Abstract
Background
Granuloma formation is an uncommon and persistent skin inflammatory condition caused by the injection of dermal fillers. The exact cause of this reaction is not well understood, but it may be associated with irritating components or abnormal immune function. Treating granulomas can be difficult. However, recent research has shown that Janus kinase (JAK) inhibitors hold promise as a potential therapy for refractory granulomatous diseases.
Objectives
The aim was to evaluate the efficacy and safety of tofacitinib as a treatment for granulomas secondary to filler injection and the possible mechanisms were discussed and summarized.
Methods
This study focuses on three cases of patients who experienced granuloma formation after receiving filler injections and were subsequently treated with tofacitinib. The efficacy and safety of the treatment were evaluated using parameters such as photographs and monitoring for any adverse reactions. In addition, a literature review was conducted to explore the underlying mechanisms and potential effects of tofacitinib.
Results
All three cases recovered from swelling and nodules without side effects through the off-label use of oral tofacitinib. Existing data review reveals some approaches for cutaneous granulomatous disorders like inhibiting macrophage activation and downregulation of the JAK–STAT pathway.
Conclusion
This report emphasizes the effectiveness of JAK inhibitors in treating granulomas caused by filler injections. Recent advancements in understanding the underlying mechanisms of granulomatous reactions have paved the way for JAK inhibitors to be regarded as a promising treatment choice. However, further research is necessary to fully assess the safety and long-term effectiveness of using tofacitinib for granuloma treatment.
Keywords:
1. Introduction
Soft-tissue augmentation through the use of fillers is a popular technique for facial rejuvenation. Alongside the commonly employed hyaluronic acid (HA), an increasing array of novel filling materials have been utilized in cosmetic procedures in recent years. These include calcium hydroxyapatite (CaHA), poly-L-Lactic acid (PLLA), polycaprolactone (PCL), among others (Citation1). However, it is important to note that some of these materials may carry a higher risk of sensitization, granuloma formation, and other filler-related complications (Citation2). Foreign body granuloma is a chronic inflammatory reaction with complicated etiologies which can develop as a result of dermal filler injections, exhibiting diverse clinical and histologic characteristics that depend on the specific type of filler used. Intralesional corticosteroid injections are the mainstay treatment for foreign body granulomas resulting from filler injections (Citation1). Corticosteroids are known to disrupt the activities of fibroblasts, macrophages, giant cells, and collagen synthesis at the local level (Citation3). Other therapies that have been reported include the injection of 5-Fluorouracil, the systemic use of allopurinol, cyclosporine and steroids, as well as hyperbaric therapy (Citation4,Citation5). However, as a result of the extended degradation cycle of certain filling materials and the adjuvant effect stemming from unique material properties and immune factors, recurrent swelling and other immune-mediated reactivation of inflammatory response can be induced by triggering events, such as infections, vaccines, and similar factors (Citation6). Consequently, these granuloma disorders present significant treatment challenges, necessitating the development of more efficient and targeted therapeutic approaches. There are some recent developments and findings for JAK in treating granulomatous disorders and other macrophage activation diseases. There are no reported cases of JAK inhibitors used in immune reactions such as foreign body granuloma after cosmetic injection.
2. Materials and methods
A total of three cases diagnosed with foreign body granuloma and treated with tofacitinib were reviewed and summarized (n = 3). Infection, hypersensitivity, and autoimmune disease were ruled out as potential causes in all three patients. The effectiveness of tofacitinib in these cases was evaluated based on patient satisfaction with the treatment and subjective evaluations by physicians. A comprehensive literature search was conducted using keywords such as ‘granuloma’, ‘macrophage’, and ‘Janus kinase (JAK) inhibitors’.
3. Case reports
3.1. Case 1
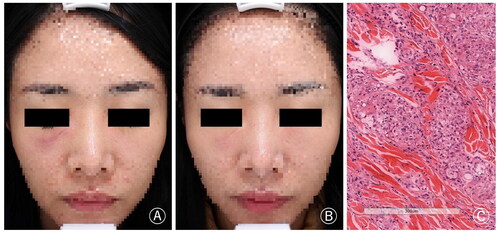
A 31-year-old woman underwent collagen (type unknown) filler injection for bilateral tear trough rejuvenation at a beauty salon eight months ago and there was no abnormal symptoms initially. However, after two months, nodules and swelling developed in the bilateral infraorbital region, especially on the right side. Despite the use of oral antibiotics and corticosteroids, the symptoms remained unresolved. She sought further treatment at our clinic, and histopathological examination confirmed the diagnosis of foreign body granuloma. Finally, after excluding contraindications, the patient was treated with tofacitinib 5 mg/day. The symptoms were relieved after approximately one month of medication, with no reported drug-related side effects and no symptom recurrence after discontinuing the medication. The patient is currently under regular monitoring and follow-up ().
