Abstract
Background: Mycosis fungoides (MF) is the most common type of cutaneous T-cell lymphoma.
Objectives: This study was conducted to evaluate efficacy and safety of interferon (IFN) α-2a combined with phototherapy for early-stage MF.
Methods: Thirteen patients with early-stage MF received subcutaneous injections of IFN α-2a at 3 million IU combined with phototherapy three times per week for 6 months. Treatment efficacy was measured by changes in body surface area (BSA) score and modified severity-weighted assessment tool (mSWAT) score at 1, 3, and 6 months after treatment. Histopathologic examinations of skin lesions were performed before and after treatment.
Results: After 3 months of treatment, all 13 patients achieved a partial response, and BSA and mSWAT scores were significantly lower than those at baseline (p < 0.001). After 6 months, BSA and mSWAT scores were significantly lower than those at baseline (p < 0.001) and after 3 months (p < 0.05). Eleven patients achieved complete remission and two patients achieved a partial response (overall response rate, 100%). Histopathologic examination showed a significant decrease in the number of atypical lymphocytes in both epidermis and dermis. No severe adverse effects occurred.
Conclusion: IFN α-2a in combination with phototherapy may be an effective and safe alternative modality for early-stage MF.
Introduction
Cutaneous T-cell lymphoma (CTCL) is a subtype of primary cutaneous lymphoma caused by abnormal clonal proliferation of T lymphocytes in the skin (Citation1). Mycosis fungoides (MF) is an indolent type of CTCL with slow clinical progression. Treatment of MF primarily depends on the stage and extent of the disease. Early-stage MF includes stages IA, IB, and IIA. For early-stage MF, the treatment goal is to control skin lesions mainly by skin-directed therapies, such as topical therapy, phototherapy, and radiotherapy.
Phototherapy has been a first-line treatment for early-stage MF since 1976 (Citation2). The most commonly used forms of ultraviolet (UV) irradiation are UVA1, psoralen plus UVA (PUVA), and narrow-band (NB) UVB (Citation3). Both UVA and NB-UVB are safe, effective, and well-tolerated first-line therapies for the management of early-stage MF. Phototherapy can affect the infiltration of epidermal lymphocytes and the activity of keratinocytes, specifically by inhibiting their growth rate and inducing apoptosis (Citation3). NB-UVB can also reduce the production of proinflammatory cytokines (interleukin [IL]-1a, IL-2, IL-5, and IL-6) and increase the production of anti-inflammatory cytokines (IL-10) in the microenvironment of lesions (Citation4).
Immune function is impaired in patients with CTCL. During the past few decades, immunotherapy agents such as interferon (IFN) have gradually emerged as effective therapeutic options for CTCL. Immunotherapy can stimulate the body to produce antitumor effects, eliminate malignant T lymphocytes, and repair immune dysfunction. By potentiating rather than suppressing the immune system, immunotherapy can result in longer treatment responses than alternatives such as chemotherapy (Citation5). Research shows that IFN-α can stimulate CD8+ T cells and natural killer cells, thereby activating antitumor cytotoxicity (Citation6). In addition, IFN-α can upregulate the expression of major histocompatibility complex class I in malignant lymphocytes (Citation7) and inhibit the excessive expression of Th2 cytokines IL-4 and IL-5 in malignant T cells, helping to restore the host Th1/Th2 balance (Citation8,Citation9).
The present study involved patients with early-stage MF who met the inclusion criteria and received subcutaneous injections of IFN α-2a (3 million IU, once every other day, 3 times a week, for 3 months) combined with phototherapy (either UVB + UVA, once every other day, 3 times a week or UVA1, once a day, 5 times a week) for 6 months. The changes in the body surface area (BSA) score and modified severity-weighted assessment tool (mSWAT) score were recorded before treatment and at 1, 3, and 6 months after starting treatment. Additionally, histopathologic examinations of the skin lesions were performed before and after treatment. The overall study goal was to verify the effectiveness and safety of IFN α-2a combined with phototherapy for early-stage MF.
