Abstract
Background and objective
Brivudine has been used in herpes zoster (HZ) treatment for years, but the safety and efficacy of brivudine are inconclusive. Here we perform a meta-analysis to assess the efficacy, safety, incidence of postherpetic neuralgia of brivudine.
Methods
Data of randomized controlled Trials (RCTS) were obtained from the databases of both English (PubMed, Embase, and Cochrane Library) and Chinese (China National Knowledge Infrastructure, China Science Journal Database, and WanFang Database) literatures from inception to 12 September 2022. Meta-analyses of efficacy and safety of Brivudine for the treatment of herpes zoster for RCTS were conducted.
Results
The analyses included seven RCTS (2095 patients in experimental group and 2076 patients in control group) in the treatment of HZ with brivudine. It suggested that the brivudine group was superior to the control group in terms of efficacy (p = .0002) and incidence of postherpetic neuralgia (p = .04). But the incidence of adverse reactions has no significant difference between the brivudine and the control groups (p = .22). In addition, subgroup analysis of adverse events also showed that brivudine was about the same safety as other modalities in the treatment of HZ (p > .05).
Conclusions
Brivudine is effective for HZ. However, the evidence on the safety of brivudine is insufficient.
Systematic Review Registration:
1. Introduction
Herpes zoster (HZ) is induced by reactivation of varicella-zoster, which remains in a latent form in the sensory neurons of the spinal and cranial sensory ganglia following the infection with chickenpox (Citation1). Prevalence of HZ ranges from 0.2 to 2% in general population. HZ brings heavy financial burden and life pressure to patients (Citation2). HZ is commonly manifested by unilateral blistering rash with intense pain, which could be complicated with ophthalmic, vascular, visceral, and nervous system complications. Postherpetic neuralgia (PHN) is the most common complication, which occurs in 20% of patients with HZ and increases with age (Citation3).
Because HZ is caused by reactivation of varicella-zoster virus (VZV), antiviral agents such as valaciclovir, acyclovir, and famciclovir are commonly used in clinic (Citation4). These drugs can improve HZ and PHN to some extent. However, due to the low oral bioavailability of these conventional antiviral drugs, frequent high-dose administration is required. For the convenience of patients and to improve patient compliance, drugs with high oral bioavailability and better efficacy and safety are still needed clinically (Citation5).
Brivudine’s activity against VZV and HSV-1 depends on phosphorylation of the virus-encoded thymidine kinase (TK). In its active form (BVDU TP or BVDU 5′-triphosphate), it acts as both a substrate and inhibitor of the viral (HSV-1) DNA polymerase (Citation6). Because of its terrific high bioavailability(90%) and high specificity against HZV, brivudine has been used in the treatment of HZ in recent years (Citation7). Moreover, some studies suggest that brivudine appears to be more suitable for pediatric patients than antiviral drugs, which have more indications to consider (Citation8).
A number of studies suggest that brivudine displays better efficacy, while some studies did not show significant differences in improvement of pain score and vesicle severity between brivudine and other antiviral drugs (Citation9–11). Meta-analysis is an objective, quantitative method that could be used to improve the comprehensive and reliable evidence for clinical drug therapy through systematic review methods, thus addressing specific clinical problems (Citation12–14). Therefore, a meta-analysis was conducted to systematically evaluate the clinical efficacy, safety, and PHN in brivudine versus non-brivudine treatment (Citation11).
2. Methods
This systematic review was registered on PROSPERO(CRD42023397496).