Figure 1. (A) Nodules and swelling developed in the bilateral infraorbital region, particularly on the right side. (B) The symptoms were significantly relieved after treatment. (C) Foreign bodies were observed dispersed among inflammatory cells, epithelioid cells, and macrophages. Additionally, multinucleated foreign body giant cells were also observed (H&E × 200).
3.2. Case 2
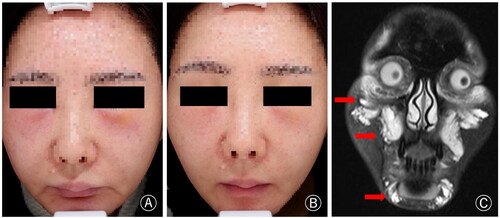
A 38-year-old woman, who received facial collagen type I filling injection at a cosmetic hospital two months ago, experienced a gradual onset of multiple nodules and facial swelling. Systemic administration of corticosteroids and antihistamines proved ineffective. Consequently, she sought further treatment at our clinic. MRI images were consistent with foreign body reaction and soft tissue swelling after filling injection. Following one month of receiving Tofacitinib at a dosage of 5 mg/day, the patient experienced significant relief from facial swelling. The patient is currently undergoing treatment follow-up, and no recurrence or side effects have been reported ().
Figure 2. (A) The patient had erythema and swelling in the central area of the face, especially in the bilateral suborbital and chin regions. (B) The symptoms significantly improved after approximately one month of treatment. (C) MRI (T2W) coronal image revealed the presence of enhancing signal and space-occupying changes with indistinct margins.
3.3. Case 3
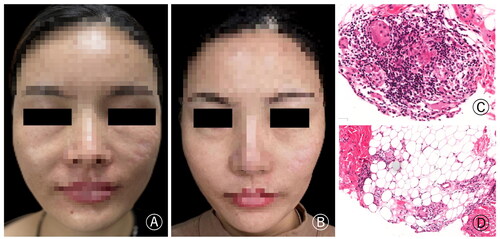
A 42-year-old woman, who underwent bilateral nasolabial fold augmentation with PLLA filler at a plastic surgery hospital six months ago, started experiencing swelling, nodules, and a burning sensation at the injection site three months after the procedure. The patient received systemic corticosteroid therapy, but it was discontinued due to significant side effects. She presented at our clinic and underwent histopathological examination, which confirmed the diagnosis of foreign body granuloma. Due to the slow efficacy of the initial 5 mg/day dosage, we adjusted the tofacitinib to 5 mg twice daily after one week. Following three weeks of comprehensive treatment, the swelling of the nasolabial fold was significantly reduced ().
Figure 3. (A) The patient had swelling and nodules at the bilateral nasolabial fold. (B) The symptoms significantly improved after treatment. (C) The histopathology showed foreign body granuloma composed of more lymphocytes, macrophages and injected foreign bodies, and there was also inflammatory cell infiltrating around the adipose tissue (H&E × 200).
4. Results
All three patients were diagnosed with foreign body granuloma following filler injections. After treatment with tofacitinib, their symptoms were alleviated to varying degrees. Prior to treatment, all three patients underwent screening for infectious diseases, and no drug-related adverse reactions were reported during the follow-up period. The literature review provided a summary and analysis of the treatment of foreign body granuloma after cosmetic injections and the potential therapeutic pathways of JAK inhibitors.
