Abstract
Purpose: Real-world data comparing long-term performance of interleukin (IL)-23 and IL-17 inhibitors in psoriasis are limited. This study compared treatment persistence and remission among patients initiating guselkumab versus IL-17 inhibitors.
Methods: Adults with psoriasis initiating guselkumab, secukinumab, or ixekizumab treatment (index date) were identified from Merative™ MarketScan® Research Databases (01/01/2016–10/31/2021). Persistence was defined as no index biologic supply gaps of twice the labeled maintenance dosing interval. Remission was defined using an exploratory approach as index biologic discontinuation for ≥6 months without psoriasis-related inpatient admissions and treatments.
Results: There were 3516 and 6066 patients in the guselkumab versus secukinumab comparison, and 3805 and 4674 patients in guselkumab versus ixekizumab comparison. At 18 months, the guselkumab cohort demonstrated about twice the persistence rate as secukinumab (hazard ratio [HR] = 2.15; p < 0.001) and ixekizumab cohorts (HR = 1.77; p < 0.001). At 6 months after index biologic discontinuation, the guselkumab cohort was 31% and 40% more likely to achieve remission than secukinumab (rate ratio [RR] = 1.31; p < 0.001) and ixekizumab cohorts (RR = 1.40; p < 0.001).
Conclusions: Guselkumab was associated with greater persistence and likelihood of remission than IL-17 inhibitors, indicating greater disease control and modification potential.
Introduction
Moderate-to-severe psoriasis is a chronic immune-mediated skin disease that generally requires long-term treatment with systemic therapy, including biologics and oral therapies, such as immunosuppressants, systemic retinoids, and apremilast (Citation1,Citation2). Biologics represent an important advancement in the management of psoriasis and generally have better efficacy and long-term safety compared with oral therapies (Citation1–3). Currently, there are four classes of biologics approved by U.S. Food and Drug Administration (FDA) for the treatment of moderate-to-severe psoriasis, notably, tumor necrosis factor (TNF) inhibitors, such as infliximab (approval in 2006) and adalimumab (approval in 2008); the interleukin (IL)-12/23 inhibitor ustekinumab (approval in 2008); IL-17 inhibitors, such as secukinumab (approval in 2015), and ixekizumab (approval in 2016); and IL-23 inhibitors, such as guselkumab (approval in 2017) and risankizumab (approval in 2019) (Citation4,Citation5).
In clinical trials, IL-23 inhibitors had greater efficacy than TNF, IL-12/23, and IL-17 inhibitors (Citation6–8). In meta-analyses, IL-17 and IL-23s were often the most efficacious, with similar rates of efficacy (Citation9–11), while IL-17s typically provided the most rapid response overall (Citation12). However, in the real-world setting, the effectiveness of biologics may vary among patients and may wane with time (Citation5,Citation13,Citation14). The relative performance of biologics in a real-world setting can be analyzed using health insurance claims data, which typically allows for a large sample size, U.S.-wide geographic coverage, and complete information on medical services and prescriptions. Specifically, persistence on a biologic can be determined from claims data by assessing gaps in treatment claims over time (Citation15). Drug persistence refers to the time from treatment initiation to discontinuation, and could be an indicator of real-world effectiveness, safety, and convenience, making it a valuable tool for helping to select a biologic therapy (Citation13,Citation14). Although continuous treatment is beneficial in the management of moderate-to-severe psoriasis (Citation16), achieving intervals of long-term treatment-free disease is a highly desirable goal (Citation2,Citation15). While claims data do not include clinical information on symptom remission, treatment patterns may be used as a proxy for this (Citation15,Citation17).
At present, real-world data evaluating the comparative effectiveness of guselkumab to IL-17 inhibitors are limited (Citation6). In a previous descriptive analysis, guselkumab-treated patients had better treatment persistence and higher rates of remission than adalimumab-, secukinumab-, or ixekizumab-treated patients (Citation17). However, unadjusted differences between treatment cohorts may have confounded results, warranting additional analyses. Furthermore, a variable therapy exposure gap proportional to the frequency of biologic administration was used to define persistence in previous work. Due to inherent differences in dosing schedules and to ensure a more robust assessment, application of a standard, fixed exposure gap for all biologics would be important to help avoid bias (Citation15). The aim of this study was to address some of the limitations of earlier reports and to conduct a more comprehensive comparison of real-world treatment persistence and remission among patients with psoriasis initiating guselkumab versus the IL-17 inhibitors, secukinumab and ixekizumab.