2.1. Search strategy
A comprehensive database search using the determined search mode was operated in PubMed, Embase, Cochrane Library, China National Knowledge Infrastructure (CNKI), China Science Journal Database (VIP), and WanFang Database from inception to 12 September 2022. The following terms and words were used in the search: ((((‘Herpes Zoster’[Mesh]) OR (((Zona[Title/Abstract]) OR (Zoster[Title/Abstract])) OR (Shingles[Title/Abstract]))) AND ((‘Herpes Zoster’[Mesh]) OR (((Zona[Title/Abstract]) OR (Zoster[Title/Abstract])) OR (Shingles[Title/Abstract])))) AND ((‘brivudine’ [Supplementary Concept]) OR (((((((((5-(2-bromoethenyl)-2′-deoxyuridine[Title/Abstract]) OR (5-(2-bromovinyl)-2′-deoxyuridine[Title/Abstract])) OR (E-5-(2-bromovinyl)-dUrd[Title/Abstract])) OR (brivudin[Title/Abstract])) OR ((E)-5-(2-bromovinyl)-2′-deoxyuridine[Title/Abstract])) OR (5-BVDU[Title/Abstract])) OR ((Z)-5-(2-bromovinyl)-2′-deoxyuridine[Title/Abstract])) OR (Z-5-(2-bromovinyl)-dUrd[Title/Abstract])) OR (Zostex[Title/Abstract])))) AND ((randomized controlled trial[Publication Type]) OR (randomized[Publication Type])). Besides, we adjusted the vocabulary and syntax to fit each database.
2.2. Inclusion and exclusion criteria
Inclusion criteria were: (1) randomized controlled clinical trials; (2) patients were diagnosed with herpes zoster; (3) patients in the observation group were treated with brivudine alone, while those in the control group were treated with medications other than brivudine; and (4) at least one of following indicators was evaluated: efficacy, adverse reaction, safety, blister time, pain relief time, incidence of PHN. Exclusion criteria included (1) non-randomized controlled clinical trials, case reports, animal experiments; (2) full text of the publication could not be obtained; (3) description of the outcome data was not clear; (4) repeatedly published literature; and (5) the control group intervention was also studied for treatment with brivudine.
2.3. Data extraction
Two researchers (LDY and CJX) read the retrieved literature independently, selected and excluded the documents according to the above inclusion and exclusion criteria. Data from literature were extracted and input into the pre-established Excel sheet (). Final analyses of the data were conducted by LDY, CJX, and ZLT.
Table 1. Characteristics of included studies.
2.4. Quality evaluation
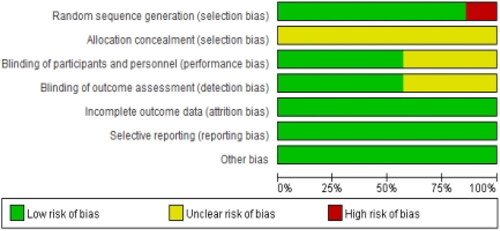
Cochrane bias risk assessment tool was used to assess the quality of the included literature in six points: random sequence generation, allocation hiding, blinding, completeness of outcome information, selective reporting, and other bias. The evaluation results in each aspect were divided into high risk, low risk, and unclear. The consequences of literature quality evaluation were described by the bias risk map.
2.5. Statistical analysis
RevMan5.4 software was used to conduct meta analysis, odd ratio (OR), and 95% confidence interval (CI) were used to analyze the included binary variables, OR is interpreted as a multiple of the exposure event in the intervention group versus the exposure event in the control group; it is important to note that odds are ratios of odds rather than probabilities or ratios of probabilities (Citation15). Standardized mean difference (SMD) and 95%CI were used to analyze the included continuous variables (Citation15,Citation16). The heterogeneity among the data included in the study was measured by I2. Since the sample size of each study was less than 5, the fixed effect model was used for the whole study (Citation15). Meta analysis, and the differences between the studies were reviewed. A subgroup analysis was carried out to determine the source of the heterogeneity if necessary. Significance was determined by the p value and 95%CI.