Patients and methods
Patients
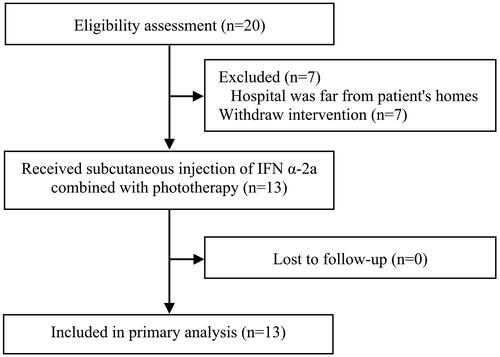
Patients with early-stage MF were recruited from the outpatient department of Peking Union Medical College Hospital, Peking Union Medical College, and the Chinese Academy of Medical Sciences from June 2022 to November 2022. Among 20 enrolled patients, 13 completed the entire treatment course (). The definitive diagnosis of early-stage MF in this study was based on standard guidelines proposed by the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC) (Citation10). The patients’ age, sex, disease course, disease location, disease staging, relevant laboratory test results, and anamnesis were recorded in detail. Among the 13 patients, 5 were previously treated with phototherapy alone, but the results were not satisfactory. Therefore, they were willing to accept the treatment plan of phototherapy combined with interferon. All patients received a detailed explanation of the study and signed a detailed informed consent form describing the study design and possible adverse effects of IFN α-2a and phototherapy. This prospective study was approved by the Medical Ethics Committee affiliated with Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences (file number: I-22PJ131).
The inclusion criteria were early-stage MF that met the ISCL-EORTC diagnostic criteria and had been confirmed by histopathology in our hospital; age of ≥18 years; ≥6 months of follow-up visits; no systemic treatments (such as retinoic acid, IFN, glucocorticoids, methotrexate, or other agents) within the past 2 months; and no phototherapy (such as UVA, UVB, or PUVA) within the past 1 month. Both male and female patients were included in the study.
Patients were excluded if they had other malignant tumors; had severe infection, such as upper respiratory tract infection, active tuberculosis, urinary tract infection, HIV infection, or other types of infection; had liver or kidney dysfunction; had any blood disease; had a history of an allergic reaction to IFN or phototherapy; had contraindications for phototherapy, such as systemic lupus erythematosus or other conditions; were pregnant or breastfeeding; or were unwilling to attend follow-up visits.
Methods
Preparation for treatment
All patients provided written or verbal consent to undergo complete blood cell counts, liver and kidney function tests, blood glucose measurement, electrocardiography, blood lipid measurement, and ultrasound examination of the superficial lymph nodes (including those in the neck, subclavian area, axillae, and groin) along with clinical evaluation of MF, including photographs.
Medications and phototherapy instrument
The following medications were used in this study: recombinant human IFN α-2a injection (3 million IU) (Shenyang Sunshine Pharmaceuticals Co., Ltd., Shenyang, China), halometasone/triclosan cream (Pro Farma AG, Baar, Canton of Zug, Switzerland; Famar SA, Athens, Greece). Phototherapy was administered using a UV phototherapy instrument (Waldmann, Villingen-Schwenningen, Germany).
Therapeutic protocol
The phototherapy options varied based on whether the patient’s skin lesions were patches or plaques. NB-UVB combined with UVA was chosen for skin lesions presenting as patchy or thin plaques. If the skin lesions were not significantly improved after 1 month of treatment, UVA1 therapy was chosen instead. However, skin lesions presenting as thick plaques were immediately treated with UVA1 therapy. The starting dose, dose increase, and frequency of phototherapy were as follows.
NB-UVB: Starting dose of 0.3 J/cm2, once every other day, three times a week, with a weekly dose increase of 0.2 J/cm2 and maximum dose of 2.8 J/cm2
UVA: Starting dose of 2.0 J/cm2, once every other day, three times a week, with a weekly dose increase of 0.5 J/cm2 and maximum dose of 8.0 to 8.5 J/cm2
UVA1: Starting dose of 20 or 40 mJ/cm2 based on the lesion thickness, once a day, five times a week, for 3 consecutive months. If the skin lesions significantly improved, NB-UVB was chosen with a starting dose of 0.3 J/cm2, once every other day, three times a week, with an increase of 0.2 J/cm2 per week and maximum dose of 2.8 J/cm2.
All patients also received subcutaneous injections of recombinant human IFN α-2a injection, 3 million IU each time, once every other day, for 3 consecutive months. The IFN α-2a therapy was stopped at the fourth month of treatment, but phototherapy was continued with no changes.