Methods
Data source
Administrative claims data were obtained from the Merative™ MarketScan® Commercial and Medicare Supplemental Research Databases (01/01/2016–10/31/2021). The Commercial database consists of employer and health plan-sourced data beneficiaries, comprising employees, their spouses, and dependents who are covered by employer-sponsored private health insurance. The Medicare Supplemental database profiles retirees with employer-sponsored Medicare Supplemental plans; only plans with both the Medicare-paid amounts and the employer-paid amounts available in claims are selected for this database. Both the Commercial and Medicare Supplemental databases cover all U.S. census regions, with a higher concentration in the South and North Central (Midwest) regions. The data contain information on demographics, member health plan eligibility, and medical and prescription drug claims. The data were de-identified and comply with the Health Insurance Portability and Accountability Act (HIPAA) regulations.
Study design
A retrospective longitudinal cohort design was used. A pairwise analysis of index biologics i.e., guselkumab versus secukinumab and guselkumab versus ixekizumab was conducted for both persistence and remission outcomes. Additionally, adherence on index biologic was described.
To analyze persistence, the index date was defined as the first observed claim for the index biologic (). The index window began on 07/13/2017 (the FDA approval date of guselkumab for plaque psoriasis (Citation18)) to ensure all three index biologics were available on the market. The index window ended 6 months before the end of data availability (05/01/2021) to allow an equal opportunity for patients in all treatment cohorts to discontinue the index biologic before the end of data availability. The baseline period comprised the 12 months before the index date and was used to report patient characteristics. The follow-up period spanned the index date to the earliest of end of data availability or end of continuous health plan eligibility and was used to evaluate persistence outcomes. To analyze adherence, fixed duration of follow-up was imposed after the index date (i.e., 6, 12, 18, and 24 months), and only patients followed for at least the same amount of time were included.
Figure 1. (A) Study design for persistence analysis. (B) Study design for remission analysis among patients who discontinued index treatment for ≥6 months.
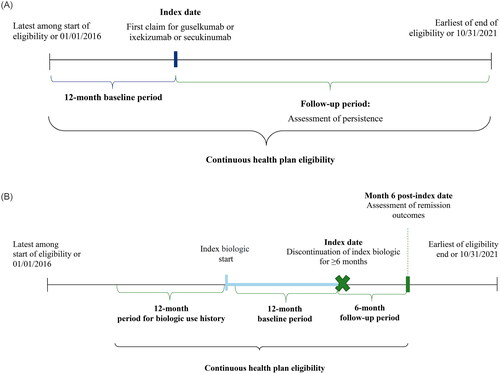
To analyze remission, the index date was defined as the date patients discontinued their index biologic for ≥6 months (). The baseline period was the 12-month period before the index date and was used to report patient characteristics; in addition, the 12-month period before initiation of the index biologic was used to report use of biologics other than the index. The follow-up period spanned 6 months after the index date and remission outcomes were evaluated at month 6 of follow-up.
Study sample
Patients were included in the persistence analysis if (1) the first observed claim for the study biologic (guselkumab, secukinumab, or ixekizumab) occurred during the intake period (i.e., 07/13/2017–05/01/2021); (2) they had ≥12 months of continuous health plan eligibility before the date of initiating the first biologic (index date); (3) they had ≥2 unique dates with diagnoses of psoriasis during the baseline period or on the index date (International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] codes: L40.0-L40.4, L40.8, and L40.9); (4) were ≥18 years old at the index date; (5) had no claims for psoriasis-indicated biologic agents other than the index agent on the index date; and (6) had no claims for either index biologic during the most recent period of continuous eligibility and before index date. Patients in the adherence analysis were additionally required to have ≥6, 12, 18, and 24 months of continuous health plan eligibility after the index date.
Patients included in the remission analysis represented a subset of those in the persistence analysis. They were required to discontinue the index biologic for ≥6 months and to have ≥6 months of continuous health plan eligibility after the discontinuation (new index) date.
Study outcomes
Imputations of days of supply
Persistence in claims data is measured based on the dates of claims and the number of days of supply (i.e., how long the dispensed medication will last) coded for each claim. Days of supply imputations may be warranted given that this information is unavailable in medical claims, and in pharmacy claims, it may deviate from the frequency of drug administration per label. Such deviation may specifically occur for biologics with maintenance dosing intervals greater than 4 weeks (e.g., guselkumab) due to restrictions on the maximum days of supply (typically 30 days) imposed by various health plans (Citation19).
In this study, the mode of administration for all biologics was subcutaneous injection. Only guselkumab had medical claims. If the first guselkumab claim was a medical claim, 28 days of supply were imputed; in all subsequent medical claims 56 days of supply were imputed. These imputations were consistent with the guselkumab dosing frequency per label.
