Abstract
Background
Lebrikizumab, a high-affinity monoclonal antibody targeting IL-13, previously demonstrated clinical efficacy in three randomized, double-blind, placebo-controlled Phase 3 trials that included adults and adolescents with moderate-to-severe atopic dermatitis (AD): ADvocate1, ADvocate2, and ADhere.
Aim
This subset analysis evaluated 16-week physician- and patient-reported outcomes of lebrikizumab in the adolescent patients enrolled in these three trials.
Methods
Eligible adolescents (≥12 to <18 years weighing ≥40kg) were randomized 2:1 to subcutaneous lebrikizumab (500 mg loading doses at baseline and Week 2 followed by 250 mg every 2 weeks) or placebo as monotherapy in ADvocate1&2, and in combination with topical corticosteroids (TCS) in the ADhere study. Week 16 analyses included clinical efficacy outcomes (IGA (0,1) with ≥2-point improvement, EASI 75, EASI 90), patient-reported Pruritus NRS ≥4-point improvement and Sleep-Loss Scale ≥2-point improvement.
Results
Pooled ADvocate1&2 16-week results in lebrikizumab (N = 67) vs placebo (N = 35) were: IGA (0,1) 46.6% vs 14.3% (p < 0.01), EASI 75 62.0% vs 17.3% (p < 0.001), EASI 90 40.7% vs 11.5% (p < 0.01), Pruritus NRS 48.9% vs 13.1% (p < 0.01), and Sleep-Loss Scale 26.9% vs 6.9% (p = 0.137). Corresponding results for ADhere, (lebrikizumab + TCS, N = 32; placebo + TCS, N = 14), were consistent.
Conclusions
Lebrikizumab treatment demonstrated efficacy in improving the signs and symptoms of AD in adolescent patients, consistent with the ADvocate and ADhere overall population results.
Introduction
The worldwide prevalence of atopic dermatitis (AD) in adolescents is approximately 10–15%, with up to 50% suffering from moderate-to-severe disease (Citation1). AD results in a high disease burden and impaired quality of life (Citation2–5). The intense pruritus, skin pain, and sleep impairment can impact daily activities and school performance. Visible AD lesions are associated with social isolation, and mental health disorders such as anxiety and depression (Citation6–9). A cross-sectional study found that 90% of adolescent patients with AD reported intense itching, 69.2% reported sleep disturbance, 60.2% reported fatigue, and 74.1% reported stress-associated skin flares during high school (Citation10). Increased clinical severity has been correlated with increased levels of psychological stress (Citation11, Citation12). Adolescents with AD have an increased risk of cutaneous infections (Citation13), rate of atopic comorbidities, and use of rescue treatments compared to adults with AD, indicating higher disease severity in this population (Citation14).
Regular use of emollients, topical corticosteroids (TCS), and calcineurin inhibitors (TCI) are standard-of-care, first-line treatment for moderate-to-severe AD in adolescents; phosphodiesterase-4 (PDE-4) inhibitors and topical ruxolitinib are newer topical options approved for adolescents with mild-to-moderate AD in some regions (Citation15, Citation16). Systemic agents approved for adolescents include the biologic dupilumab as well as the oral Janus kinase inhibitors upadacitinib and abrocitinib (Citation14, Citation17–21). However, AD remains uncontrolled in many patients and due to the broad and diverse adolescent patient population, there is an unmet need for systemic treatment options suitable for long-term management of moderate-to-severe AD (Citation22, Citation23).
Interleukin (IL)-13 is a dominant cytokine in AD pathophysiology (Citation24). Lebrikizumab is a novel, high-affinity monoclonal antibody that selectively binds to IL-13, preventing formation of the IL-13Rα1/IL-4Rα heterodimer receptor signaling complex, thus blocking downstream IL-13 signaling. Lebrikizumab allows IL-13 to bind and internalize through the IL-13 decoy receptor (IL-13Rα2), exhibits high binding affinity and a slow dissociation rate, and neutralizes IL-13 with high potency (Citation25).
