser and bleomycin therapy, as well as dyspigmentation resulting from bleomycin therapy and IPL. Alexandrite laser, in comparison to PDL, exhibits a narrower therapeutic window due to its deeper penetration and lower absorption coefficient for Hb. To achieve adequate vascular damage, higher fluence settings are required, which can lead to an increase in side effects (Citation17,Citation53,Citation61). Previous studies have reported that pulse durations exceeding 3 msec are associated with a higher incidence of side effects (Citation54,Citation55). Bleomycin therapy exerts sclerosing and cytotoxic effects on vascular and surrounding tissues, leading to damage, and potentially resulting in fibrosis (Citation62). Higher doses and improper technique can further contribute to potential side effects (Citation43). Additionally, due to the broad absorption spectrum of IPL treatment, it can cause nonspecific thermal damage to chromophores other than Hb, such as water and melanin (Citation63). Consequently, dyspigmentation and other side effects may arise (Citation64).
The major strengths of this study include the extensive sensitivity analyses that confirmed the robustness of the study results, the scientific evidence that could explain our estimated effectiveness, and the combination of direct and indirect evidence using the NMA approach. However, there are several limitations that need to be addressed. First, this study was based on six observational studies and only two RCTs, with a small number of patients in each study. In addition, more than half were published before 2010. Improvements in treatment techniques and access to care over the years may have partially enhanced patient outcomes. Second, although the transitivity assumption could be inferred from the coherence of the included studies’ core designs, methodological heterogeneity among studies was still another potential source of intransitivity. Third, statistical evidence of heterogeneity was detected in several treatment pairs, and therefore a random-effects model was employed to address this specific issue. Finally, our study did not include several interesting studies due to the absence of comparative controls, disconnected network of treatment, or incompliance outcome definitions, such as those on PDT, that looked at a different level of clearing, such as a clearing of more than 20%.
Conclusions
According to the SUCRA ranking, this SR and NMA indicate that IPL and 585 nm LPDL are more effective than 585 nm SPDL for treating difficult-to-treat PWS. However, it is important to note that the confidence rating for the effect estimates is very low, suggesting that the actual effectiveness of the treatment modalities included in the study could differ significantly. Therefore, it is necessary to conduct additional well-designed prospective studies or clinical trials to obtain a higher level of evidence and establish more reliable clinical recommendations.
Supplemental Material
Download PDF (661.1 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
Additional information
Funding
References
- Rydh M, Malm M, Jernbeck J, et al. Ectatic blood vessels in port-wine stains lack innervation: possible role in pathogenesis. Plast Reconstr Surg. 1991;87(3):1–8. doi: 10.1097/00006534-199103000-00003.
- Smoller BR, Rosen S. Port-wine stains. A disease of altered neural modulation of blood vessels? Arch Dermatol. 1986;122(2):177–179. doi: 10.1001/archderm.1986.01660140067019.
- Frigerio A, Wright K, Wooderchak-Donahue W, et al. Genetic variants associated with port-wine stains. PLoS One. 2015;10(7):e0133158. doi: 10.1371/journal.pone.0133158.
- Tan W, Wang J, Zhou F, et al. Coexistence of eph receptor B1 and ephrin B2 in port-wine stain endothelial progenitor cells contributes to clinicopathological vasculature dilatation. Br J Dermatol. 2017;177(6):1601–1611. doi: 10.1111/bjd.15716.
- Vural E, Ramakrishnan J, Cetin N, et al. The expression of vascular endothelial growth factor and its receptors in port-wine stains. Otolaryngol Head Neck Surg. 2008;139(4):560–564. doi: 10.1016/j.otohns.2008.07.015.
- Fölster-Holst R, Shukla R, Kassir M, et al. Treatment update of port-wine stain: a narrative review. J Drugs Dermatol. 2021;20(5):515–518.
- Jacobs AH, Walton RG. The incidence of birthmarks in the neonate. Pediatrics. 1976;58(2):218–222. doi: 10.1542/peds.58.2.218.
- Lorenz S, Maier C, Segerer H, et al. Skin changes in newborn infants in the first 5 days of life. Hautarzt. 2000;51(6):396–400. doi: 10.1007/s001050051140.
- Garden JM, Bakus AD. Laser treatment of port-wine stains and hemangiomas. Dermatol Clin. 1997;15(3):373–383. doi: 10.1016/s0733-8635(05)70447-2.