For pharmacy claims, the distribution of days of supply for all study biologics was assessed to decide if imputations were necessary. For guselkumab, 25.6% of pharmacy claims in the maintenance phase were coded with 28–31 days of supply, while the median time to next claim was 54 days. Thus, the second and subsequent guselkumab pharmacy claims coded with 28–31 days in the data were imputed to 56 days, in accordance with the label and the median time to the next claim. Only 0.2% of secukinumab and 0.3% of ixekizumab pharmacy claims during the maintenance phase had days of supply coded with <28 days. Therefore, no imputations were made for secukinumab and ixekizumab pharmacy claims, as coded days of supply in the data were aligned with their respective labels (i.e., 28 days in the maintenance phase).
Persistence
Two definitions of persistence were considered. The primary definition was based on a gap in index biologic supply proportional to the dosing frequency of each biologic. Specifically, persistence was defined as the absence of gaps in index biologic supply of at least twice the labeled maintenance dosing interval (i.e., >120 days for guselkumab (Citation20), >60 days for secukinumab (Citation21) and ixekizumab (Citation22)). The secondary (sensitivity) definition was based on a fixed gap for each of the index biologics. Persistence was defined as the absence of gaps in index biologic supply of >120 days for each of the index biologics. The discontinuation date was the last day with index biologic supply before the gap.
Adherence
Proportion of days covered (PDC) is the measure recommended for assessment of adherence to chronic therapies (Citation23). PDC was defined as the sum of non-overlapping days of supply of the index agent divided by a fixed period (i.e., 6, 12, 18, and 24 months) among patients followed for at least the same fixed duration of time. PDC, including proportion of patients with PDC ≥80%, was described at 6-month intervals.
Remission
Remission of disease was identified among patients who discontinued the index biologic for ≥6 months. The discontinuation date was the last day with the index biologic supply (i.e., the date of the last claim plus days of supply of the last claim) before the gap of ≥6 months until the next claim or the earliest of end of data availability or end of continuous health plan eligibility. Remission was defined as an absence of any psoriasis-related inpatient (IP) admissions, claims for psoriasis-related topical (topical corticosteroids, antipsoriatics, and retinoids), non-biologic systemic (immunosuppressant agents, apremilast, phototherapy, systemic retinoids, and laser therapy), or other biologic agents (anti-TNF, anti-IL-17, anti-IL-12/23, and anti-IL-23) during the 6-month period after the index biologic discontinuation date. Partial remission or remission of moderate-to-severe psoriasis was defined similarly but allowed patients to have claims for topical agents, which may be used for milder disease.
Apart from remission, other reasons may account for remaining off treatment, such as pregnancy, cancer, or a surgical procedure (Citation17). To understand whether these events may have disproportionally driven discontinuation of the index biologic and remaining off treatment in any of the cohorts, the number and proportion of patients with ≥1 diagnosis of pregnancy or cancer or ≥1 surgical procedure during the baseline or follow-up periods were additionally reported for each cohort.
Statistical analysis
Entropy balancing (e-balance) (Citation24) was used to balance potential confounders between guselkumab and each of the control cohorts (i.e., secukinumab or ixekizumab). The control cohorts were each reweighted so that the overall distribution of potential confounders in the resulting cohorts was the same as in the guselkumab cohort with respect to mean and variance (for continuous variables) or proportions (for binary variables). E-balance preserved the original sample size for all cohorts and enforced reweighted units to achieve balance while keeping the weights as close as possible to the base weights. Cohorts were balanced based on the following baseline characteristics: demographics, index year, select psoriasis-related comorbidities and treatments, and all-cause pharmacy and medical costs. The balance of baseline characteristics was assessed using standardized differences, where a standardized difference of <10% indicated a well-balanced characteristic (Citation25).
The e-balance procedure was repeated for the subsets of patients from each cohort included in the remission analysis. In addition to baseline characteristics, the use of non-index biologics measured 12 months prior to treatment initiation was also included in the weighting scheme of the e-balance procedure.
Persistence
Persistent time was evaluated using survival analysis from the index date until the earliest of either the end of the follow-up period or index biologic discontinuation. Patients who did not discontinue their index biologic during the follow-up period were censored on the last day of the index biologic supply before the end of the follow-up period. Probability of persistence was described with Kaplan–Meier (KM) analysis and median time of persistence (i.e., time when ≥50% of patients remained persistent) was reported, when reached. Probability of persistence at 3, 6, 12, 18, and 24 months of follow-up was compared with log-rank tests. Unadjusted weighted Cox proportional hazard models were used to compare rates of persistence at 3, 6, 12, 18, and 24 months of follow-up.
Adherence
Adherence was described using means, standard deviations, and medians for continuous variables and frequencies and proportions for categorical variables. No statistical comparisons were conducted.