Lebrikizumab has been approved in the European Union for the treatment of moderate-to-severe AD in adults and adolescents 12 years and older with a body weight of at least 40 kg who are candidates for systemic therapy. Lebrikizumab has previously demonstrated robust efficacy in adult and adolescent patients with moderate-to-severe AD in three Phase 3 trials: two 52-week monotherapy studies (ADvocate1 [NCT04146363] and ADvocate2 [NCT04178967]) and a 16-week combination study with TCS (ADhere [NCT04250337]) (Citation26, Citation27). However, the adolescent data have not been separately analyzed and reported previously. The aim of this analysis is therefore to report the 16-week physician- and patient-reported outcomes (PROs) of lebrikizumab in adolescent patients with moderate-to-severe AD from the ADvocate1, ADvocate2, and ADhere trials.
Materials and methods
Study design and patients
ADvocate1, ADvocate2, and ADhere were randomized, double-blind, placebo-controlled, parallel-group, Phase 3 trials to evaluate the efficacy and safety of lebrikizumab in adult and adolescent patients with moderate-to-severe AD. ADvocate 1/2 were identically designed monotherapy studies with 16-week induction treatment periods and 36-week maintenance treatment periods (Citation26). ADhere was a 16-week topical corticosteroids (TCS) combination study. In all three studies, patients were randomized in a 2:1 ratio to subcutaneous lebrikizumab (loading doses of 500 mg at baseline and Week 2 followed by 250 mg every 2 weeks) or placebo. Patients in ADhere were instructed to use low-to mid-potency TCS at baseline but were allowed to taper or stop TCS use as needed, and TCS treatment could be resumed at the patients’ discretion.
Eligibility criteria for the described studies have been previously published (Citation26, Citation27). Eligible patients for this analysis included adolescents (≥12 to <18 years, weighing ≥40 kg) with moderate-to-severe AD for at least 1 year (as defined by the American Academy of Dermatology Consensus Criteria), an Eczema Area and Severity Index score (EASI) ≥16, an Investigator’s Global Assessment (IGA) score of ≥3, and Body Surface Area involvement of ≥10%. Full inclusion and exclusion criteria are outlined in the primary manuscripts.
Outcomes/endpoints
Efficacy was reported using the percentage of patients achieving IGA score of 0 or 1 and ≥2-point improvement from baseline to Week 16, the percentage of patients achieving at least 75% improvement in the Eczema Area and Severity Instrument (EASI 75) from baseline to Week 16, the percentage of patients achieving at least 90% improvement in EASI (EASI 90), and the least squares mean percentage change from baseline in EASI total score to Week 16. The impact of symptoms was reported by the percentage of patients with Pruritus Numeric Rating Scale (NRS) ≥4-point improvement from baseline to Week 16 among patients with Pruritus NRS ≥4 at baseline, and the percentage of patients with Sleep-Loss Scale ≥2-point improvement from baseline to Week 16 among patients with Sleep-Loss Scale ≥2 at baseline. Pruritus NRS is a patient-reported question that assesses itching at its worst over the past 24 h and ranges from 0 (no itch) to 10 (worst itch imaginable). The Sleep-Loss Scale measures the extent of sleep loss due to itch interference during the previous night. Patients rate their sleep interference due to itch based on a 5-point Likert scale ranging from 0 (not at all) to 4 (unable to sleep at all).
Statistical analysis
Data for adolescents in the ADvocate1 and ADvocate2 studies were pooled, while data from the ADhere study were analyzed separately. Data subsequent to the use of rescue medication or the discontinuation of treatment due to lack of efficacy were imputed as non-response. Rescue medication is defined as topical or systemic treatment in the induction phase of the ADvocate studies and as high potency topical or systemic treatment in the ADhere study. Treatment discontinuation due to other reasons was set to missing. Other missing data were imputed with the use of multiple imputation (MI). Binary outcomes were analyzed by means of a Cochran-Mantel-Haenszel test with adjustment for the stratification factors (study [for pooled ADvocate1 and ADvocate2 only], geographic region, and disease severity). Continuous outcomes were analyzed using the analysis of covariance (ANCOVA), with treatment group, baseline value, and the stratification factors included in the model. Analyses for the pooled ADvocate1/ADvocate2 and ADhere studies were performed on a modified population that excluded ten adolescents (from a single study site) whose eligibility could not be confirmed (three adolescent patients from ADvocate2 and seven adolescent patients from ADhere).