Halometasone/triclosan cream was topically applied to the lesions twice a day. To avoid adverse reactions such as skin atrophy caused by long-term use, the halometasone/triclosan cream was applied for 2 weeks and then stopped for 1 week; this treatment regimen was continued for 3 to 6 consecutive months. If the skin lesions had not completely subsided after this time, the halometasone/triclosan cream could be continued for another month.
Treatment monitoring
Clinical evaluation of the efficacy of therapy was based on the percentage change of skin lesions in the total BSA (TBSA). The TBSA of patches or plaques in 12 body areas was measured using the area of patches or plaques in hand units, which was set at 1% per unit. The BSA score was determined by the same clinician during the entire follow-up course.
The mSWAT was used to monitor the skin tumor load in patients with MF/Sézary syndrome. The TBSA of patches, plaques, and tumors in 12 body areas was measured using the area of lesions in hand units, which was set at 1% per unit. The percentage of TBSA for each lesion type was multiplied by a number (patch = 1, plaque = 2, tumor = 4), then summed to obtain the mSWAT score (Citation11,Citation12). The mSWAT score was determined by the same clinician during the entire follow-up course.
The clinical efficacy evaluation criteria were defined as follows (Citation11,Citation12).
Complete remission (CR): 100% clearance of the skin lesion
Partial response (PR): ≥50% decrease in the mSWAT score compared with baseline
Stable disease (SD): <50% decrease to <25% increase in the mSWAT score compared with baseline
Progressive disease (PD): ≥25% increase in the mSWAT score compared with baseline or ≥50% increase in the sum of the maximum diameters of pathologically positive lymph nodes compared with baseline
The overall response rate (ORR) was calculated as CR + PR. CR and PR were confirmed through repeated evaluation after ≥4 weeks.
After 6 months of treatment, with the patient’s consent, another pathological examination of the skin tissue was performed to observe the changes in the skin lesions from before to after treatment. This examination mainly involved observation of the infiltration of atypical lymphocytes, the presence of Pautrier’s abscesses.
Laboratory testing was performed prior to treatment and every 2 months during treatment. Adverse events during treatment were reported by the patients and clinicians. Patients who completed the 6-month visit were included in the efficacy analysis.
Statistical analysis
Data were analyzed by SPSS Version 27.0 (IBM Corp., Armonk, NY, USA). The BSA and mSWAT scores are expressed as mean ± standard deviation. Analysis of variance was used to compare repeated measurement data. A P value of <0.05 indicated statistical significance.
Results
Patient characteristics
This study included 20 patients with early-stage MF who met the diagnostic criteria (stage IA, n = 6; stage IB, n = 12; stage IIA, n = 2). However, seven patients withdrew from the study because of the inconvenience of coming to our hospital for treatment. The remaining 13 patients (stage IA, n = 3; stage IB, n = 8; stage IIA, n = 2) completed treatment and follow-up (). Among the 13 cases, there were a total of 2 patients with hypopigmented MF. The patients’ characteristics, including sex, age, disease course, stage, and others, are shown in .
Table 1. Baseline characteristics of 13 patients with early-stage MF.
Clinical efficacy evaluation
After 1 month of treatment, all 13 patients had achieved SD and none had experienced progression. There were no significant differences in the BSA or mSWAT scores compared with before treatment (p > 0.05). One patient (case 8) whose skin lesions were not significantly improved after 1 month of treatment, then treated with UVB + UVA1. After 3 months of treatment, all 13 patients had achieved PR and none had experienced progression. The BSA and mSWAT scores were significantly lower than those before treatment (p < 0.001). After 6 months of treatment, the BSA and mSWAT scores were significantly lower than those before treatment (p < 0.001) and those at 3 months of treatment (p < 0.05). Eleven patients (including 2 patients with hypopigmented MF) achieved CR and two achieved PR, and the ORR was 100%. It should be pointed out that among the 5 patients who previously had poor response with phototherapy alone, there were 1 case of PR and 4 cases of CR after 6 months of treatment of phototherapy combined with IFN α-2a. The improvement in the BSA and mSWAT scores and the grading of efficacy are shown in and . The clinical improvement from before to after treatment is shown in and .
Figure 2. Improvement in (a) BSA score and (b) mSWAT score in patients with early-stage MF from before to after treatment. ***p < 0.001 vs. before treatment; #p < 0.05 vs. after 3 months of treatment.