Remission
Remission rates at month 6 of follow-up were described using frequencies and proportions and compared between cohorts using modified Poisson regressions with robust error variance. Modified Poisson regressions were used instead of logistic regressions, since the incidence of both remission and partial remission was well above 10% in all cohorts, and logistic regressions would overestimate the relative risk associated with a binary outcome in such circumstances (Citation26).
Results
Persistence analysis
Baseline characteristics
A total of 3516 and 6066 patients were included in the guselkumab versus secukinumab comparison, respectively, and 3805 and 4674 patients were included in the guselkumab versus ixekizumab comparison, respectively (Supplementary Figure S1 and S2). After e-balancing, across all cohorts, patients had a mean age of 48 years and 48% were female. During the baseline period, use of biologics was observed in 37.4% of patients in the guselkumab versus secukinumab comparison and in 41.1% of patients in the guselkumab versus ixekizumab comparison ().
Table 1. Baseline characteristics for persistence analysis.
Persistence of guselkumab versus secukinumab
Using the primary definition of persistence, the median time to discontinuation was 29.2 months for the guselkumab cohort and 10.7 months for the secukinumab cohort (). At 18 months of follow-up, probability of persistence was 61.5% for the guselkumab cohort and 33.4% for the secukinumab cohort (p < 0.001). At 18 months, persistence was 2.2 times higher for the guselkumab cohort than persistence for the secukinumab cohort (p < 0.001). Trends remained consistent at 24 months; however, results at this timepoint should be interpreted with caution as the proportion of patients at risk was below 10% in the secukinumab cohort, suggesting that these results may be less reliably estimated (Citation27).
Figure 2. (A) Persistence in weighted guselkumab and secukinumab cohorts using primary definition.1 (B) Persistence in weighted guselkumab and ixekizumab cohorts using primary definition.1
CI: Confidence interval.
Notes:
1Time to treatment discontinuation (or persistence end) was measured from the index date until the discontinuation date. The primary gap definition was >120 days for guselkumab or >60 days for secukinumab and ixekizumab. Patients who did not discontinue during the follow-up period were censored on the last day of the index agent supply before the end of follow-up.
2Patients at risk of having the event are patients who have not had the event and have not been lost to follow-up at that point in time.
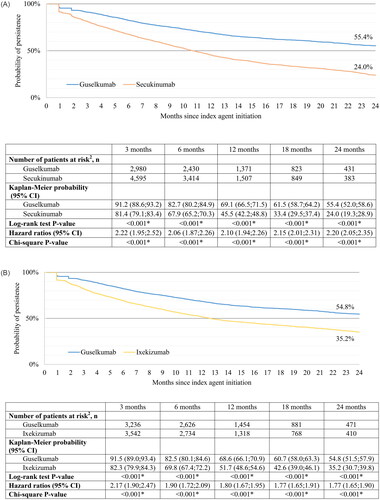
Similar results were obtained using the secondary definition of persistence based on the fixed 120-day exposure gap. Specifically, persistence for the guselkumab cohort was 1.6 times greater than persistence for the secukinumab cohort at 18 months (p < 0.001; Supplementary Figure S3 (A)).
Persistence of guselkumab versus ixekizumab
Using the primary definition of persistence, the median time to discontinuation was 28.9 months for the guselkumab cohort and 12.6 months for the ixekizumab cohort (). At 18 months of follow-up, probability of persistence was 60.7% for the guselkumab cohort and 42.6% for the ixekizumab cohort (p < 0.001). At 18 months, persistence for the guselkumab cohort was 1.8 times higher than persistence for the ixekizumab cohort (p < 0.001). Trends remained consistent at 24 months; however, the proportion of patients at risk at this timepoint was below 10% in the ixekizumab cohort suggesting that these results may be less reliably estimated (Citation27).
Similar results were observed using the secondary definition of persistence based on the fixed 120-day exposure gap. Specifically, persistence for the guselkumab cohort was 1.2 times greater than persistence for the ixekizumab cohort at 18 months (p < 0.001; Supplementary Figure S3 (B)).
Adherence analysis
Overall, the mean PDC and the proportion of patients with PDC ≥80% trended higher for guselkumab compared to secukinumab and ixekizumab cohorts (Supplementary Table S1).
At month 24 of the follow-up period, in the guselkumab versus secukinumab cohorts, the mean PDC was 60% versus 54%; the proportion of patients with PDC ≥80% was 35% versus 27%, respectively. In the guselkumab versus ixekizumab cohorts, the mean PDC was 60% versus 54%; the proportion of patients with PDC ≥80% was 35% versus 29%, respectively.