Results
Baseline demographics and disease characteristics
Analyses presented here include the adolescent subpopulation from the pooled ADvocate1 and ADvocate2 studies (lebrikizumab, N = 67; placebo, N = 35) and the ADhere study (lebrikizumab + TCS, N = 32; placebo + TCS, N = 14). In the pooled ADvocate studies, the mean (standard deviation [SD]) age of adolescent patients in the lebrikizumab group was 14.4 (1.6) years and 15.0 (1.7) years in the placebo group. In ADhere, the mean (SD) age was 14.4 (1.5) in lebrikizumab + TCS and 14.9 (1.5) in placebo + TCS. The mean (SD) duration since AD onset was 11.2 (4.6) years in the lebrikizumab group and 12.0 (4.3) in the placebo group in ADvocate 1/2 and 12.1 (3.8) years in lebrikizumab + TCS and 11.4 (4.5) years in placebo + TCS in ADhere. The percentage of females in ADvocate 1/2 was 56.7% (lebrikizumab) and 57.1% (placebo). In ADhere, female sex was reported as 46.9% (lebrikizumab + TCS) and 57.1% (placebo + TCS). At baseline, 38.8% (lebrikizumab) and 31.4% (placebo) of patients in ADvocate 1/2 and 25.0% (lebrikizumab + TCS) and 21.4% (placebo + TCS) of patients in ADhere had severe AD, based on IGA score. The mean EASI was 29.2 (lebrikizumab) and 28.8 (placebo) in ADvocate 1/2 and 28.3 (lebrikizumab + TCS) and 27.5 (placebo + TCS) in ADhere. Disease characteristics of the patients in the adolescent subpopulation of the pooled ADvocate1 and ADvocate2 and ADhere studies at baseline were balanced and similar to the ADvocate 1/2 and ADhere overall populations ().
Table 1. Baseline demographics and disease characteristics in the adolescent mITT population.
Physician-reported clinical signs
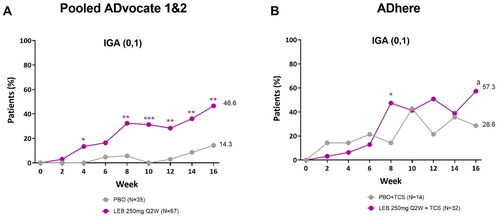
Combined adolescent results from the pooled ADvocate1 and ADvocate2 studies showed that a statistically higher percentage of patients receiving lebrikizumab (46.6%) achieved an IGA score of 0 or 1 with ≥2-point improvement from baseline at Week 16 compared to the placebo group (14.3%, p < 0.01), with statistical significance achieved as early as Week 4 (p < 0.05, ). In ADhere, 57.3% of patients in the lebrikizumab + TCS group achieved an IGA score of 0 or 1 with ≥2-point improvement from baseline compared to 28.6% of patients in the placebo + TCS group (p = 0.104, ).
Figure 1. Adolescent time-course response for IGA (0, 1) with ≥2-point improvement from baseline.
Percentage of patients (%) with IGA (0, 1) and ≥2-point reduction from baseline to Week 16 in the ADvocate (A) and ADhere (B) studies. *p < 0.05, **p < 0.01, ***p < 0.001, ap = 0.104 vs PBO using the Cochran-Mantel-Haenszel test adjusted by study (only for pooled ADvocate1 and ADvocate2), geographic region, and disease severity. IGA = Investigator’s Global Assessment; LEB = lebrikizumab; PBO = placebo; Q2W = every 2 weeks; TCS = topical corticosteroids.
