Abstract
Background
Biologics are essential in treating psoriasis. In recent years, the pathogenesis exploration and development of new target drugs have provided a more complete evidence-based foundation for the biological treatment of psoriasis. This study aims to use bibliometrics to analyze the research status and development trends of biologics in psoriasis.
Methods
The bibliometric analysis of publications related to biologics in psoriasis from 2004 to 2023 was conducted using the Web of Science Core Collection (WoSCC) database as the search data source. To perform the bibliometric analysis and create visual knowledge graphs, CiteSpace, the Bibliometrix R package, and VOSviewers were utilized.
Results
The study included a total of 3800 articles. The United States had the highest number of publications. The leading authors and institutions were Steven R. Feldman and the University of Manchester, respectively, in the global partnership. The cluster plot divided all keywords into 11 categories. Currently, Secukinumab and Guselkumab are representative biological agents being studied due to their considerable efficacy and long-term safety.
Conclusions
Targeted therapy has emerged as a significant trend in the current treatment of psoriasis. Early and active use of biologics can effectively control disease progression, prevent or delay the occurrence of comorbidities, and may even alter the natural course of psoriasis. However, further investigation is required to fully understand the specific mechanisms of psoriasis and the use of biological agents.
1. Introduction
Psoriasis is a chronic and relapsing inflammatory disease that results from a combination of genetic and environmental factors. In addition to skin symptoms, inflammation can also affect multiple organs and systems throughout the body [Citation1]. James G. Krueger and his team were the pioneers of using biological agents to treat psoriasis [Citation2]. Since then, more and more biological agents have been proven effective against psoriasis [Citation3,Citation4]. Over the years, psoriasis treatment has evolved from traditional broad-spectrum immunosuppressive therapy to targeted therapy and then to precise targeted therapy [Citation5].
As research has intensified, a large body of research data has accumulated in the field of biologic treatment of psoriasis. However, gaps in research remain regarding specific site involvement, management of comorbidities, relapse after drug discontinuation and the long-term benefits of early intervention with biologic agents. Bibliometrics refers to the interdisciplinary science that uses mathematical and statistical methods to quantitatively analyze all knowledge carriers [Citation6]. It is a comprehensive knowledge system that integrates mathematics, statistics and linguistics and focuses on quantification. In today’s era of big data, bibliometrics can help scientists and clinicians sort out the research context and hot spots in a given research area, predict research trends, and greatly improve their research efficiency [Citation7]. Previous researchers conducted a bibliometric analysis of the 100 most cited articles in the field of psoriasis vulgaris and biologics since 1991. However, it is important to note that this analysis has limitations due to the small number of articles included [Citation8]. Therefore, it cannot fully demonstrate the development trend of this field. In light of this, we conducted a bibliometric analysis of the research on biologics in psoriasis over the past 20 years. The aim of our research is to predict the next direction of research and provide more guidance on how to treat psoriasis so that patients can get the maximum benefit.
2. Methods
2.1. Data sources and filtering strategies
To prevent errors resulting from database updates, we conducted a search of WoSCC database on January 5, 2024, using the following search formula: TS=(psoriasis) AND TS=(biological OR biologicals OR biological agent OR biological agents OR biologic OR biologics OR biological product OR biological products OR biopreparate OR bio-preparate OR biological preparation OR biological preparations). A total of 6010 articles were retrieved by limiting the publication time to 2004-01-01 to 2023-12-31 and the article types to original articles and reviews written in English. After reading the titles and abstracts, articles not related to psoriasis and biologics were excluded, along with retracted and duplicated articles. Finally, 3800 articles were included.
2.2. Bibliometric analysis
CiteSpace is a visual citation analysis software that has been gradually developed in the context of scientometrics and data visualization. VOSviewer is a software tool for the construction and visualization of bibliometric networks. Bibliometrix is a mature tool based on the R language. In this study, CiteSpace software and the Bibliometrix R package were used to analyze the authors, institutions, countries, journals, references, and keywords of the included research articles. Co-occurrence, cluster analysis, and visualization of the results were performed using VOSviewer.
3. Results
3.1. Overall situation
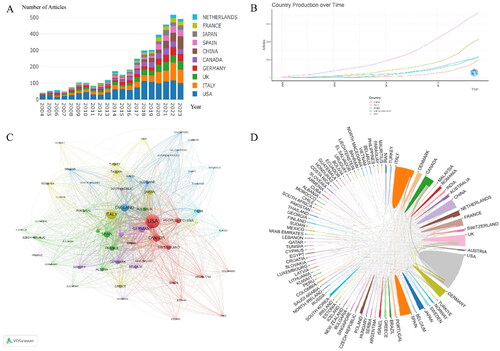
A total of 6010 documents (Supplementary S1) were retrieved from the WoSCC database, and 2210 documents were excluded after screening (Supplementary S2). displays the annual number of published articles, indicating that from 2004 to 2017, the number of research articles on biological agents in psoriasis did not exceed 200 per year, indicating a stable development stage. However, from 2018 to 2023, there were more than 200 articles published each year, indicating a rapid development stage. Although some articles published in 2023 have not yet been included, the figure shows an overall upward trend in published articles. Over the past 20 years, 90 countries have published relevant literature on the use of biological agents in psoriasis. displays the top five countries/regions with the highest number of publications. The United States has consistently ranked first in the number of publications in this field, while China, as the only developing country, ranks fifth. Analysis of the national cooperation diagram in and the chord diagram of inter-country cooperation in reveals that the United States and Italy are dominant in this field.