Remission analysis
Baseline characteristics
A total of 915 patients in the guselkumab cohort and 2694 patients in the secukinumab cohort discontinued index treatment for ≥6 months while remaining continuously enrolled in a health plan and were included in the remission analysis. Similarly, 1019 and 1434 patients were included in guselkumab versus ixekizumab cohorts, respectively. The mean age across cohorts was similar to that in the persistence analysis (48 years), but a slightly higher proportion of patients were female (51%; ).
Table 2. Baseline characteristics for remission analysis.
The definition of remission required patients to have no psoriasis-related IP admissions and treatments during the follow-up period. During the baseline period, the proportion of patients with ≥1 psoriasis-related IP admission was 0.9% versus 1.3% in the guselkumab versus secukinumab cohorts, and 1.1% versus 0.7% in the guselkumab versus ixekizumab cohorts, respectively. Topical therapies were used during the baseline period by 52.1% and 51.1% of patients in the guselkumab versus secukinumab cohorts and by 55.6% and 57.5% in the guselkumab versus ixekizumab cohorts, respectively. Non-biologic therapies (i.e., immunosuppressant agents, apremilast, phototherapy, systemic retinoids, and laser therapy) were used by 26.9% of patients in the guselkumab versus secukinumab cohorts and 25.4% in the guselkumab versus ixekizumab cohorts. In addition, during the 12 months before index treatment initiation, 41.3% of patients in the guselkumab versus secukinumab cohorts, and 45.9% of patients in the guselkumab versus ixekizumab cohorts, used a prior biologic; TNF inhibitors were the most commonly used biologics across all cohorts.
Remission with guselkumab versus secukinumab
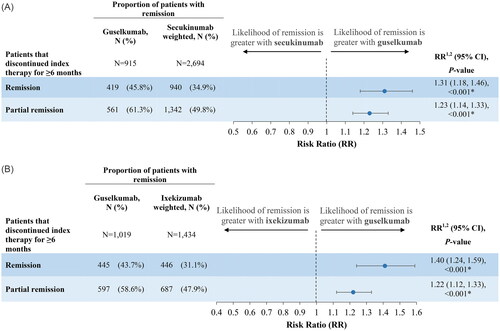
At month 6 of follow-up, 45.8% of patients in the guselkumab cohort versus 34.9% of patients in the secukinumab cohort achieved remission, while 61.3% versus 49.8% of patients in the guselkumab and secukinumab cohorts achieved partial remission, respectively (). The guselkumab cohort was 31% more likely to achieve remission and 23% more likely to achieve partial remission than the secukinumab cohort (p < 0.001).
Figure 3. (A) Remission rates in guselkumab versus secukinumab cohorts. (B) Remission rates in guselkumab versus ixekizumab cohorts.
CI: Confidence interval; RR: Risk ratio.
Notes:
1The risk ratio indicates the relative risk of remission for the guselkumab cohort, relative to the secukinumab and ixekizumab cohorts. A risk ratio >1 indicates that the risk of remission is greater among patients having used guselkumab. A risk ratio <1 indicates that the risk of remission is greater among patients in the control cohort. A risk ratio of 1 indicates that risk of remission is the same between guselkumab and the control cohort.
2Risk ratios, 95% CI, and p values are estimated based on a modified Poisson regression with robust error variance.
Proportions of patients with a diagnosis of pregnancy, cancer, or a surgical procedure during the baseline or follow-up periods, which along with remission also could explain discontinuation of a biologic and remaining untreated, were similar in the guselkumab versus secukinumab cohorts (19.8% versus 19.1%; driven by cancer and surgery in equal parts).
Remission on guselkumab versus ixekizumab
At month 6 of follow-up, 43.7% of patients in the guselkumab cohort versus 31.1% of patients in the ixekizumab cohort achieved remission, while 58.6% versus 47.9% of patients in the guselkumab and ixekizumab cohorts achieved partial remission, respectively (). The guselkumab cohort was 40% more likely to achieve remission and 22% more likely to achieve partial remission than the ixekizumab cohort (p < 0.001).
Proportions of patients with a diagnosis of pregnancy, cancer, or a surgical procedure during the baseline and follow-up periods were similar in the guselkumab versus ixekizumab cohorts (20.3% versus 18.1%; driven by cancer and surgery in equal parts).
Discussion
In these pairwise analyses comparing guselkumab to IL-17 inhibitors among commercially insured U.S. patients with psoriasis, persistence was higher for the guselkumab cohort compared to the secukinumab and ixekizumab cohorts at 18 months after therapy initiation, using both the variable and fixed treatment gap definitions. In addition, guselkumab was associated with a higher likelihood of achieving remission and partial remission, than secukinumab and ixekizumab, using the proxy definition of remission proposed in this study.