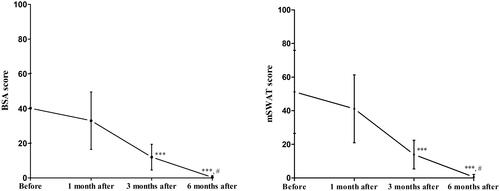
Figure 3. Changes in skin lesions on the back from before to after treatment in Patient 1. (a) Before treatment. (b) Two weeks after starting treatment. (c) One month after starting treatment. (d) Six months after starting treatment.
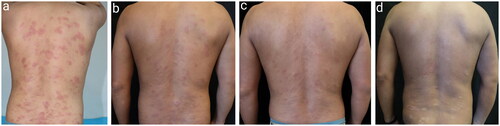
Figure 4. Changes in skin lesions on the abdomen, back, thighs, and upper arms from before to after treatment in Patient 18. (a1–a5) Before treatment. (b1–b5) One month after starting treatment. (c1–c5) Three months after starting treatment. (d1–d5) Six months after starting treatment.
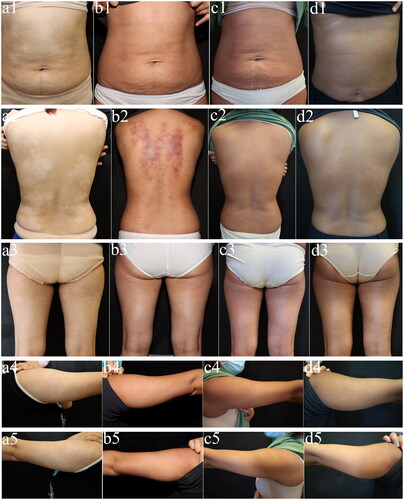
Table 2. Clinical response in patients with early-stage mycosis fungoides after treatment.
Histopathologic changes in skin lesions before and after treatment
The histopathologic changes in the skin lesions of patients with MF from before to after 6 months of treatment are shown in and . Before treatment, an atypical lymphocytic epidermotropic phenomenon was observed, which manifested as follows.
Figure 5. Histopathologic changes in skin lesions from before to after treatment (hematoxylin–eosin staining). (a, b) Before treatment, ×10, ×20. (c, d) Six months after starting treatment, ×10, ×20.
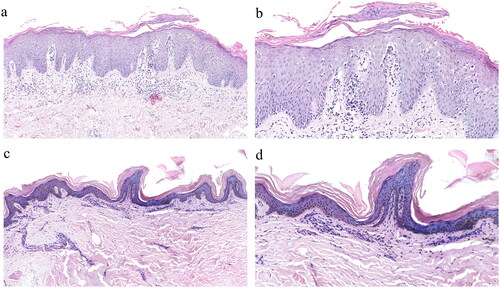
Figure 6. Histopathologic changes in skin lesions from before to after treatment (hematoxylin–eosin staining). (a, b) Before treatment, ×10, ×20. (c, d) Six months after starting treatment, ×10, ×20.
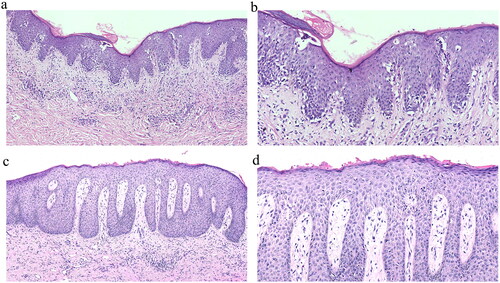
MF cells: Atypical lymphocytes with large volume and a gyriform nucleus appearing in the epidermis
Hollow cells: Abnormal lymphocytes with surrounding keratinocytes forming a clear gap, showing a hollow halo shape
Linear arrangement of cells: Atypical lymphocytes arranged in a linear or clustered pattern at the junction of the epidermis and within the dermis
Paget-like distribution: Distribution of single lymphocytes throughout the epidermis, resembling changes observed in Paget’s disease
Pautrier’s microabscesses: Accumulation of atypical lymphocytes in the epidermis. Notably, after treatment, the atypical lymphocytes infiltrating the epidermis and dermis significantly decreased in number or disappeared, and Pautrier’s microabscesses were rare.