In the pooled ADvocate studies, 62.0% of patients in the lebrikizumab group achieved EASI 75 at Week 16 compared to 17.3% of patients in the placebo group (p < 0.001). Statistical significance was achieved as early as Week 4 (p < 0.01, ). Similarly, in ADhere, significant differences were seen between the lebrikizumab + TCS (88.0%) and placebo + TCS group (57.1%) in patients achieving EASI 75 at Week 16 (p < 0.05), with significant differences seen as early as Week 8 (p < 0.01, ).
Figure 2. Adolescent time-course response for EASI clinical outcomes.
Percentage of patients (%) achieving EASI 75 in ADvocate (A) and ADhere (B) from baseline to Week 16; percentage of patients (%) achieving EASI 90 in ADvocate (C) and ADhere (D) from baseline to Week 16; EASI percentage change from baseline through 16 weeks in ADvocate (E) and ADhere (F). *p < 0.05, **p < 0.01, ***p < 0.001, ap = 0.113 vs PBO using the Cochran-Mantel-Haenszel test adjusted by study (for pooled ADvocate1 and ADvocate2 only), geographic region, and disease severity. EASI 75 = 75% improvement from baseline in Eczema Area and Severity Index score; EASI 90 = 90% improvement from baseline in Eczema Area and Severity Index score; LEB = lebrikizumab; LSM = least squares mean; PBO = placebo; Q2W = every 2 weeks; TCS = topical corticosteroids.
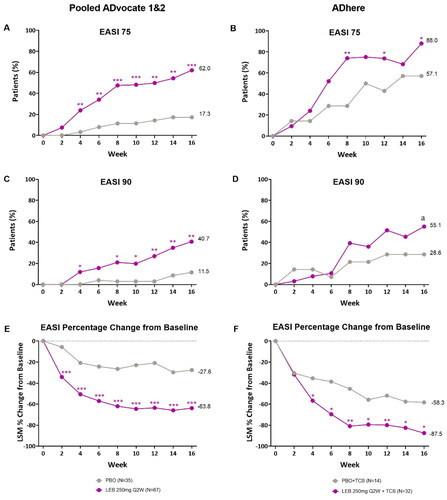
In the pooled ADvocate studies, significant differences were observed in EASI 90 responses, with 40.7% of patients in the lebrikizumab group achieving EASI 90 at Week 16 compared to 11.5% of patients in the placebo group (p < 0.01). Statistical significance was achieved as early as Week 4 (p < 0.05, ). In the ADhere study a numerical, but not statistically significant difference was observed where 55.1% of patients in the lebrikizumab + TCS group achieved EASI 90 at Week 16 compared to 28.6% in the placebo + TCS group (p = 0.113, ).
There was also a significant difference in EASI percentage change from baseline between lebrikizumab and placebo groups at Week 16 in the pooled ADvocate (−63.8% vs −27.6%, p < 0.001) and in ADhere (−87.5% vs −58.3%, p < 0.05) studies. Significance was achieved as early as Week 2 in pooled ADvocate studies (p < 0.001, ) and as early as Week 4 in the ADhere study (p < 0.05, ).
Patient-reported outcomes
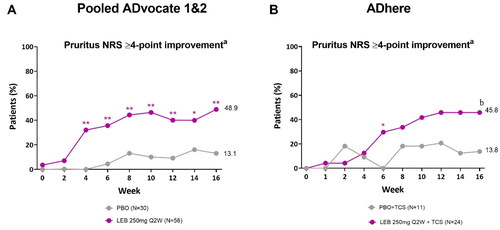
Lebrikizumab every 2 weeks (Q2W) provided clinically meaningful improvements in pruritus at Week 16 as measured by the proportion of patients with Pruritus NRS ≥4 at baseline reporting ≥4-point improvement from baseline in Pruritus NRS. In pooled ADvocate1 and ADvocate2, a significantly greater proportion of patients receiving lebrikizumab (48.9%) vs placebo (13.1%) reported a Pruritus NRS ≥4-point improvement from baseline at Week 16, with significance achieved as early as Week 4 (p < 0.01, ). The corresponding proportions at Week 16 in ADhere were 45.8% and 13.8%, respectively (p = 0.088, ).