This study builds upon existing literature that descriptively reported higher persistence and rates of remission associated with guselkumab treatment versus IL-17 inhibitors (Citation15,Citation17). This study adjusted for differences between guselkumab, secukinumab, and ixekizumab-treated patients based on observable characteristics and used both variable- and fixed-gap definitions of persistence which produced results consistent with previous findings, and suggests higher drug performance of guselkumab relative to IL-17 inhibitors (Citation17,Citation28). Similar trends were observed in real-world European studies, where patients who received IL-23s had higher drug survival, a decreased probability of drug interruption, and better long-term effectiveness than those receiving IL-17s, such as secukinumab (Citation29,Citation30). Results related to remission and partial remission additionally suggest that a substantial proportion of patients with moderate-to-severe psoriasis may be able to achieve these treatment goals with modern biologic treatment, yet IL-23 blockade with guselkumab may be associated with greater disease modification potential than IL-17 inhibition therapy.
Estimates of persistence in claims data vary according to the definition of the maximum permitted gap in treatment, which usually depends on the dosing frequency of the medication analyzed (Citation15). This represents a challenge when a study includes medications with varying dosing frequencies, such as biologics. A variable treatment gap that is proportional to the dosing frequency of each biologic may favor biologics with less frequent dosing, such as guselkumab (Citation15,Citation20). Conversely, a fixed treatment gap length may favor biologics with more frequent dosing, such as secukinumab or ixekizumab (Citation15,Citation21,Citation22). In this study, both a variable gap (i.e., twice the labeled dosing frequency) and a fixed treatment gap (i.e., 120-day gap) were used for a balanced approach to estimating persistence. The results of this study confirm that irrespective of the gap definition used, guselkumab demonstrated higher persistence rates than IL-17 inhibitors.
At present, clear guidelines regarding treatment discontinuation do not exist and the decision to discontinue treatment usually relies on discussion between the patient and physician (Citation31). Although continuous treatment with biologics is generally recommended, intermittent treatment or treatment discontinuation following skin clearance may be of interest to some patients (Citation2,Citation15). In a study evaluating outcomes following treatment discontinuation in select patients who achieved remission, almost half had sustained remission or reinitiated only topical treatment after 2 years (Citation32). Thus, in the absence of precise information on disease control from claims data, lack of treatment change following discontinuation may be an acceptable proxy of remission (Citation15). The definition of remission used in this study remains exploratory in nature. Discontinuation of a biologic may occur for several reasons, including lack of efficacy, economic burden, or tolerability (Citation33). Alternative reasons for treatment discontinuation may include pregnancy, cancer diagnosis, surgical procedure, or other patient factors (Citation15,Citation17). In this study, similar proportions of patients with occurrences of pregnancy, cancer, or surgery were observed in each cohort during the baseline and follow-up periods, suggesting that these events were not the main drivers behind differences in the proxy of remission between the cohorts. While recognizing the limitations of the proxy of remission used in this study, long-term, treatment-free disease control could be a realistic potential goal for patients and their physicians (Citation15).
Limitations
This analysis is associated with additional limitations. First, the results of this analysis may not be generalized to uninsured patients or patients with noncommercial insurance. Second, in this study, persistence and remission outcomes were based on administrative claims data. Prescription fills captured in the data base do not guarantee that the medication received was taken as prescribed, which may result in an overestimation of persistence. Third, the proxy definition of remission used in this study is exploratory in nature. The remission of psoriasis should be defined based on objective assessments of psoriasis lesions, such as psoriasis area and severity index (PASI); however, finding a large real-world source of data with this information remains a challenge. Claims data do not provide information on reasons for remaining off biologic therapy and cannot definitively attribute the treatment-free period to remission; other reasons may exist (e.g., pregnancy, upcoming surgery, and cancer) (Citation15). Finally, the results of this study may be subject to residual confounding. For example, e-balancing did not directly control for psoriasis severity and disease duration as these data were not available in health insurance claims. Nonetheless, e-balancing accounted for other covariates that may serve as a proxy for disease severity, such as prior biologic exposure, comorbidities, and healthcare costs, which may help mitigate the potential impact of this limitation.
Conclusions
In this pairwise analysis, patients with psoriasis who received guselkumab had greater rates of persistence than patients who received secukinumab and ixekizumab. While data on clinical assessment of psoriasis lesions was unavailable to study remission, based on a proxy definition relying on treatment patterns, patients who received guselkumab were also more likely to achieve remission and partial remission. The results of this study may suggest greater drug performance and disease modification potential with guselkumab relative to IL-17 inhibitors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Compliance with ethics guidelines
All data were de-identified and complied with HIPAA regulations; therefore, institutional review board approval was not required.