3.2. Analysis of author, institution, and journal
A total of 12,977 authors from 4,376 institutions and 530 journals have contributed to this field. presents the top 10 authors in this field, with six from the United States, two from Italy, and two from Taiwan, China, and the United Kingdom. present the cluster and collaboration diagrams of authors and institutions, respectively. They indicate that Steven R. Feldman and the University of Manchester are the most significant contributors to global collaborations. displays the annual publication volume of the top five journals, including the top five published articles. displays the citation path between journals, with the citing journal on the left and the cited journal on the right. The results indicate that journals with a Molecular/Biology/Genetics orientation are cited by journals with a Molecular/Biology/Immunology and Dentistry/Dermatology/Surgery orientation, while journals with a Health/Nursing/Medicine orientation are cited by journals with a Dentistry/Dermatology/Surgery orientation.
Figure 2. Analysis of author, institution, and journal. (A) Cluster diagram of inter-author collaborations; (B) Cluster diagram of inter-agency co-operation; (C) Annual publications in the top 5 journals in terms of number of publications; (D) Dual-image overlay of biologics-related journals in psoriasis.
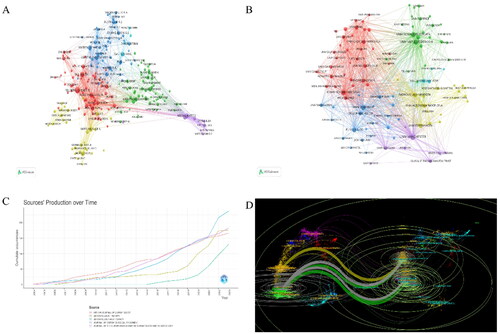
Table 1. The top ten authors by number of publications.
3.3. Analysis of reference
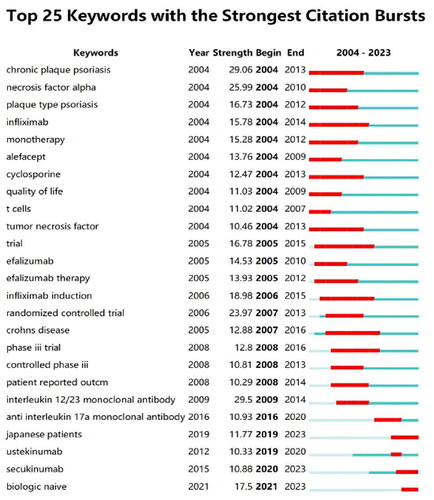
In research fields, a document’s influence and authority increase with its frequency of citation. The centrality of a cited document also indicates its significance in the field. However, it is important to note that recent hot spots with zero centrality may also be significant, even if they have not been extensively studied. Therefore, a node’s importance cannot be dismissed solely based on its centrality. A total of 19,631 references from 893 journals have been identified, each with more than three citations. and list the top 5 cited documents and the top 5 cited documents with centrality, respectively. The reference keyword clustering results reveal the content sources of research related to biological agents in psoriasis. displays the cluster analysis results of the reference literature, inciuding 13 key clusters, which are: ‘Brodalumab,’ ‘Efalizumab,’ ‘Ustekinumab,’ ‘Drug Survival,’ ‘Persistence,’ ‘Psoriatic Arthritis,’ ‘Spondyloarthritis,’ ‘ Guselkumab,’ ‘Infliximab,’ ‘COVID-19,’ ‘Pediatric Psoriasis,’ ‘Polymorphisms,’ and ‘Nail Psoriasis’. The timeline relationship of key clusters is shown in , with the current main focus being on ‘Psoriatic Arthritis’ and ‘Guselkumab’. displays the 25 documents with the highest burst citation intensity. Among them, ‘Langley RG, 2014’ achieved the highest burst value (93.29) during 2015–2019. The article assesses the effectiveness and safety of Secukinumab in treating moderate to severe plaque psoriasis and confirms IL-17A as a significant therapeutic target for this condition [Citation9].
Figure 3. Analysis of reference. (A) Cluster analysis diagram for references; (B) Reference timeline chart; (C) Outbreak of cited references.