- Ashinoff R, Geronemus RG. Flashlamp-pumped pulsed dye laser for port-wine stains in infancy: earlier versus later treatment. J Am Acad Dermatol. 1991;24(3):467–472. doi: 10.1016/0190-9622(91)70075-d.
- Grillo E, González-Muñoz P, Boixeda P, et al. Alexandrite laser for the treatment of resistant and hypertrophic port wine stains: a clinical, histological and histochemical study. Actas Dermosifiliogr. 2016;107(7):591–596. doi: 10.1016/j.ad.2016.04.016.
- Rajaratnam R, Laughlin SA, Dudley D. Pulsed dye laser double-pass treatment of patients with resistant capillary malformations. Lasers Med Sci. 2011;26(4):487–492. doi: 10.1007/s10103-011-0913-2.
- Periyasamy MK, Sekar CS, Rai R. Effectiveness of dual sequential wavelength laser in the treatment of portwine stains – a retrospective study. Indian Dermatol Online J. 2019;10(4):418–421. doi: 10.4103/idoj.IDOJ_483_18.
- Han Y, Ying H, Zhang X, et al. Retrospective study of photodynamic therapy for pulsed dye laser-resistant port-wine stains. J Dermatol. 2020;47(4):348–355. doi: 10.1111/1346-8138.15238.
- Taquin H, Lacour JP, Le Duff F, et al. Treatment of resistant port-wine stains with bosentan and pulsed dye laser: a pilot prospective study. J Eur Acad Dermatol Venereol. 2016;30(8):1432–1434. doi: 10.1111/jdv.13275.
- Zhang M, Wu Q, Lin T, et al. Hematoporphyrin monomethyl ether photodynamic therapy for the treatment of facial port-wine stains resistant to pulsed dye laser. Photodiagnosis Photodyn Ther. 2020;31:101820. doi: 10.1016/j.pdpdt.2020.101820.
- Tierney EP, Hanke CW. Alexandrite laser for the treatment of port wine stains refractory to pulsed dye laser. Dermatol Surg. 2011;37(9):1268–1278. doi: 10.1111/j.1524-4725.2011.02079.x.
- Alster TS, Tanzi EL. Combined 595-nm and 1,064-nm laser irradiation of recalcitrant and hypertrophic port-wine stains in children and adults. Dermatol Surg. 2009;35(6):914–919. doi: 10.1111/j.1524-4725.2009.01155.x.
- Artzi O, Mehrabi JN, Heyman L, et al. Treatment of port wine stain with tixel-induced rapamycin delivery following pulsed dye laser application. Dermatol Ther. 2020;33(1):e13172.
- Walker EP, Butler P h, Pickering JW, et al. Histology of port wine stains after copper vapour laser treatment. Br J Dermatol. 1989;121(2):217–223. doi: 10.1111/j.1365-2133.1989.tb01801.x.
- Lanigan SW. Port-wine stains unresponsive to pulsed dye laser: explanations and solutions. Br J Dermatol. 1998;139(2):173–177. doi: 10.1046/j.1365-2133.1998.02351.x.
- Lipner SR. Topical adjuncts to pulsed dye laser for treatment of port wine stains: review of the literature. Dermatol Surg. 2018;44(6):796–802. doi: 10.1097/DSS.0000000000001507.
- Wanitphakdeedecha R, Ng JNC, Yan C, et al. Quality of life and psychological effects of port-wine stain: a review of literature. Clin Cosmet Investig Dermatol. 2021;14:681–690. doi: 10.2147/CCID.S315804.
- van der Horst CMAM, de Borgie CAJM, Knopper JL, et al. Psychosocial adjustment of children and adults with port wine stains. Br J Plast Surg. 1997;50(6):463–467. doi: 10.1016/S0007-1226(97)90335-0.
- Kwiek B, Ambroziak M, Osipowicz K, et al. Treatment of previously treated facial capillary malformations: results of single-center retrospective objective 3-dimensional analysis of the efficacy of large spot 532 nm lasers. Dermatol Surg. 2018;44(6):803–813. doi: 10.1097/DSS.0000000000001447.
- Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. doi: 10.7326/M14-2385.
- Liu J, Zhou J, Hu D, et al. Retrospective analysis of hemoporfin-mediated photodynamic therapy in the treatment of naïve port-wine stains. Photodiagnosis Photodyn Ther. 2022;39:103003. doi: 10.1016/j.pdpdt.2022.103003.