Adverse reactions
All 13 patients reported mild influenza-like symptoms, including fatigue, headache, arthralgia, and muscle aches accompanied by a fever (38.5 °C–39.5 °C) during the first to third subcutaneous injections of IFN. However, these symptoms resolved within 24 h after rectal administration of a 30-mg indomethacin suppository and physical cooling. Three of the 13 patients reported obvious erythema accompanied by a burning sensation during phototherapy and within the first 48 h afterward. In these patients, the phototherapy was suspended for 1 week and the whole body was moisturized; the erythema and burning sensation spontaneously resolved thereafter. The treatment was continued as planned, and the symptoms did not recur. One patient showed a slight increase in liver enzymes (alanine aminotransferase and aspartate aminotransferase) on biochemical examination. IFN treatment was stopped for 1 week, and the biochemical examination showed normalization of the liver enzymes; treatment was then continued. Another patient developed occasional mild pain in the joints of both hands after 3 months of treatment, and the pain was more obvious in the morning. IFN therapy had been discontinued, and no obvious abnormalities were found in a routine blood examination, biochemistry examination, anti-nuclear antibody spectrum, and rheumatoid factors. The pain gradually improved during follow-up, and the phototherapy was continued. After 6 months of treatment, all 13 patients had varying degrees of pigmentation on their skin. No other severe adverse reactions were reported.
Discussion
MF is an indolent type of CTCL, and its etiology is unknown. The natural course of MF can reach 20 to 30 years or more, and its incidence rate increases with age. It is most commonly seen in patients of advanced age, with the highest incidence rate found in patients of >70 years. The incidence is higher among men than women (male:female incidence rate ratio of MF/Sézary syndrome = 1.57) and among Black than White patients (Black:White incidence rate ratio of MF = 1.55) (Citation13). Among the 20 patients with early-stage MF who met the diagnostic criteria in the present study, however, only 1 was >70 years of age. The patients’ mean age was 39.05 ± 14.93 years, and the male:female ratio was 9:11. This differs from the literature and may be related to the small sample size. In addition, the patients tended to be younger, which may have been related to the continuous improvement in the quality of medical care in China and the increasing understanding of MF. Such advancements will benefit patients with respect to early diagnosis and treatment, thereby improving their prognosis.
Unified standards for the treatment of MF are still lacking. Treatment of MF is planned mainly according to the stage and extent of the disease (Citation14). In the early phases, nonaggressive options represent the first-line strategy (e.g. local corticosteroids, psoralen, and UVA irradiation) (Citation14). Regardless of which treatment option is chosen, more than 80% of patients with early-stage disease will no longer experience disease progression (Citation15). Therefore, unless the disease progresses or worsens, patients with early-stage MF should be treated and maintained with conservative skin-directed therapies (Citation16).
IFN has antiviral, antitumor, and immunomodulatory effects (Citation17). Three types of IFN are available for clinical use: IFN-α, IFN-β, and IFN-γ. Gökşin et al. (Citation18) evaluated the response to IFN α-2a treatment as monotherapy in patients with stage IB MF. IFN α-2a was administered subcutaneously at a dose of 3 × 106 units three times weekly as initially described. According to the patients’ clinical tolerance, the dose was increased every 4 weeks to 6–9 million units. CR, PR, and PD occurred in 11 (44%), 12 (48%), and 2 (8%) patients, respectively. CR was accomplished at a mean of 16.1 ± 9.8 weeks. The most common adverse effect was fatigue, which occurred at an acceptable incidence of 12%.
In the present study, patients with early-stage MF were administered IFN α-2a combined with UVB + UVA/UVA1 phototherapy, and the results showed that after 1 month of treatment, the BSA and mSWAT scores were not significantly different from those before treatment. After 3 months of treatment, however, the BSA and mSWAT scores were significantly lower than those before treatment. After 6 months of treatment, the BSA and mSWAT scores were significantly lower than those before treatment and after 3 months of treatment. Among the 13 patients, 11 (84.62%) reached CR, 2 (15.38%) reached PR, and the ORR was 100%. Histopathological examination showed that after treatment, the epidermal infiltration by atypical lymphocytes had significantly decreased and that the number of Pautrier’s microabscesses had subsided, indicating that IFN α-2a combined with phototherapy was effective for early-stage MF.