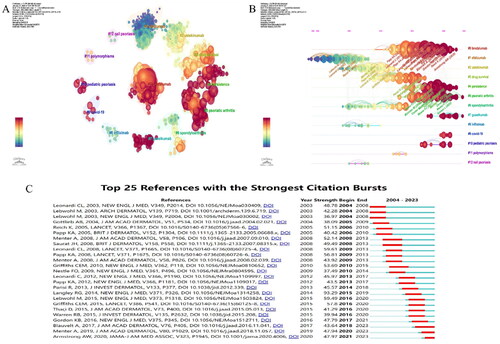
Table 2. The top five references with cited frequency.
Table 3. The top five references with the centrality.
3.4. Analysis of research hotspots
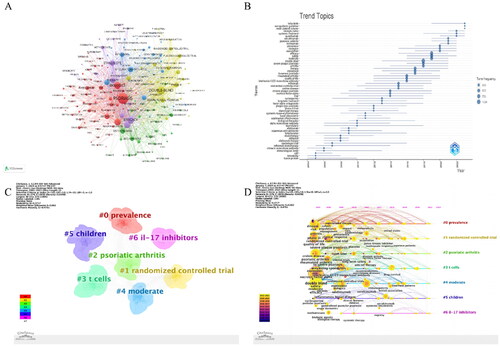
The results of the keyword analysis of the included articles indicate the current research trends in this field. A total of 6265 keywords were tagged in 3800 documents, with 189 keywords appearing more than 30 times. shows the keyword co-occurrence map, while displays the time trend of research topics. The research topics have evolved from ‘Fusion Protein,’ which was the earliest focus, to ‘Tofacitinib,’ which is the latest focus, involving a total of eight biological agents. The order of appearance has been adjusted to reflect the chronological order of the agents. These eight biological agents are ‘Infliximab,’ ‘Efalizumab,’ ‘Alefaceptm,’ ‘Adalimumab,’ ‘Etanercept,’ ‘Ixekizumab,’ ‘Secukinumab,’ and ‘Guselkumab’. Furthermore, the current trend in popular research topics indicates that Secukinumab and Guselkumab are the primary biological agents being studied. It is important to note that these findings are objective and based solely on the data presented. displays the results of the cluster analysis of keywords, which includes a total of 7 key clusters: ‘Prevalence,’ ‘Randomized Controlled Trial,’ ‘Psoriatic Arthritis,’ ‘T-Cells,’ ‘Moderate,’ ‘Children,’ and ‘IL-17 Inhibitors’. shows the contribution timeline and average year of each keyword to each cluster, calculated based on the publication date of each keyword. The current main focus is on ‘Prevalence,’ ‘T-Cells,’ ‘Psoriatic Arthritis,’ and ‘IL-17 Inhibitors’. To effectively reflect the period of main research content and themes and reveal development trends, CiteSpace’s ‘Burstness’ function can be used. lists the first 25 of these, showing the duration and intensity of the bursts.
4. Discussion
Based on keyword analysis, burst analysis and cluster analysis, we identified the following major research themes.
4.1. Epidemiology
Based on statistics, Taiwan, China has the lowest incidence of adult psoriasis, while Italy has the highest. Psoriasis is more prevalent in high-income areas of Western Europe, Central Europe, North America, and southern Latin America [Citation10]. Our research indicates that there is rapid development in the study of biological agents in psoriasis, with the United States and Italy leading international cooperation. The countries with the highest number of published articles are predominantly developed, with China being the only developing country. This highlights the uneven distribution of psoriasis across different geographical areas [Citation10] and suggests that racial/ethnic disparities may be linked to its onset [Citation11,Citation12].
4.2. Targeted treatments for psoriasis
Biological agents have gone through two eras in the treatment of psoriasis. The first generation includes Etanercept, Infliximab, Adalimumab, Golimumab, and Certolizumab, which target TNF-α. The second-generation of biological drugs primarily targets multiple factors, including IL-12, IL-17, and IL-23. Both Alefacept and Efalizumab are T-cell activation inhibitors. They have been withdrawn from the market due to the emergence of adverse reactions [Citation13]. Ustekinumab is a fully human IgG1κ monoclonal antibody that targets the p40 subunit shared by IL-12 and IL-23. It inhibits the differentiation of Th1 and Th17 cells and the subsequent cascades mediated by IL-12 and IL-23 [Citation14]. There are currently five IL-17 pathway inhibitors available in the market. These include Secukinumab, Ixekizumab, Netakimab, Bimekizumab and Brodalumab [Citation15,Citation16]. Guselkumab, Risankinumab, and Tislezumab are biological agents that target IL-23. All three can bind to the p19 subunit of IL-23, inhibiting the activation of Th17 cells and reducing downstream cytokine production. This leads to a reduction in downstream inflammatory responses [Citation16]. Currently, new targets, structures, and types of biological agents are being developed, providing more options for the treatment of psoriasis [Citation16,Citation17].