3. Results
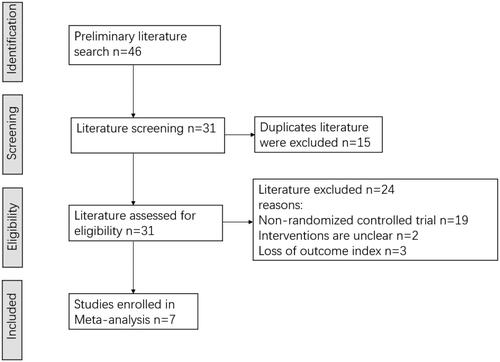
3.1. Literature search
A total of seven literatures were finally included (Citation10,Citation17–22), all of which were randomized controlled clinical trials. There were a total of 4171 patients, including 2095 cases in the observation group and 2076 cases in the control group. The literature screening process is shown in . In six of these studies (Citation10,Citation17,Citation19–22), patients in the observation group were orally given brivudine at a dose of 125 mg daily for one week. In one study (Citation18), patients in the observation group were orally given brivudine at a dose of 125 mg every 6 h for five days. The control group of two studies was treated with oral valacyclovir (Citation10,Citation20), while patients were intravenously or orally treated with acyclovir in the other five studies (Citation17–19,Citation21,Citation22). The outcome measures and the basic characteristics of these researches are shown in .
3.2. Quality evaluation
Among the included literatures, one study was divided into groups according to the treatment method (Citation20), three studies were randomly divided into groups according to the random number table (Citation10,Citation21,Citation22), and three studies were only proposed random without specifying the specific random method (Citation17–19). None of the studies was involved in allocation concealment. Blinding was not mentioned in three studies (Citation20–22) (, ).
Table 2. Methods and quality evaluation of included studies.
3.3. Outcome indicators
3.3.1. Primary indicators
3.3.1.1. Efficacy
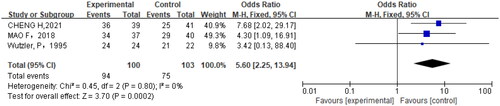
Among the seven articles included in the analysis, the effectiveness was used as the outcome index in three studies (Citation18,Citation20,Citation22) (p = .80, I2=0%). Analysis with the fixed effect model showed that the efficacy of oral brivudine was superior to other treatment methods for the treatment of herpes zoster (OR = 5.60, 95% CI = 2.25,13.94, p = .0002) vs. other treatments ().
3.3.1.2. Blister time
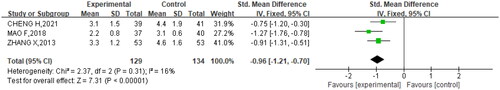
Among the seven studies, three studies (Citation20–22) mentioned the blister time (p = .31, I2= 16%). Again, analysis with fixed effect model showed that oral brivudine significantly shortened blister time (SMD= −0.96, 95% CI= −1.21, −0.70, p < .00001) vs. other treatments ().
3.3.1.3. Pain relief time
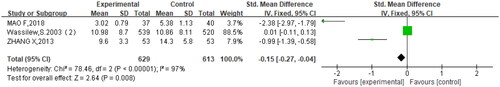
In the seven studies, three studies (Citation19–21) mentioned pain relief time. A high heterogeneity was observed across studies (p < .00001, I2 = 97%). The results displayed that oral brivudine significantly shortened pain relief time in comparison to other treatments (SMD= −0.15, 95% CI= −0.27, −0.04, p = .008) ().
3.3.2. Secondary indicators
3.3.2.1. The incidence of postherpetic neuralgia
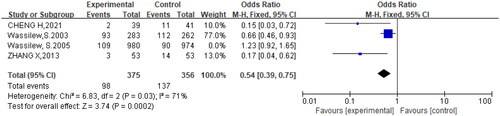
Four studies (Citation10,Citation17,Citation21,Citation22) compared the incidence of PHN between the brivudine and other treatment groups. Among them, acyclovir was used in the control group in three studies (Citation17,Citation21,Citation22) and valacyclovir treatment was used as controls in one study (Citation10). The results showed that the incidence of PHN did not differ significantly between brivudine and other treatments (OR = 0.84, 95% CI:=0.68,1.05, p = .0003). Sensitivity analysis was conducted and the source of heterogeneity was tested by eliminating the studies one by one. After excluding the study by Wassilew et al. (Citation10), the heterogeneity decreased (p = .03, I2=71%), and brivudine significantly decreased the incidence of PHN compared to other treatments (OR = 0.54, 95% CI= 0.39,0.75, p = .03). The outcomes of the forest map showed that the incidence of PHN treated with brivudine was lower than that treated with acyclovir ().