IFN α-2b combined with PUVA can be an effective treatment option for patients with MF who are resistant to conventional treatment such as PUVA or local corticosteroids and show good tolerance, especially patients with stage IB, IIA, and IIB MF (Citation19). In this study, 5 patients who were previously treated with phototherapy alone and received poor response, but had good results (1 case of PR, 4 cases of CR) after 6 months of treatment of phototherapy combined with IFN α-2a, showing that the combination of phototherapy and IFN can be considered in patients with early disease who did not respond or partially responded to phototherapy alone. Combination therapy is effective for both early and advanced stages of MF, with a more significant therapeutic effect in early MF. One patient in the present study developed a coin-sized protruding nodule on the left lateral leg after 1 month of treatment, but IFN therapy combined with phototherapy was continued. Two weeks later, the nodule subsided and the patient’s other skin lesions improved, indicating that IFN combined with phototherapy can have good therapeutic effects for some patients with active disease.
The adverse reactions of IFN are often mild. Those reported in the literature mainly include fatigue, myalgia, arthralgia, fever, nausea, headache, dry eyes, and dry mouth (Citation20) and usually occur during or shortly after treatment. In addition, mental symptoms such as depression, anxiety, and even suicidal tendencies can also occur (Citation21). The short-term side effects of phototherapy are generally mild and self-limiting; they include erythema, edema, pruritus, pain, purpura, transient petechiae, blisters, and crusting and occur during treatment or within the first 24 h after treatment (Citation22). The main long-term side effects include pigmentary disorders, photoaging, cataracts, and carcinogenesis (Citation22).
All 13 patients in the present study reported mild influenza-like symptoms during the first to third sessions of IFN treatment. Three patients developed erythema accompanied by a noticeable burning sensation after phototherapy. Only one patient showed a slight increase in liver enzymes. Another patient developed occasional mild pain in both hands after 3 months of treatment. All of these adverse reactions gradually resolved, and no other serious adverse reactions were observed, showing that the combination of IFN α-2a and phototherapy is safe for treating MF.
Because MF is a rare disease, one of the main limitations of this study is the small sample size and short follow-up period. Additionally, this was a single-center, non-randomized controlled trial. Multicenter randomized controlled clinical studies with larger sample sizes and long-term follow-up should be performed in the future.
Conclusion
IFN α-2a in combination with phototherapy may be an effective and safe alternative modality for treatment of early-stage MF, and can also be considered in patients with early disease who did not respond or partially responded to phototherapy alone.
Ethics statement
All patients were informed of the purpose of the study and signed a detailed informed consent form describing the study design and possible adverse effects of interferon α-2a or phototherapy. At all stages of the project, the basic principles of the Declaration of Helsinki were taken into account, and the implementation protocol was investigated and approved by the Ethics Committee of Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences (file number: I-22PJ131).
Author contributions statement
Tao Wang and Yuehua Liu contributed to the study conception and design. Material preparation, data collection and analysis were performed by Hongbin Song, Zhonghui Hu, Shiyu Zhang, Lu Yang, Jindi Feng, and Lu Lu. The first draft of the manuscript was written by Hongbin Song. All authors commented on previous versions of the manuscript. All authors read, approved the final manuscript, and agree to be accountable for all aspects of the work.
Acknowledgment
We thank Angela Morben, DVM, ELS, from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Larocca CA, Leboeuf NR. Overview of cutaneous T-cell lymphomas [J]. Hematol Oncol Clin North Am. 2019;33(4):1–9. doi: 10.1016/j.hoc.2019.04.004.
- Pattamadilok B, Poomputsar T. A retrospective, descriptive study of patients with mycosis fungoides treated by phototherapy (oral PUVA, NB-UVB) with a twice-weekly regimen at the institute of dermatology, Bangkok, Thailand, with an experiential timeline of 13 years [J]. Photodermatol Photoimmunol Photomed. 2021;37(1):49–55. doi: 10.1111/phpp.12611.
- Dogra S, Mahajan R. Phototherapy for mycosis fungoides [J]. Indian J Dermatol Venereol Leprol. 2015;81(2):124–135. doi: 10.4103/0378-6323.152169.
- Sigmundsdottir H, Johnston A, Gudjonsson JE, et al. Narrowband-UVB irradiation decreases the production of pro-inflammatory cytokines by stimulated T cells [J]. Arch Dermatol Res. 2005;297(1):39–42. doi: 10.1007/s00403-005-0565-9.