5. Discussion
As the cosmetic injection market continues to develop, more and more non-HA substances have been applied in filling injection, in addition, the injection of illegal substances is still being carried out. Granulomatous changes become an emerging concern which severely affecting appearance and social interaction. Foreign body granuloma is a type of granulomatous tissue reaction that occurs in response to the presence of nonself organization. Theoretically, foreign body reaction also belongs to type IV hypersensitivity reaction, which is mainly mediated by T lymphocytes (Citation7). The common clinical symptoms are chronic subcutaneous nodules and induration. Histopathological examination is the standard of diagnosis which consists of macrophage, epithelioid cells, multinuclear giant cells, and foreign body structures. Lemperle and Gauthier-Hazan (Citation8). divided foreign body granulomas into three types based on pathological characteristics and clinical features: cystic granulomas, edematous granulomas, and sclerosing granulomas. The common treatment for foreign body granuloma is intralesional corticosteroid injections. In Systemic therapy, systemic steroid, minocycline, colchicines, and cyclosporine has been reported. In intralesional injections, intralesional 5-Fluorouracil, colchicine, antibiotic, hyaluronidase and others have also been reported to successfully treat nodules and swelling caused by filling foreign bodies (Citation9,Citation10). There have also been several reports of successful treatment using laser or focused ultrasound (Citation11,Citation12), but the number is small and the specific efficacy needs further study. It is worth noting that there are some side effects for several commonly used treatments, such as corticosteroid-induced dermal atrophy, centripetal obesity and opportunistic infection. More importantly, some non-HA fillers lack effective lytic enzymes and the degradation cycle is long, continue to stimulate the body to produce foreign body reaction. Therefore, in the absence of effective dissolving enzymes in some fillers, how to inhibit the activation of foreign body reaction is the key to treatment.
The injection of filler material induces an immune response, initiating the activation of macrophages, which are essential in the body’s defense mechanism against foreign substances (Citation13). Subsequently, macrophages release chemical signals to attract other immune cells, including lymphocytes and fibroblasts, to the injection site. These immune cells, along with the remaining macrophages, cluster around the foreign material, resulting in the formation of a granuloma (Citation14). As time progresses, the granuloma can become surrounded by fibrous tissue, creating a more distinct structure that impacts the appearance and imposes a psychological burden (Citation15).
Emerging evidence indicates that JAK inhibition holds promise as a molecularly targeted therapy for granulomatous disorders. On the one hand, some studies indicate that an overproduction of inflammatory cytokines, such as interferon gamma (IFN-γ), and subsequent constitutive activation of the JAK-STAT pathway may be the feature of these granulomatous disorders. Consequently, the use of JAK inhibitors, which effectively block these signaling pathways, has demonstrated remarkable improvements in sarcoidosis patients (Citation16). Additionally, JAK inhibitors have also shown potential in treating other inflammatory disorders characterized by macrophage activation, such as hemophagocytic lymphohistiocytosis, Crohn’s disease, granuloma annulare, and necrobiosis lipoidica (Citation17). Herein, we present three cases of refractory granuloma formation following filler injection, which were effectively managed through the oral administration of tofacitinib at a dose of 5 mg once daily. The substances injected by these patients do not consist solely of pure HA; rather, they contain PCL, PLLA, and other components that lack lysozyme. One the one hand, these long-term foreign substances will perpetuate the activation of the macrophage-T cell pathway, creating a feedback loop that leads to the persistence or even exacerbation of nodules and swelling. On the other hand, according to the ‘adjuvant effect’ pathogenesis theory (Citation18), it is believed that the filler is not directly recognized as an antigen, but rather it enhances the activity and function of immune cells such as T lymphocytes. This, in turn, strengthens the local innate and acquired immune responses, making them more sensitive to external factors such as infection, temperature changes, and vaccines (Citation6). As a result, symptoms are repeatedly stimulated and aggravated.
On the whole, the JAK inhibitors like tofacitinib, not only impede the signaling pathway involved in granuloma formation but also hinder a wide range of intracellular inflammatory signaling pathways. Therefore, considering the two crucial pathogenic mechanisms mentioned above, tofacitinib can effectively serve as a therapeutic intervention for granuloma after dermal filler injection In theory. There are several limitations in this report, due to the intricate mechanism involved in the pathogenesis of foreign body granuloma after filler injections, further comprehensive clinical studies are required to determine the exact efficacy of tofacitinib treatment. Additionally, the specific dosage and long-term safety of tofacitinib need to be validated by a larger body of scientific clinical data.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Dong J, Gantz M, Goldenberg G. Efficacy and safety of new dermal fillers. Cutis. 2016;98(5):1–6.