4.3. Psoriasis and COVID-19
There is a bidirectional link between psoriasis and infection. However, it is unclear whether individuals with psoriasis are at a higher risk of COVID-19 infection or if treatment with biologics affects the protective effect of COVID-19 vaccines [Citation18]. Some studies suggest that the use of biologics in psoriasis patients is not associated with an increased risk of COVID-19 infection or worse outcomes [Citation19]. Regardless of the biologic therapy used, there is no increased risk of severe COVID complications. Studies have reported worsening of psoriasis in patients after receiving the COVID-19 vaccine [Citation20]. However, these studies do not provide clear evidence of a causal link between COVID-19 vaccination and psoriasis, and vaccination should not be discouraged [Citation19].
4.4. Early intervention and disease modification
Psoriasis relapses in most patients occur mainly in situ after treatment cessation, indicating a potential link between local skin immune inflammation memory and psoriasis recurrence [Citation21]. Tissue-resident memory T-cells (TRM) are believed to play a crucial role in immune memory and psoriasis recurrence. Follow-up studies have shown that T-cells with immune memory function remain in the skin lesions after psoriasis remission. On the one hand, vascular endothelial cells can accept cytokines, vasoactive substances, metabolites and mechanical signals in the psoriasis microenvironment to regulate vascular function; on the other hand, vascular endothelial cells can actively mediate vascular function through transcriptional regulation and vascular secretory function. Psoriasis is an inflammatory disease [Citation22]. IL-23 can activate and expand CD8 + TRM, which produces IL-17A and induces the recurrence of psoriasis [Citation23,Citation24]. Complete removal of skin lesions can significantly improve the quality of life and work efficiency of psoriasis patients. Due to the limited efficacy of traditional systemic therapeutic drugs in clearing skin and the safety concerns associated with long-term use, there has been a general trend toward earlier application of biological agents [Citation25–27]. Early intervention to block IL-23 and IL-17A can reduce resident memory T-cells, prevent the establishment of psoriasis skin tissue memory, and help patients achieve sustained clearance of psoriasis skin lesions [Citation21]. Additionally, early active treatment can effectively control disease progression, prevent or delay the onset of co-morbidities and even alter the natural history of psoriasis [Citation28].
4.5. Drug retention rate
Although various biological agents have been proven to be both effective and safe, some patients have not achieved the expected results and have experienced varying degrees of toxic reactions in the short or long term. This variability in response may be influenced by genetic factors, such as genetic polymorphisms involved in the pathological environment, metabolism, or drug mechanism of action, which can potentially affect the effectiveness and toxicity of biological therapies [Citation29]. Furthermore, biologics are immunogenic and may result in the production of anti-drug antibodies, leading to secondary failure. These factors contribute to a decrease in the drug retention rate of biological agents in patients, ultimately reducing their efficacy. Real-world studies have shown that Secukinumab has a high three-year drug retention rate in the treatment of psoriasis. This is beneficial for long-term treatment, and the drug retention rate is higher among patients who are new to biologic treatment [Citation30,Citation31]. The BADBIR study found that drug survival was associated with Psoriatic Arthritis, nail involvement, prior biological exposure, and race and ethnicity [Citation32]. Further research revealed that Guselkumab has a higher drug retention rate than the other drugs, with Ustekinumab following closely behind [Citation32]. This confirms that IL-23 inhibitors are advantageous for long-term treatment of psoriasis [Citation28,Citation33–36].
4.6. Pediatric psoriasis
Psoriasis often occurs in infancy in non-adults. The disease develops in more than one-third of cases by age 2 years, and approximately 1% of children in the United States develop the disease during adolescence [Citation37]. The reported prevalence rate in children aged 1–18 years in Europe is 0.17%–1.5% [Citation38]. In children, psoriasis is often caused by infection, stress, skin trauma, and an increased body mass index (BMI). Due to the imperfect functions of organs such as the liver and kidney, drug metabolism is slow, and adverse reactions are prone to occur. Therefore, the safety of psoriasis drugs in children is of particular importance. Currently, Adalimumab, Ixekizumab, Secukinumab, Etanercept, and Ustekinumb are approved for use in pediatric patients over 6 years old as new targeted drugs in the treatment of psoriasis. Adalimumab can also be used for patients over 4 years old.
4.7. Comorbidities and psoriasis at specific sites
A Clinical scholars generally believe that the severity of skin symptoms is directly proportional to the level of inflammation. However, recent basic researches have found that patients with mild to moderate psoriasis and limited affected areas may also have severe inflammation and a higher risk of comorbidities [Citation39–42]. In whole-genome analysis, genes related to psoriasis include LCE3BC and HLACw6. Other genes related to psoriasis comorbidities, such as IL12B, 5p31, TNFAIP3, and IL23R, are associated with psoriatic arthritis, spondyloarthritis, inflammatory bowel disease, asthma, and metabolic syndrome [Citation43–45]. Research indicates that psoriasis comorbidities impose a significant burden on patients that accumulates over time and lasts a lifetime [Citation46]. For patients with moderate to severe psoriasis, particularly those with rapidly progressive disease and high-risk or existing comorbidities, biologics may be a more effective treatment option [Citation25,Citation27].