- Weiner DM, Durgin JS, Wysocka M, et al. The immunopathogenesis and immunotherapy of cutaneous T cell lymphoma: current and future approaches [J]. J Am Acad Dermatol. 2021;84(3):597–604. doi: 10.1016/j.jaad.2020.12.026.
- Yoo EK, Cassin M, Lessin SR, et al. Complete molecular remission during biologic response modifier therapy for Sézary syndrome is associated with enhanced helper T type 1 cytokine production and natural killer cell activity [J]. J Am Acad Dermatol. 2001;45(2):208–216. doi: 10.1067/mjd.2001.116345.
- Dinarello CA, Mier JW. Lymphokines [J]. N Engl J Med. 1987;317(15):940–945. doi: 10.1056/NEJM198710083171506.
- Suchin KR, Cassin M, Gottleib SL, et al. Increased interleukin 5 production in eosinophilic sézary syndrome: regulation by interferon alfa and interleukin 12 [J]. J Am Acad Dermatol. 2001;44(1):28–32. doi: 10.1067/mjd.2001.109853.
- Rook AH, Heald P. The immunopathogenesis of cutaneous T-cell lymphoma [J]. Hematol/Oncol Clin North Am. 1995;9(5):997–1010. doi: 10.1016/S0889-8588(18)30054-6.
- Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and sezary syndrome: a proposal of the international society for cutaneous lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC) [J]. Blood. 2007;110(6):1713–1722. doi: 10.1182/blood-2007-03-055749.
- Olsen EA, Kim YH, Kuzel TM, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma [J]. J Clin Oncol. 2007;25(21):3109–3115. doi: 10.1200/JCO.2006.10.2434.
- Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the international society for cutaneous lymphomas, the United States cutaneous lymphoma consortium, and the cutaneous lymphoma task force of the European Organisation for Research and Treatment of Cancer [J]. J Clin Oncol. 2011;29(18):2598–2607. doi: 10.1200/JCO.2010.32.0630.
- Imam MH, Shenoy PJ, Flowers CR, et al. Incidence and survival patterns of cutaneous T-cell lymphomas in the United States [J]. Leuk Lymphoma. 2013;54(4):752–759. doi: 10.3109/10428194.2012.729831.
- Cerroni L. Mycosis fungoides-clinical and histopathologic features, differential diagnosis, and treatment [J]. Semin Cutan Med Surg. 2018;37(1):2–10. doi: 10.12788/j.sder.2018.002.
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal [J]. J Clin Oncol. 2010;28(31):4730–4739. doi: 10.1200/JCO.2009.27.7665.
- Edelson RL. Outsmarting cutaneous T-cell lymphoma cells by decoding the language they speak: focusing past and present insights on future prospects [J]. Clin Lymphoma Myeloma Leuk. 2010;10 Suppl 2:S59–S62. doi: 10.3816/CLML.2010.s.008.
- Lazear HM, Schoggins JW, Diamond MS. Shared and distinct functions of type I and type III interferons [J]. Immunity. 2019;50(4):907–923. doi: 10.1016/j.immuni.2019.03.025.
- GöKŞIN Ş, İmren IG, Cenk H, et al. The use of interferon-α2a as monotherapy in stage IB patients with mycosis fungoides: a retrospective chart review of patient outcomes [J]. Dermatol Ther. 2022;35(4):e15344. doi: 10.1111/dth.15344.
- Olisova OY, Megna M, Grekova EV, et al. PUVA and interferon α2b combined therapy for patients with mycosis fungoides at different stages of the disease: a seven-year retrospective study in Russia [J]. J Eur Acad Dermatol Venereol. 2019;33(2):e72–e4. doi: 10.1111/jdv.15212.
- Cotler SJ, Wartelle CF, Larson AM, et al. Pretreatment symptoms and dosing regimen predict side-effects of interferon therapy for hepatitis C [J]. J Viral Hepat. 2000;7(3):211–217. doi: 10.1046/j.1365-2893.2000.00215.x.
- Trask PC, Esper P, Riba M, et al. Psychiatric side effects of interferon therapy: prevalence, proposed mechanisms, and future directions [J]. J Clin Oncol. 2000;18(11):2316–2326. doi: 10.1200/JCO.2000.18.11.2316.
- Zhang P, Wu MX. A clinical review of phototherapy for psoriasis [J]. Lasers Med Sci. 2018;33(1):173–180. doi: 10.1007/s10103-017-2360-1.