- Oh H, Lee S, Na J, et al. Comparative evaluation of safety and efficacy of a novel hyaluronic acid-polynucleotide/poly-L-lactic acid composite dermal filler. Aesthetic Plast Surg. 2021;45(4):1792–1801. doi: 10.1007/s00266-021-02295-3.
- Lee JM, Kim YJ. Foreign body granulomas after the use of dermal fillers: pathophysiology, clinical appearance, histologic features, and treatment. Arch Plast Surg. 2015;42(2):232–239. doi: 10.5999/aps.2015.42.2.232.
- Lemperle G, Morhenn V, Charrier U. Human histology and persistence of various injectable filler substances for soft tissue augmentation. Aesthetic Plast Surg. 2020;44(4):1348–1360. doi: 10.1007/s00266-020-01827-7.
- Ghareeb FM, Hassan MSA, El Nahas MA, et al. Complicated facial fillers: management algorithm. Plast Reconstr Surg Glob Open. 2022;10(7):e4445. doi: 10.1097/GOX.0000000000004445.
- Owczarczyk-Saczonek A, De Boulle K. Hyaluronic acid fillers and ASIA syndrome: case studies. Clin Cosmet Investig Dermatol. 2023;16:2763–2771. [Published correction appears in Clin Cosmet Investig Dermatol. 2023 Nov 15;16:3321–3322]. doi: 10.2147/CCID.S419716.
- Lucey P, Goldberg DJ. Complications of collagen fillers. Facial Plast Surg. 2014;30(6):615–622. doi: 10.1055/s-0034-1396904.
- Lemperle G, Gauthier-Hazan N. Foreign body granulomas after all injectable dermal fillers: part 2. Treatment options. Plast Reconstr Surg. 2009;123(6):1864–1873. doi: 10.1097/PRS.0b013e3181858f4f.
- Aivaliotis M, Kontochristopoulos G, Hatziolou E, et al. Successful colchicine administration in facial granulomas caused by cosmetic implants: report of a case. J Dermatolog Treat. 2007;18(2):112–114. doi: 10.1080/08910600601149619.
- Lemperle G, Duffy DM. Treatment options for dermal filler complications. Aesthet Surg J. 2006;26(3):356–364. doi: 10.1016/j.asj.2006.04.002.
- Ostezan L, Peck J. Radial sound (shockwave) therapy resolves delayed-onset nodules following injection of hyaluronic acid dermal filler: a case study. J Clin Aesthet Dermatol. 2021;14(12 Suppl 1):S15–S17.
- Hong JK, Sun B, Shin SH, et al. Late-onset filler-induced granuloma after polycaprolactone-based filler treated with High-Intensity focused ultrasound and quantum molecular resonance technology. Dermatol Surg. 2022;48(6):693–694. doi: 10.1097/DSS.0000000000003424.
- Bonnema H, Popa ER, van Timmeren MM, et al. Distribution patterns of the membrane glycoprotein CD44 during the foreign-body reaction to a degradable biomaterial in rats and mice. J Biomed Mater Res A. 2003;64(3):502–508. doi: 10.1002/jbm.a.10404.
- Modarressi A, Nizet C, Lombardi T. Granulomas and nongranulomatous nodules after filler injection: different complications require different treatments. J Plast Reconstr Aesthet Surg. 2020;73(11):2010–2015. doi: 10.1016/j.bjps.2020.08.012.
- Kunjur J, Witherow H. Long-term complications associated with permanent dermal fillers. Br J Oral Maxillofac Surg. 2013;51(8):858–862. doi: 10.1016/j.bjoms.2013.06.013.
- Wang A, Singh K, Ibrahim W, et al. The promise of JAK inhibitors for treatment of sarcoidosis and other inflammatory disorders with macrophage activation: a review of the literature. Yale J Biol Med. 2020;93(1):187–195.
- Damsky W, Thakral D, McGeary MK, et al. Janus kinase inhibition induces disease remission in cutaneous sarcoidosis and granuloma annulare. J Am Acad Dermatol. 2020;82(3):612–621. doi: 10.1016/j.jaad.2019.05.098.
- Cohen Tervaert JW, Martinez-Lavin M, Jara LJ, et al. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) in 2023. Autoimmun Rev. 2023;22(5):103287. doi: 10.1016/j.autrev.2023.103287.