- Li DC, Nong X, Hu Z, et al. Efficacy and related factors analysis in HMME-PDT in the treatment of port wine stains. Photodiagnosis Photodyn Ther. 2020;29:101649. doi: 10.1016/j.pdpdt.2020.101649.
- Shi W, Wang J, Lin Y, et al. Treatment of port wine stains with pulsed dye laser: a retrospective study of 848 cases in Shandong province, People’s Republic of China. Drug Des Devel Ther. 2014;8:2531–2538.
- Xiao Q, Li Q, Yuan KH, et al. Photodynamic therapy of port-wine stains: long-term efficacy and complication in Chinese patients. J Dermatol. 2011;38(12):1146–1152. doi: 10.1111/j.1346-8138.2011.01292.x.
- Kono T, Sakurai H, Takeuchi M, et al. Treatment of resistant port-wine stains with a variable-pulse pulsed dye laser. Dermatol Surg. 2007;33(8):951–956. doi: 10.1111/j.1524-4725.2007.33197.x.
- Khandpur S, Sharma VK. Assessment of efficacy of the 595-nm pulsed dye laser in the treatment of facial port-wine stains in Indian patients. Dermatol Surg. 2016;42(6):717–726. doi: 10.1097/DSS.0000000000000723.
- Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898.
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919.
- Chaimani A, Higgins JPT, Mavridis D, et al. Graphical tools for Network Meta-Analysis in STATA. PLoS One. 2013;8(10):e76654. doi: 10.1371/journal.pone.0076654.
- Nikolakopoulou A, Higgins JPT, Papakonstantinou T, et al. CINeMA: an approach for assessing confidence in the results of a Network Meta-Analysis. PLoS Med. 2020;17(4):e1003082. doi: 10.1371/journal.pmed.1003082.
- Chang CJ, Kelly KM, Van Gemert MJC, et al. Comparing the effectiveness of 585-nm vs 595-nm wavelength pulsed dye laser treatment of port wine stains in conjunction with cryogen spray cooling. Lasers Surg Med. 2002;31(5):352–358. doi: 10.1002/lsm.10102.
- Woo WK, Jasim ZF, Handley JM. Evaluating the efficacy of treatment of resistant port-wine stains with variable-pulse 595-nm pulsed dye and 532-nm Nd: YAG lasers. Dermatol Surg. 2004;30(2 Pt 1):158–162; discussion 162. doi: 10.1046/j.1076-0512.2003.30055.x.
- Yung A, Sheehan-Dare R. A comparative study of a 595-nm with a 585-nm pulsed dye laser in refractory port wine stains. Br J Dermatol. 2005;153(3):601–606. doi: 10.1111/j.1365-2133.2005.06707.x.
- Yang MU, Yaroslavsky AN, Farinelli WA, et al. Long-pulsed neodymium:yttrium-aluminum-garnet laser treatment for port-wine stains. J Am Acad Dermatol. 2005;52(3 Pt 1):480–490. doi: 10.1016/j.jaad.2004.10.876.
- Babilas P, Schreml S, Eames T, et al. Split-face comparison of intense pulsed light with short- and long-pulsed dye lasers for the treatment of port-wine stains. Lasers Surg Med. 2010;42(8):720–727. doi: 10.1002/lsm.20964.
- Carlsen BC, Wenande E, Erlendsson AM, et al. A randomized side-by-side study comparing alexandrite laser at different pulse durations for port wine stains. Lasers Surg Med. 2017;49(1):97–103. doi: 10.1002/lsm.22532.
- Horbach SER, Wolkerstorfer A, Jolink F, et al. Electrosclerotherapy as a novel treatment option for hypertrophic capillary malformations: a randomized controlled pilot trial. Dermatol Surg. 2020;46(4):491–498. doi: 10.1097/DSS.0000000000002191.
- Cinkara G, Langbroek GB, van der Horst CMAM, et al. Therapeutic strategies for untreated capillary malformations of the head and neck region: a systematic review and Meta-Analyses. Am J Clin Dermatol. 2021;22(5):603–614. doi: 10.1007/s40257-021-00616-5.
- Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220(4596):524–527. doi: 10.1126/science.6836297.
- Tan OT, Murray S, Kurban AK. Action spectrum of vascular specific injury using pulsed irradiation. J Invest Dermatol. 1989;92(6):868–871. doi: 10.1111/1523-1747.ep12696885.
- Anderson RR, Parrish JA. Microvasculature can be selectively damaged using dye lasers: a basic theory and experimental evidence in human skin. Lasers Surg Med. 1981;1(3):263–276. doi: 10.1002/lsm.1900010310.