Authors’ contributions
All authors reviewed and approved the final content of this manuscript.
Previous presentations
Part of the results was included in presentations at the Fall Clinical Dermatology Conference 2023, October 19–22, 2023, Las Vegas, Nevada, and Fall Clinical Dermatology Conference, October 20–23, 2022 in Las Vegas, Nevada. were included as part of the response to the letter to the editor of Dermatology and Therapy – Re: Blauvelt et al. on Long-Term Psoriasis Control with Guselkumab, Adalimumab, Secukinumab, or Ixekizumab in the USA.
Medical writing, editorial, and other assistance
Medical writing assistance was provided by Roxanne Wosu, an employee of Analysis Group, Inc., a consulting company that has provided paid consulting services to Janssen Scientific Affairs, LLC which funded the development and conduct of this study and manuscript.
Supplemental Material
Download MS Word (269.6 KB)Disclosure statement
Timothy Fitzgerald and Rachel Teneralli are employees of Janssen Scientific Affairs, LLC, and stockholders of Johnson & Johnson. Maryia Zhdanava, Dominic Pilon, Aditi Shah, Lilian Diaz, and Patrick Lefebvre are employees of Analysis Group, Inc., a consulting company that has provided paid consulting services to Janssen Scientific Affairs, LLC. Steven R. Feldman received research, speaking and/or consulting support from Janssen Scientific Affairs, LLC and is the founder and majority owner of www.DrScore.com [drscore.com] and founder and part owner of Causa Research.
Data availability statement
The data that support the findings of this study are available from the Merative™ MarketScan® Research Databases, but restrictions apply to the availability of these data, which were used under license for this study, and so are not publicly available. Any researchers interested in obtaining the data used in this study can access database under a license agreement, including the payment of appropriate license fee.
Additional information
Funding
References
- Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1–11.
- Gisondi P, Del Giglio M, Girolomoni G. Treatment approaches to moderate to severe psoriasis. Int J Mol Sci. 2017;18(11):2427.
- Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2022;5(5):CD011535.
- Abbvie Inc. SKYRIZI® (rizankizumab) prescribing information. 2023. Available from: https://www.rxabbvie.com/pdf/skyrizi_pi.pdf
- Menter A, Cordoro KM, Davis DMR, et al. Joint American academy of Dermatology-National psoriasis foundation guidelines of care for the management and treatment of psoriasis in pediatric patients. J Am Acad Dermatol. 2020;82(1):161–201. doi: 10.1016/j.jaad.2019.08.049.
- Sawyer LM, Malottki K, Sabry-Grant C, et al. Assessing the relative efficacy of interleukin-17 and interleukin-23 targeted treatments for moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response. PLoS One. 2019;14(8):e0220868. doi: 10.1371/journal.pone.0220868.
- Menter A, Krueger GG, Paek SY, et al. Interleukin-17 and interleukin-23: a narrative review of mechanisms of action in psoriasis and associated comorbidities. Dermatol Ther (Heidelb). 2021;11(2):385–400. doi: 10.1007/s13555-021-00483-2.
- Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. The Lancet. 2019;394(10201):831–839. doi: 10.1016/S0140-6736(19)31773-8.
- Wright E, Yasmeen N, Malottki K, et al. Assessing the quality and coherence of network meta analyses of biologics in plaque psoriasis: what does all this evidence synthesis tell Us? Dermatol Ther (Heidelb). 2021;11(1):181–220.
- Armstrong A, Fahrbach K, Leonardi C, et al. Efficacy of bimekizumab and other biologics in moderate to severe plaque psoriasis: a systematic literature review and a network Meta-Analysis. Dermatol Ther (Heidelb). 2022;12(8):1777–1792. doi: 10.1007/s13555-022-00760-8.
- Armstrong AW, Puig L, Joshi A, et al. Comparison of biologics and oral treatments for plaque psoriasis: a meta-analysis. JAMA Dermatol. 2020;156(3):258–269.
- Warren RB, See K, Burge R, et al. Rapid response of biologic treatments of moderate-to-severe plaque psoriasis: a comprehensive investigation using bayesian and frequentist network meta-analyses. Dermatol Ther (Heidelb). 2020;10(1):73–86.
- Lin PT, Wang SH, Chi CC. Drug survival of biologics in treating psoriasis: a meta-analysis of real-world evidence. Sci Rep. 2018;8(1):16068.
- No DJ, Inkeles MS, Amin M, et al. Drug survival of biologic treatments in psoriasis: a systematic review. J Dermatol Treat. 2018;29(5):460–466. doi: 10.1080/09546634.2017.1398393.