Psoriasis can cause skin damage not only on the limbs and trunk, but also on special areas such as the scalp, fingernails, and even the genitals. The survey data revealed that 50% of patients had scalp involvement, 36% had genital involvement, and 14% had involvement of the palms and soles [Citation47]. Studies have also identified the calf as the most common residual and difficult-to-treat site, in addition to the traditionally considered special sites [Citation48]. According to the PSOHO study, a greater proportion of patients achieved complete clearance with Risankizumab compared to other biological agents such as Secukinumab, Guselkumab, Adalimumab, and Ustekinumab [Citation49]. Several studies have shown rapid and stable efficacy of Ezekizumab in specific areas such as the scalp, nails, palmoplantar and genital areas [Citation50,Citation51].
4.8. Psoriatic arthritis
Psoriatic arthritis (PsA) has been clinically understood for centuries, from the first discovery of joint erosion in the thirteenth century AD to the connection with psoriasis in 1860, and the formal naming of PsA as an independent disease in 1964. Approximately one-third of psoriasis patients have undiagnosed PsA. However, there is still a common delay in diagnosing PsA [Citation52]. A delay in diagnosis of more than six months can lead to impaired joint function and long-term deterioration of body function [Citation53]. Biological agents can effectively control the symptoms of psoriasis while delaying its progression and reducing the occurrence of PsA. Secukinumab significantly reduces inflammatory markers and maintains stable BMI and blood lipid levels in patients with PsA [Citation54,Citation55]. Additionally, it improves coronary atherosclerosis in PsA patients and alleviates anxiety and depression in patients with moderate to severe psoriasis and PsA [Citation56]. IL-23 inhibitors are considered the most effective in delaying the onset of arthritis and improving joint pain and skin lesions [Citation57,Citation58]. Guselkumab has the potential to prevent the progression of psoriasis to PsA in patients with moderate to severe psoriasis who are at high risk of short-term progression to PsA [Citation59]. For psoriasis patients with abnormal entheseal inflammation on ultrasound, the entheseal inflammation score decreased by 42.2% from baseline at week 24 of Ustekinumab treatment and by 47.5% at week 52 of treatment [Citation60].
4.9. Strengths and limitations
Several biologics are approved for the treatment of psoriasis. However, in-depth research into the specific targeting of immune pathways and increasing clinical evidence for targeted drugs indicate that their efficacy is variable. Our review provides important information and insights into research directions and trends in biologics for the treatment of psoriasis. However, it is important to note that this article has certain limitations. This study focuses solely on English literature in WoSCC database. It is important to note that the lack of relevant Chinese literature may result in incomplete findings. Additionally, recently published documents were at a disadvantage in the citation analysis due to the shorter period during which they could be cited. Nonetheless, these results provide a useful summary of knowledge for researchers interested in this field and offer scholars scientific research ideas and reference directions.
5. Conclusions
The number of academic articles on biologics for the treatment of psoriasis has generally increased over the years, indicating the importance researchers attach to this particular topic. Currently, there are four challenges that require attention in the field of psoriasis: early intervention and disease modification, management of comorbidities, disease progression and long-term management, and management of special or difficult-to-treat areas. Early use of biologics is the best treatment option for psoriasis. Secukinumab and Guselkumab are receiving much attention. In addition, further clinical practice is needed to address issues such as loss of efficacy and immune phenotype switching that may occur with biologic therapy.
Ethics statement
Review and/or approval by an ethics committee was not needed for this study because this study was an analysis based on the literature and did not involve human or animal studies.
Authors’ contributions
Yingdong Wang: Conceptualization, Methodology, Investigation, Writing – Original Draft, Writing – Review & Editing. Junchen Li: Conceptualization, Investigation. Chenqi Guo: Methodology, Investigation. Guojing Yang: Methodology. Haiyue Lin: Investigation. Yu Zhang: Writing – Review & Editing, Funding Acquisition, Resources, Supervision.
Supplemental Material
Download ()Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability statement
All the results found during this study are available in this article.
Additional information
Funding
References
- McInnes IB, Gravallese EM. Immune-mediated inflammatory disease therapeutics: past, present and future. Nat Rev Immunol. 2021;21(10):1–11. doi: 10.1038/s41577-021-00603-1.
- Krueger JG. The immunologic basis for the treatment of psoriasis with new biologic agents. J Am Acad Dermatol. 2002;46(1):1–26. doi: 10.1067/mjd.2002.120568.
- Chamian F, Lin SL, Lee E, et al. Alefacept (anti-CD2) causes a selective reduction in circulating effector memory T cells (tem) and relative preservation of Central memory T cells (tcm) in psoriasis. J Transl Med. 2007;5(1):27. doi: 10.1186/1479-5876-5-27.