Figure 3. Adolescent time-course response for pruritus NRS ≥4-point improvementa.
Percentage of patients with a Pruritus NRS score of ≥4 points at baseline (%) achieving Pruritus NRS ≥4-point improvement from baseline in the ADvocate (A) and ADhere (B) studies. *p < 0.05, **p < 0.01, bp = 0.088 vs PBO using the Cochran-Mantel-Haenszel test adjusted by study (for pooled ADvocate1 and ADvocate2 only), geographic region, and disease severity. LEB = lebrikizumab; NRS = Numeric Rating Scale; PBO = placebo; Q2W = every 2 weeks; TCS = topical corticosteroids.
aPatients with baseline Pruritus NRS score ≥4.
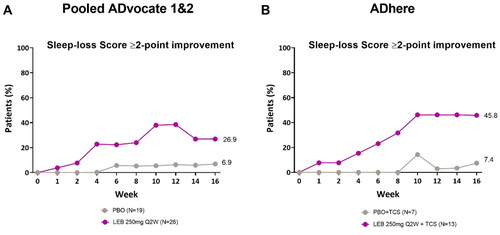
In the pooled ADvocate studies, 26.9% of patients (n = 7) in the lebrikizumab group and 6.9% of patients (n = 1) in the placebo group reported a ≥ 2-point reduction from baseline in the Sleep-Loss Scale at Week 16 (). In the ADhere study, 45.8% of patients (n = 6) in the lebrikizumab + TCS group and 7.4% of patients (n = 1) in the placebo + TCS group reported a ≥ 2-point reduction from baseline in the Sleep-Loss Scale at Week 16 ().
Figure 4. Adolescent time-course response for sleep-loss scale ≥2-point improvement.
Percentage of patients (%) with a Sleep-Loss score of ≥2 points at baseline achieving a ≥2-point improvement from baseline in the ADvocate (A) and ADhere (B) studies. The Cochran-Mantel-Haenszel test adjusted by study (for pooled ADvocate1 and ADvocate2 only), geographic region, and disease severity. LEB = lebrikizumab; PBO = placebo; Q2W = every 2 weeks; TCS = topical corticosteroids.
Discussion
Adolescent patients with AD have high disease burden and limited treatment options, which can negatively impact quality of life. Adolescents with moderate-to-severe AD may benefit from systemic rather than topical medication alone, which is time-consuming, often difficult to maintain and often insufficient to control this degree of inflammation (Citation28). To date, one targeted biologic agent (dupilumab) and 2 Janus kinase (JAK) inhibitors (upadacitinib and abrocitinib), have been approved for adolescents with moderate-to-severe AD in the US (Citation14, Citation18, Citation21, Citation29). Lebrikizumab has recently been approved for adolescents with moderate-to-severe disease in the EU.
The Phase 3, adolescent subgroups analyses presented here showed meaningful clinical and patient-reported AD efficacy improvements after treatment with lebrikizumab 250 mg Q2W with or without TCS. Statistically significant improvements were seen in the pooled ADvocate 1/2 studies in skin clearance measurements and itch improvement at Week 16 and as early as Week 4. The EASI percentage change from baseline was significantly different from placebo by Week 2. In the ADhere adolescent subgroup analysis, significant differences were seen between the lebrikizumab + TCS and placebo + TCS groups for EASI 75 and EASI percentage change from baseline at Week 16. Significant differences were seen for IGA (0,1) and EASI 75 at Week 8 and Pruritus NRS 4-point improvement at Week 6. Concomitant TCS use in the ADhere study had an expected impact on efficacy results in both the lebrikizumab and placebo groups.
Efficacy in the adolescent population described here is in line with the overall results of Phase 3 lebrikizumab studies in adults and adolescents with improvements in skin clearance, itch, and itch interference on sleep, with or without TCS.