- Fitzgerald T, Zhdanava M, Pilon D, et al. Response to the letter to the editor: long-term psoriasis control with guselkumab, adalimumab, secukinumab, or ixekizumab in the USA. Dermatol Ther (Heidelb). 2023;3:2917–2923.
- Gordon KB, Armstrong AW, Foley P, et al. Guselkumab efficacy after withdrawal is associated with suppression of serum IL-23-Regulated IL-17 and IL-22 in psoriasis: VOYAGE 2 study. J Invest Dermatol. 2019;139(12):2437–2446 e1. doi: 10.1016/j.jid.2019.05.016.
- Fitzgerald T, Zhdanava M, Pilon D, et al. Long-Term psoriasis control with guselkumab, adalimumab, secukinumab, or ixekizumab in the USA. Dermatol Ther (Heidelb). 2023;13(4):1053–1068. doi: 10.1007/s13555-023-00910-6.
- Johnson & Johnson. Janssen announces US FDA approval of tremfya™ (guselkumab) for the treatment of moderate to severe plaque psoriasis. Horsham (PA): Janssen Biotech, Inc.; 2017. [cited 2020 December 3]. Available from: https://www.jnj.com/media-center/press-releases/janssen-announces-us-fda-approval-of-tremfya-guselkumab-for-the-treatment-of-moderate-to-severe-plaque-psoriasis
- Xu C, Ferrante SA, Fitzgerald T, et al. Inconsistencies in the days supply values reported in pharmacy claims databases for biologics with long maintenance intervals. J Manag Care Spec Pharm. 2023;29(1):90–100.
- Janssen Biotech Inc. TREMFYA (guselkumab) injection, for subcutaneous use. 2019. Available from: https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/TREMFYA-pi.pdf
- Novartis Pharmaceuticals Corporation. COSENTYX (secukinumab) injection, for subcutaneous use. 2015. Available from: https://www.novartis.com/us-en/sites/novartis_us/files/cosentyx.pdf
- Eli Lilly and Co. TALTZ (ixekizumab) injection, for subcutaneous use. 2019. Available from: https://pi.lilly.com/us/taltz-uspi.pdf
- Pharmacy Quality Alliance. PQA adherence measures 2022. 2022. [updated April 19, 2022; cited 2024 March 25]. Available from: https://www.pqaalliance.org/adherence-measures
- Hainmueller J. Entropy balancing for causal effects: a multivariate reweighting method to produce balanced samples in observational studies. Polit Anal. 2012;20(1):25–46. doi: 10.1093/pan/mpr025.
- Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Statist- Simulat Comput. 2009;38(6):1228–1234. doi: 10.1080/03610910902859574.
- Gnardellis C, Notara V, Papadakaki M, et al. Overestimation of relative risk and prevalence ratio: misuse of logistic modeling. Diagnostics (Basel). 2022;12(11):2851.
- Pocock SJ, Clayton TC, Altman DG. Survival plots of time-to-event outcomes in clinical trials: good practice and pitfalls. Lancet. 2002;359(9318):1686–1689. doi: 10.1016/S0140-6736(02)08594-X.
- Xu C, Teeple A, Wu B, et al. Treatment adherence and persistence of seven commonly prescribed biologics for moderate to severe psoriasis and psoriatic arthritis in a U.S. commercially insured population. J Dermatolog Treat. 2022;33(4):2270–2277. doi: 10.1080/09546634.2021.1950600.
- Mastorino L, Dapavo P, Susca S, et al. Drug survival and clinical effectiveness of secukinumab, ixekizumab, brodalumab, guselkumab, risankizumab, tildrakizumab for psoriasis treatment. J Dtsch Dermatol Ges. 2024;22(1):34–42.
- Torres T, Puig L, Vender R, et al. Drug survival of interleukin (IL)‑17 and IL‑23 inhibitors for the treatment of psoriasis: a retrospective multi‑country, multicentric cohort study. Am J Clin Dermatol. 2022;23(6):891–904.
- Dao DD, Pixley JN, Feldman SR. When should systemic biologic therapy for psoriasis be discontinued? J Dermatolog Treat. 2023;34(1):2173516.
- Nielsen ML, Thein D, Rasmussen MK, et al. Trajectories and prognosis after discontinuation of biologics due to remission in psoriasis: a nationwide cohort study. J Am Acad Dermatol. 2023;88(6):1378–1381.
- Li Y, Lu JJ, Zhong XY, et al. Drug survival outcomes associated with the real-world use of ixekizumab, secukinumab, guselkumab, and adalimumab for the treatment of plaque psoriasis in China: a 52-Week Single-Center retrospective study. CCID. 2022;15:2245–2252. doi: 10.2147/CCID.S387759.