- Haider AS, Cohen J, Fei J, et al. Insights into gene modulation by therapeutic TNF and IFNgamma antibodies: TNF regulates IFNgamma production by T cells and TNF-regulated genes linked to psoriasis transcriptome. J Invest Dermatol. 2008;128(3):655–666. doi: 10.1038/sj.jid.5701064.
- Schlapbach C, Conrad C. TYK-ing all the boxes in psoriasis. J Allergy Clin Immunol. 2022;149(6):1936–1939. doi: 10.1016/j.jaci.2022.03.014.
- Chen C, Song M. Visualizing a field of research: a methodology of systematic scientometric reviews. PLoS One. 2019;14(10):e0223994. doi: 10.1371/journal.pone.0223994.
- Kokol P, Blažun Vošner H, Završnik J. Application of bibliometrics in medicine: a historical bibliometrics analysis. Health Info Libr J. 2021;38(2):125–138. doi: 10.1111/hir.12295.
- Chen CH, Chien TW, Yu-Chieh Ho S, et al. Predicting article citations using data from 100 top-cited publications in the field of psoriasis vulgaris and biological agents (PVBA) since 1991: a bibliometric analysis. Medicine (Baltimore). 2022;101(30):e29396. doi: 10.1097/MD.0000000000029396.
- Langley RG, Elewski BE, Lebwohl M, FIXTURE Study Group., et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338. doi: 10.1056/NEJMoa1314258.
- Parisi R, Iskandar IYK, Kontopantelis E, and Global Psoriasis Atlas., et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369:m1590. doi: 10.1136/bmj.m1590.
- Shao K, Hooper J, Feng H. Racial and ethnic health disparities in dermatology in the United States. Part 2: disease-specific epidemiology, characteristics, management, and outcomes. J Am Acad Dermatol. 2022;87(4):733–744. doi: 10.1016/j.jaad.2021.12.062.
- Narla S, Heath CR, Alexis A, et al. Racial disparities in dermatology. Arch Dermatol Res. 2023;315(5):1215–1223. doi: 10.1007/s00403-022-02507-z.
- Molloy ES, Calabrese LH. Therapy: Targeted but not trouble-free: efalizumab and PML. Nat Rev Rheumatol. 2009;5(8):418–419. doi: 10.1038/nrrheum.2009.142.
- Erichsen CY, Jensen P, Kofoed K. Biologic therapies targeting the interleukin (IL)-23/IL-17 immune axis for the treatment of moderate-to-severe plaque psoriasis: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2020;34(1):30–38. doi: 10.1111/jdv.15879.
- Bellinato F, Gisondi P, Girolomoni G. Latest advances for the treatment of chronic plaque psoriasis with biologics and oral small molecules. Biologics. 2021;15:247–253. doi: 10.2147/BTT.S290309.
- Strychalski ML, Brown HS, Bishop SC. Cytokine modulators in plaque Psoriasis - A review of current and prospective biologic therapeutic approaches. JAAD Int. 2022;9:82–91. doi: 10.1016/j.jdin.2022.08.008.
- Guo J, Zhang H, Lin W, et al. Signaling pathways and targeted therapies for psoriasis. Signal Transduct Target Ther. 2023;8(1):437. doi: 10.1038/s41392-023-01655-6.
- Damiani G, Allocco F, Malagoli P, Young Dermatologists Italian Network. COVID-19 vaccination and patients with psoriasis under biologics: real-life evidence on safety and effectiveness from italian vaccinated healthcare workers. Clin Exp Dermatol. 2021;46(6):1106–1108. doi: 10.1111/ced.14631.
- Campanati A, Diotallevi F, Martina E, et al. Treatment of moderate to severe psoriasis during the COVID-19 pandemic: lessons learned and opportunities. J Clin Med. 2022;11(9):2422. doi: 10.3390/jcm11092422.
- Piccolo V, Russo T, Mazzatenta C, et al. COVID vaccine-induced pustular psoriasis in patients with previous plaque type psoriasis. J Eur Acad Dermatol Venereol. 2022;36(5):e330–e332. doi: 10.1111/jdv.17918.
- Iversen L, Eidsmo L, Austad J, et al. Secukinumab treatment in new-onset psoriasis: aiming to understand the potential for disease modification - rationale and design of the randomized, multicenter STEPIn study. J Eur Acad Dermatol Venereol. 2018;32(11):1930–1939. doi: 10.1111/jdv.14979.
- Li Q, Pang B, Dang E, et al. Endothelial dysfunction in psoriasis: an integrative review. J Invest Dermatol. 2024. S0022-202X(24)00171-4. Advance online publication. doi: 10.1016/j.jid.2024.02.013.
- Ho AW, Kupper TS. T cells and the skin: from protective immunity to inflammatory skin disorders. Nat Rev Immunol. 2019;19(8):490–502. doi: 10.1038/s41577-019-0162-3.
- Cheuk S, Wikén M, Blomqvist L, et al. Epidermal Th22 and Tc17 cells form a localized disease memory in clinically healed psoriasis. J Immunol. 2014;192(7):3111–3120.), doi: 10.4049/jimmunol.1302313.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–1072. doi: 10.1016/j.jaad.2018.11.057.