Lebrikizumab will potentially add to available systemic treatment options for adolescent patients who are not sufficiently controlled or cannot tolerate topical treatment, with a consistent safety profile as already reported in the overall Phase 3 program. Q4W maintenance dosing may offer an important option in this patient population by providing convenience and potentially increasing adherence and compliance to treatment (Citation30–33).
Limitations
This post hoc analysis was conducted based on the subpopulation without multiplicity controls. Nominal p values were reported. Additionally, PRO analyses were conducted with limited sample sizes, particularly in the ADhere adolescent subpopulation. The small sample size in the Sleep-Loss ≥2 improvement analyses was due to inclusion of only adolescents with a baseline Sleep-Loss score of ≥2. We acknowledge that post hoc analyses with small sample sizes are often exploratory. We treat the findings as preliminary insights that may warrant further investigation in larger samples.
Conclusions
In pooled adolescent data from lebrikizumab monotherapy trials, clinically meaningful improvements in skin symptoms and itch were observed at Week 16, with significance first reported at Week 4. The 16-week EASI 75 response rate was significantly higher with lebrikizumab in combination with TCS as compared to TCS only. These findings are in line with the ADvocate and ADhere overall population results. Thus, lebrikizumab both in monotherapy and in combination with TCS showed robust clinical efficacy in adolescents with moderate-to-severe AD and may represent a new systemic treatment option for this population.
Acknowledgments
Eli Lilly and Company would like to thank the clinical trial participants and their caregivers, without whom this work would not be possible. Writing and process support were provided by Niamh Wiley, PhD, of Eli Lilly and Company.
Disclosure statement
AH reported grants to The University of Texas Health Science Center McGovern Medical School − Houston from Genetech, Demira, Pfizer, Lilly, Amgen, AbbVie; Honoraria received from Demira, Pfizer, Arcutis, Dermavant, Almirall S.A., Incyte, Galderma, Janssen, Leo Pharma, Sun Pharma, UCB; DSMB: Regeneron-Sanofi, GSK, Ortho Dermatologics; CF is Chief Investigator of the UK National Institute for Health Research-funded TREAT (ISRCTN15837754) and SOFTER (Clinicaltrials.gov: NCT03270566) trials as well as the UK-Irish Atopic Eczema Systemic Therapy Register (A-STAR; ISRCTN11210918) and a Principal Investigator in the European Union (EU) Horizon 2020-funded BIOMAP Consortium (Home | BIOMAP (biomap-imi.eu)). He also leads the EU Trans-Foods consortium and European Dermatology Forum treatment guideline for atopic dermatitis. He is Section Editor of the British Journal of Dermatology and receives an honorarium for this role. CF has spoken at Almirall S.A., Bioderma, and Sanofi educational events, and his department has received funding from Sanofi Genzyme and Pfizer for skin microbiome work; HCH is a consultant and/or advisory board member and/or speaker for AbbVie, Amgen, Bausch Health, Celgene, Dermavant, Eli Lilly and Company, Galderma, GlaxoSmithKline, Janssen, Leo Pharma, Novartis, Pfizer, Regeneron/Sanofi, Sun Pharma, and UCB. Received research grants for investigator services from AbbVie, Akros, Amgen, Arcutis, Boehringer Ingelheim, Bristol-Meyers-Squibb, Celgene, Cutanea, Dermira, Dermavant, DS Biopharma, Eli Lilly and Company, Evelo, Galderma, GlaxoSmithKline, Incyte, Janssen, Leo Pharma, Medimmune, Novartis, Pfizer, Regeneron/Sanofi, Roche, and UCB; AI is a consultant/advisory board member/DSMB for AbbVie, Novartis, Regeneron, Sanofi, Pfizer, Eli Lilly and Company, Benevolent AI, LEO, Arena. He has received research grants from AbbVie and Pfizer, is on the board of directors of the International Eczema Council, provides research support to Regeneron, and is in the speakers bureau for AbbVie, Regeneron, Sanofi Genzyme and Eli Lilly and Company; EP, HE, SP, and ZD are employees of Eli Lilly and Company; ES is a speaker, consultant, and/or investigator for AbbVie, Alphyn, ASLAN Pharmaceuticals, Boehringer Ingelheim, Cara Therapeutics, Incyte, Leo, NobelPharma, Novan, Novartis, Pfizer, Pierre Fabre, Regeneron, Sanofi Genzyme, UCB, and Verrica and a Data Safety Monitoring committee member for Leo, Novan, Pfizer, UCB, and Esperare; SW is a speaker, advisory board member, and/or investigator for AbbVie, Almirall S.A., Boehringer, Galderma, Kymab, LEO Pharmaceuticals, Eli Lilly and Company, Novartis, Pfizer, Regeneron, and Sanofi. He has received research grants from Sanofi, Leo, Pfizer, and La Roche Posay.