3.3.2.2. Safety
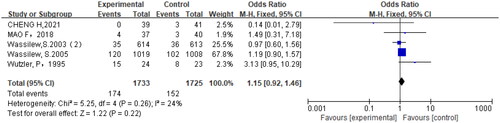
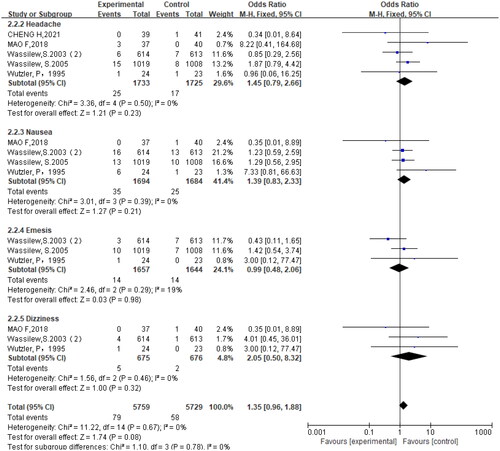
Six studies (Citation10,Citation18–22) reported adverse reactions in the trial, including headache, nausea, vomiting, dizziness, gastrointestinal reaction, proteinuria, etc. The results showed no significant difference in the incidence of adverse reactions between the brivudine-treated and the control groups (OR = 1.15, 95% CI = 0.92, 1.46, p = .22) (). Further subgroup analysis also revealed no significant difference in the incidence of adverse reactions between brivudine-treated and the control groups (p > .05) ().
4. Discussion
Herpes zoster (HZ) is a prevalent dermatosis in the aged population with a lifetime risk of 20%∼30% (Citation23). HZ is reduced by reactivation of VZV, which could spread along the peripheral nerves to the skin, leading to unilateral banded erythema and blisters (Citation23). Timely antiviral therapy against VZV is crucial in HZ treatment, that can effectively reduce formation of new lesions, accelerate healing and lower risk of PHN (Citation24). Various antiviral agents have been exploited for the treatment of alpha, beta, and gamma types of herpes viridae. Some agents, including acyclovir, valaciclovir, famciclovir, and brivudine, have been approved for HZ treatment caused by VZV (alpha type of Herpesviridae) (Citation25).
Valacyclovir and Acyclovir are the nucleoside analogues and can specifically block viral DNA replication in the affected cells. Acyclovir was mainly used for HZ in 1982. But it can cause renal toxicity (Citation26). Valacyclovir, the prodrug of acyclovir, provides the superiority of a three-to fivefold increase in acyclovir bioavailability (Citation27). Brivudine can also block DNA polymerase and inhibit the replication of herpes zoster virus without renal toxicity. But the interval between the end of brivudine and the start of 5-fluorouracil or similar anti-cancer drugs must be at least four weeks.(Citation28,Citation29). With increased availability of various antiviral agents for HZ, it has become increasingly challenge for clinicians to compare across the available treatments and make appropriate decision.
In this study, we performed a comprehensive assessment of the clinical efficacy, safety, and PHN of brivudine, acyclovir, valaciclovir, and famciclovir for the treatment of HZ. A total of seven studies including 4171 patients were analyzed and pooled. Results showed that brivudine had better efficacy for HZ and shorter recovery time than acyclovir and valaciclovir. There were no significant differences in adverse reactions among acyclovir, valaciclovir and brivudine (p > .05). Moreover, brivudine can advance the clinical efficacy and prevent the occurrence of posthumous neuralgia while ensuring the safety in herpes zoster treatment. As to remission of postherpetic neuralgia, brivudine can more effectively lower posthumous neuralgia than acyclovir and valaciclovir.