- Nast A, Altenburg A, Augustin M, et al. German S3-Guideline on the treatment of psoriasis vulgaris, adapted from EuroGuiDerm - Part 1: Treatment goals and treatment recommendations. J Dtsch Dermatol Ges. 2021;19(6):150–934. doi: 10.1111/ddg.14508.
- Smith CH, Yiu ZZN, Bale T, and British Association of Dermatologists’ Clinical Standards Unit., et al. British association of dermatologists guidelines for biologic therapy for psoriasis 2020: a rapid update. Br J Dermatol. 2020;183(4):628–637. doi: 10.1111/bjd.19039.
- Mehta H, Mashiko S, Angsana J, et al. Differential changes in inflammatory mononuclear phagocyte and T-Cell profiles within psoriatic skin during treatment with Guselkumab vs. Secukinumab. J Invest Dermatol. 2021;141(7):1707–1718.e9. doi: 10.1016/j.jid.2021.01.005.
- Membrive Jiménez C, Pérez Ramírez C, Sánchez Martín A, et al. Influence of genetic polymorphisms on response to biologics in moderate-to-severe psoriasis. J Pers Med. 2021;11(4):293. doi: 10.3390/jpm11040293.
- Augustin M, Sator PG, von Kiedrowski R, and SERENA study group., et al. Secukinumab demonstrated sustained retention, effectiveness and safety in a real-world setting in patients with moderate-to-severe plaque psoriasis: long-term results from an interim analysis of the SERENA study. J Eur Acad Dermatol Venereol. 2022;36(10):1796–1804. doi: 10.1111/jdv.18329.
- Yiu ZZN, Mason KJ, Hampton PJ, and BADBIR Study Group., et al. Drug survival of adalimumab, ustekinumab and secukinumab in patients with psoriasis: a prospective cohort study from the british association of dermatologists biologics and immunomodulators register (BADBIR). Br J Dermatol. 2020;183(2):294–302. doi: 10.1111/bjd.18981.
- Yiu ZZN, Becher G, Kirby B, and BADBIR Study Group., et al. Drug survival associated with effectiveness and safety of treatment with Guselkumab, Ixekizumab, Secukinumab, Ustekinumab, and Adalimumab in patients with psoriasis. JAMA Dermatol. 2022;158(10):1131–1141. doi: 10.1001/jamadermatol.2022.2909.
- Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394(10201):831–839. doi: 10.1016/S0140-6736(19)31773-8.
- Reich K, Gordon KB, Strober BE, et al. Five-year maintenance of clinical response and health-related quality of life improvements in patients with moderate-to-severe psoriasis treated with Guselkumab: results from VOYAGE 1 and VOYAGE 2. Br J Dermatol. 2021;185(6):1146–1159. doi: 10.1111/bjd.20568.
- Gordon KB, Armstrong AW, Foley P, et al. Guselkumab efficacy after withdrawal is associated with suppression of serum IL-23-Regulated IL-17 and IL-22 in psoriasis: VOYAGE 2 study. J Invest Dermatol. 2019;139(12):2437–2446.e1. doi: 10.1016/j.jid.2019.05.016.
- Rivera R, Martorell A, López A, et al. Maintenance of response following discontinuation of Guselkumab and Secukinumab in spanish patients who participated in the ECLIPSE study. J Eur Acad Dermatol Venereol. 2021;35(1):e65–e67. doi: 10.1111/jdv.16809.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American academy of Dermatology-National psoriasis foundation guidelines of care for the management and treatment of psoriasis in pediatric patients. J Am Acad Dermatol. 2020;82(1):161–201. doi: 10.1016/j.jaad.2019.08.049.
- Peris K, Fortina AB, Bianchi L, et al. Update on the management of pediatric psoriasis: an italian consensus. Dermatol Ther (Heidelb). 2022;12(8):1753–1775. doi: 10.1007/s13555-022-00758-2.
- Kim J, Bissonnette R, Lee J, et al. The spectrum of mild to severe psoriasis vulgaris is defined by a common activation of IL-17 pathway genes, but with key differences in immune regulatory genes. J Invest Dermatol. 2016;136(11):2173–2182. doi: 10.1016/j.jid.2016.04.032.
- Gottlieb AB, Merola JF, Cirulli J, et al. Characteristics of patients with psoriasis treated with apremilast in the corrona psoriasis registry. Dermatol Ther (Heidelb). 2021;11(1):253–263. doi: 10.1007/s13555-020-00479-4.
- Boehncke WH, Brembilla NC. Unmet needs in the field of psoriasis: pathogenesis and treatment. Clin Rev Allergy Immunol. 2018;55(3):295–311. doi: 10.1007/s12016-017-8634-3.