Data availability statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available for request 6 months after the indication studied has been approved in the United States and Europe and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Additional information
Funding
References
- Silverberg JI, Barbarot S, Gadkari A, et al. Atopic dermatitis in the pediatric population: a cross-sectional, international epidemiologic study. Ann Allergy Asthma Immunol. 2021;126(4):1–9. doi:10.1016/j.anai.2020.12.020.
- Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396(10247):345–360. doi:10.1016/S0140-6736(20)31286-1.
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121(3):340–347. doi:10.1016/j.anai.2018.07.006.
- Vakharia PP, Chopra R, Sacotte R, et al. Burden of skin pain in atopic dermatitis. Ann Allergy Asthma Immunol. 2017;119(6):548–552.e3. doi:10.1016/j.anai.2017.09.076.
- Ricci G, Bellini F, Dondi A, et al. Atopic dermatitis in adolescence. Dermatol Reports. 2012;4(1):e1. doi:10.4081/dr.2012.e1.
- Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134(7):1847–1854. doi:10.1038/jid.2014.70.
- Slattery MJ, Essex MJ, Paletz EM, et al. Depression, anxiety, and dermatologic quality of life in adolescents with atopic dermatitis. J Allergy Clin Immunol. 2011;128(3):668–671. doi:10.1016/j.jaci.2011.05.003.
- Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013;24(5):476–486. doi:10.1111/pai.12095.
- Fasseeh AN, Elezbawy B, Korra N, et al. Burden of atopic dermatitis in adults and adolescents: a systematic literature review. Dermatol Ther (Heidelb). 2022;12(12):2653–2668. doi:10.1007/s13555-022-00819-6.
- Brenninkmeijer EEA, Legierse CM, Sillevis Smitt JH, et al. The course of life of patients with childhood atopic dermatitis. Pediatr Dermatol. 2009;26(1):14–22. doi:10.1111/j.1525-1470.2008.00745.x.
- Saunes M, Smidesang I, Holmen TL, et al. Atopic dermatitis in adolescent boys is associated with greater psychological morbidity compared with girls of the same age: the young‐HUNT study. Br J Dermatol. 2007;156(2):283–288. doi:10.1111/j.1365-2133.2006.07688.x.
- Oh SH, Bae BG, Park CO, et al. Association of stress with symptoms of atopic dermatitis. Acta Derm Venereol. 2010;90(6):582–588. doi:10.2340/00015555-0933.
- Sanclemente G, Hernandez N, Chaparro D, et al. Epidemiologic features and burden of atopic dermatitis in adolescent and adult patients: a cross-sectional multicenter study. World Allergy Organ J. 2021;14(12):100611. doi:10.1016/j.waojou.2021.100611.
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156(1):44–56. doi:10.1001/jamadermatol.2019.3336.
- Hoy SM. Ruxolitinib cream 1.5%: a review in mild to moderate atopic dermatitis. Am J Clin Dermatol. 2023;24(1):143–151. doi:10.1007/s40257-022-00748-2.
- Sideris N, Paschou E, Bakirtzi K, et al. New and upcoming topical treatments for atopic dermatitis: a review of the literature. J Clin Med. 2022;11(17):4974. doi:10.3390/jcm11174974.