- Van Gemert MJC, Welch AJ, Amin AP. Is there an optimal laser treatment for port wine stains? Lasers Surg Med. 1986;6(1):76–83. doi: 10.1002/lsm.1900060116.
- Dierickx CC, Casparian JM, Venugopalan V, et al. Thermal relaxation of port-wine stain vessels probed in vivo: the need for 1-10-millisecond laser pulse treatment. J Invest Dermatol. 1995;105(5):709–714. doi: 10.1111/1523-1747.ep12324514.
- Reynolds N, Exley J, Hills S, et al. The role of the lumina intense pulsed light system in the treatment of port wine stains–a case controlled study. Br J Plast Surg. 2005;58(7):968–980. doi: 10.1016/j.bjps.2005.04.006.
- Faurschou A, Togsverd-Bo K, Zachariae C, et al. Pulsed dye laser vs. intense pulsed light for port-wine stains: a randomized side-by-side trial with blinded response evaluation. Br J Dermatol. 2009;160(2):359–364. doi: 10.1111/j.1365-2133.2008.08993.x.
- Ozdemir M, Engin B, Mevlitoğlu I. Treatment of facial port-wine stains with intense pulsed light: a prospective study. J Cosmet Dermatol. 2008;7(2):127–131. doi: 10.1111/j.1473-2165.2008.00375.x.
- Izikson L, Nelson JS, Anderson RR. Treatment of hypertrophic and resistant port wine stains with a 755 nm laser: a case series of 20 patients. Lasers Surg Med. 2009;41(6):427–432. doi: 10.1002/lsm.20793.
- McGill DJ, MacLaren W, Mackay IR. A direct comparison of pulsed dye, alexandrite, KTP and Nd: YAG lasers and IPL in patients with previously treated capillary malformations. Lasers Surg Med. 2008;40(6):390–398. doi: 10.1002/lsm.20638.
- Li L, Kono T, Groff WF, et al. Comparison study of a long-pulse pulsed dye laser and a long-pulse pulsed alexandrite laser in the treatment of port wine stains. J Cosmet Laser Ther. 2008;10(1):12–15. doi: 10.1080/14764170701817023.
- Chowdhury MM, Harris S, Lanigan SW. Potassium titanyl phosphate laser treatment of resistant port-wine stains. Br J Dermatol. 2001;144(4):814–817. doi: 10.1046/j.1365-2133.2001.04138.x.
- Apfelberg DB, Bailin P, Rosenberg H. Preliminary investigation of KTP/532 laser light in the treatment of hemangiomas and tattoos. Lasers Surg Med. 1986;6(1):38. doi: 10.1002/lsm.1900060110.
- van der Horst CM, Koster PH, de Borgie CA, et al. Effect of the timing of treatment of port-wine stains with the flash-lamp-pumped pulsed-dye laser. N Engl J Med. 1998;338(15):1028–1033. doi: 10.1056/NEJM199804093381504.
- Savas JA, Ledon JA, Franca K, et al. Pulsed dye laser-resistant port-wine stains: mechanisms of resistance and implications for treatment. Br J Dermatol. 2013;168(5):941–953. doi: 10.1111/bjd.12204.
- Morelli JG, Weston WL, Huff JC, et al. Initial lesion size as a predictive factor in determining the response of port-wine stains in children treated with the pulsed dye laser. Arch Pediatr Adolesc Med. 1995;149(10):1142–1144. doi: 10.1001/archpedi.1995.02170230096014.
- Jasim ZF, Handley JM. Treatment of pulsed dye laser-resistant port wine stain birthmarks. J Am Acad Dermatol. 2007;57(4):677–682. doi: 10.1016/j.jaad.2007.01.019.
- Horbach SER, Rigter IM, Smitt JHS, et al. Intralesional bleomycin injections for vascular malformations: a systematic review and Meta-Analysis. Plast Reconstr Surg. 2016;137(1):244–256. doi: 10.1097/PRS.0000000000001924.
- Cai Y, Zhu Y, Wang Y, et al. Intense pulsed light treatment for inflammatory skin diseases: a review. Lasers Med Sci. 2022;37(8):3085–3105. doi: 10.1007/s10103-022-03620-1.
- Babilas P, Schreml S, Szeimies RM, et al. Intense pulsed light (IPL): a review. Lasers Surg Med. 2010;42(2):93–104. doi: 10.1002/lsm.20877.