- de Hooge M, Ishchenko A, De Craemer AS, et al. Extent of axial damage in psoriatic arthritis and spondyloarthritis: comparative data from the BEPAS and (be-)GIANT multicentre cohorts. RMD Open. 2023;9(2):e002994. doi: 10.1136/rmdopen-2023-002994.
- Patel HA, Revankar RR, Pedroza ST, et al. The genetic susceptibility to psoriasis and the relationship of linked genes to our treatment options. Int J Mol Sci. 2023;24(15):12310. doi: 10.3390/ijms241512310.
- Giang NH, Lien NTK, Trang DT, et al. Associations of A20, CYLD, cezanne and JAK2 genes and immunophenotype with psoriasis susceptibility. Medicina (Kaunas). 2023;59(10):1766. doi: 10.3390/medicina59101766.
- Zhu D, Yao S, Wu H, et al. A transcriptome-wide association study identifies novel susceptibility genes for psoriasis. Hum Mol Genet. 2021;31(2):300–308. doi: 10.1093/hmg/ddab237.
- Bhatti ZU, Salek MS, Finlay AY. Major life changing decisions and cumulative life course impairment. J Eur Acad Dermatol Venereol. 2011;25(2):245–246; author reply 246. doi: 10.1111/j.1468-3083.2010.03930.x.
- Kaeley GS, Eder L, Aydin SZ, et al. Nail psoriasis: diagnosis, assessment, treatment options, and unmet clinical needs. J Rheumatol. 2021;48(8):1208–1220. doi: 10.3899/jrheum.201471.
- Hjuler KF, Iversen L, Rasmussen MK, et al. Localization of treatment-resistant areas in patients with psoriasis on biologics. Br J Dermatol. 2019;181(2):332–337. doi: 10.1111/bjd.17689.
- Piaserico S, Riedl E, Pavlovsky L, et al. Comparative effectiveness of biologics for patients with moderate-to-severe psoriasis and special area involvement: week 12 results from the observational psoriasis study of health outcomes (PSoHO). Front Med (Lausanne). 2023;10:1185523. doi: 10.3389/fmed.2023.1185523.
- Egeberg A, Kristensen LE, Vender R, et al. Sustained resolution of nail psoriasis through 5 years with ixekizumab: a Post-Hoc analysis from UNCOVER-3. Acta Derm Venereol. 2022;102:adv00787. doi: 10.2340/actadv.v102.2269.
- Egeberg A, Kristensen LE, Puig L, et al. Network meta-analyses comparing the efficacy of biologic treatments for achieving complete resolution of nail psoriasis at 24-28 and 48-52 weeks. J Dermatolog Treat. 2023;34(1):2263108. doi: 10.1080/09546634.2023.2263108.
- Karmacharya P, Wright K, Achenbach SJ, et al. Diagnostic delay in psoriatic arthritis: a population-based study. J Rheumatol. 2021;48(9):1410–1416. doi: 10.3899/jrheum.201199.
- Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015;74(6):1045–1050. doi: 10.1136/annrheumdis-2013-204858.
- Simopoulou T, Tsiogkas SG, Zafiriou E, et al. Secukinumab, ixekizumab, bimekizumab and brodalumab for psoriasis and psoriatic arthritis. Drugs Today (Barc). 2023;59(3):135–167. doi: 10.1358/dot.2023.59.3.3419557.
- Alegre-Sancho JJ, Núñez-Monje V, Campos-Fernández C, et al. Real-world effectiveness and persistence of secukinumab in the treatment of patients with psoriatic arthritis. Front Med (Lausanne). 2023;10:1294247. doi: 10.3389/fmed.2023.1294247.
- Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in psoriasis longitudinal assessment and registry (PSOLAR). J Am Acad Dermatol. 2018;78(1):70–80. doi: 10.1016/j.jaad.2017.08.051.
- Rosenthal YS, Schwartz N, Sagy I, et al. Incidence of psoriatic arthritis among patients receiving biologic treatments for psoriasis: a nested Case-Control study. Arthritis Rheumatol. 2022;74(2):237–243. doi: 10.1002/art.41946.
- Gisondi P, Bellinato F, Targher G, et al. Biological disease-modifying antirheumatic drugs may mitigate the risk of psoriatic arthritis in patients with chronic plaque psoriasis. Ann Rheum Dis. 2022;81(1):68–73. doi: 10.1136/annrheumdis-2021-219961.
- Zabotti A, Giovannini I, McGonagle D, et al. Arthritis interception in patients with psoriasis treated with Guselkumab. Dermatol Ther (Heidelb). 2022;12(1):5–8. doi: 10.1007/s13555-021-00650-5.
- Savage L, Goodfield M, Horton L, et al. Regression of peripheral subclinical enthesopathy in therapy-Naive patients treated with ustekinumab for moderate-to-severe chronic plaque psoriasis: a Fifty-Two-week, prospective, Open-Label feasibility study. Arthritis Rheumatol. 2019;71(4):626–631. doi: 10.1002/art.40778.