- Wollenberg A, Blauvelt A, Guttman‐Yassky E, et al. Tralokinumab for moderate‐to‐severe atopic dermatitis: results from two 52‐week, randomized, double‐blind, multicentre, placebo‐controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol. 2021;184(3):437–449. doi:10.1111/bjd.19574.
- Simpson EL, Papp KA, Blauvelt A, et al. Efficacy and safety of upadacitinib in patients with moderate to severe atopic dermatitis: analysis of follow-up data from the measure up 1 and measure up 2 randomized clinical trials. JAMA Dermatol. 2022;158(4):404–413. doi:10.1001/jamadermatol.2022.0029.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(8):863–873. doi:10.1001/jamadermatol.2020.1406.
- Ferreira S, Guttman-Yassky E, Torres T. Selective JAK1 inhibitors for the treatment of atopic dermatitis: focus on upadacitinib and abrocitinib. Am J Clin Dermatol. 2020;21(6):783–798. doi:10.1007/s40257-020-00548-6.
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE Mono-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396(10246):255–266. doi:10.1016/S0140-6736(20)30732-7.
- Thibodeaux Q, Smith MP, Ly K, et al. A review of dupilumab in the treatment of atopic diseases. Hum Vaccin Immunother. 2019;15(9):2129–2139. doi:10.1080/21645515.2019.1582403.
- Stölzl D, Weidinger S, Drerup K. A new era has begun: treatment of atopic dermatitis with biologics. Allergol Select. 2021;5(01):265–273. doi:10.5414/ALX02259E.
- Bieber T. Interleukin‐13: targeting an underestimated cytokine in atopic dermatitis. Allergy. 2020;75(1):54–62. doi:10.1111/all.13954.
- Okragly A, Ryuzoji A, Daniels M, et al. Comparison of the affinity and in vitro activity of lebrikizumab, tralokinumab, and cendakimab. 4th inflammatory skin disease summit, New York. Exp Dermatol. 2021;30(Suppl. 2):3–43. November 2–6, 2021. doi:10.1111/exd.14457.
- Silverberg JI, Guttman-Yassky E, Thaçi D, et al. Two phase 3 trials of lebrikizumab for moderate-to-severe atopic dermatitis. N Engl J Med. 2023;388(12):1080–1091. doi:10.1056/NEJMoa2206714.
- Simpson EL, Gooderham M, Wollenberg A, et al. Efficacy and safety of lebrikizumab in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis: a randomized clinical trial (ADhere). JAMA Dermatol. 2023;159(2):182–191. doi:10.1001/jamadermatol.2022.5534.
- Bandyopadhyay D. A treatise on topical corticosteroids in dermatology. Use, misuse and abuse. Indian J Dermatol Venereol Leprol. 2018;84:761–762. doi:10.4103/ijdvl.IJDVL_755_18.
- Ghamrawi R, Bell KA, Balogh EA, et al. Current and emerging biologics for the treatment of pediatric atopic dermatitis. Expert Opin Biol Ther. 2020;20(12):1435–1445. doi:10.1080/14712598.2021.1840548.
- Patel N, Feldman SR. Adherence in atopic dermatitis. Adv Exp Med Biol. 2017;1027:139–159. doi:10.1007/978-3-319-64804-0_12.
- Kelly KA, Ewulu A, Emmerich VK, et al. Refractory pediatric psoriasis and atopic dermatitis: the importance of therapeutical adherence and biological management. Biomedicines. 2021;9(8):958. doi:10.3390/biomedicines9080958.
- Freitas E, Guttman-Yassky E, Torres T. Tralokinumab for the treatment of atopic dermatitis. Am J Clin Dermatol. 2021;22(5):625–638. doi:10.1007/s40257-021-00613-8.
- Krejci-Manwaring J, Tusa MG, Carroll C, et al. Stealth monitoring of adherence to topical medication: adherence is very poor in children with atopic dermatitis. J Am Acad Dermatol. 2007;56(2):211–216. doi:10.1016/j.jaad.2006.05.073.