The results of current study showed that the brivudine group was superior to the control group in terms of efficacy, blister time, pain relief time. A previous study based on varicella zoster activity showed that brivudine inhibits VZV replication 200 to 1000 times more than acyclovir and peniclovir (Citation30). Another clinical retrospective analysis comparing the combined efficacy of valaciclovir, famciclovir and brivudine indicated that brivudine relieves pain faster in severe pain cases and is more convenient to use (Quaque die, Peros) (Citation11), which is consistent with the findings in the present study. Brivudine is almost completely absorbed by the gastrointestinal tract, with half-life of 16h (Citation31,Citation32). Therefore, brivudine is the most convenient treatment mode compared with other antiviral drugs.
In any case, safety is one of the essential clinical concerns, thereby more attention should be paid to the safety and adverse effects of antiviral agents. In previous studies, the safety profile of HZ was not significantly different from that of other antiviral drugs, consistent with the results of the present study. Additionally, subgroup analysis also did not show differences in adverse effects between brivudine and others. However, these results cannot draw a solid conclusion whether brivudine is safer or not because of the small sample size, which is not suitable for analysis of some adverse events including heart palpitations, phlebitis, fatigue etc. Thus, additional clinical data are needed to ascertain the safety of brivudine.
Up to now, there are few systematic evaluations of brivudine. McDonald et al. compared the clinical efficacy of acyclovir, valaciclovir, famciclovir, and brivudine for the HZ. But due to the lack of randomized controlled clinical trials, the study on brivudine was not included in their analysis (Citation33). Wilhelmus et al. reported no significant difference in effectiveness among brivudine, acyclovir, and trifluridine following one-week treatment (Citation34). However, the results of this study showed that brivudine had an unprecedented advantage over conventional antiviral drugs in all aspects, which we believe is greatly related to the bias caused by the small sample size. For example, there were only three study data available for analysis of effectiveness. This also makes it difficult to analyze other reference criteria for shingles recovery, such as last vesicular eruption time, full crusting time. We cannot be certain that brivudine has an advantage in other indicators not analyzed. Again, the small sample size can compromise the validity of the study.
In the present study, we systematically evaluated the efficacy, safety, and incidence of PHN following the treatment of herpes zoster with brivudine. Data of the present study were from multiple countries and multiple races. On the premise of no limitation of languages and nationalities, results and conclusions based on the data of all relevant studies currently available can provide reference for clinical treatment of HZ. However, several potential limitations can interfere the results of our study. First, only seven studies fit into the inclusion criteria. The control groups which only included acyclovir and valaciclovir also have limitation. Second, bias may exist in some studies. For example, one study did not mention whether it was randomized or not, and three studies did not clarify the randomization method. Additionally, subgroup analyses of intention-to treat population analysis and per-protocol analysis were not performed in all studies to verify whether their conclusions were consistent.
5. Conclusion
Brivudine is more effective for HZ without increasing the risk of adverse reactions. Because there are only few randomized controlled clinical trials on brivudine treatment of HZ, more studies with larger sample sizes are needed to further validate the efficacy, safety of brivudine for the treatment of HZ.
Authors’ contributions
Litao Zhang and Jiaxing Chen are responsible for the conception and design of the study. Jiaxing Chen, Dongyun Lei, and Peng Cao are assigned to the acquisition of data and analysis. Dongyun Lei, Jiaxing Chen, and Junchen He wrote and revised the manuscript. All authors read and approved the final manuscript. Jiaxing Chen, Dongyun Lei, Junchen He, and Peng Cao are joint first authors.
Acknowledgments
The authors kindly thank the editors and reviewers for their useful comments.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Xia Wang CP, Gong J, Liu X, et al. Enhancing the enrichment of Pharmacophore-Based target prediction for the polypharmacological profiles of drugs. J Chem Inf Model. 2016;56(1):1–9. doi: 10.1007/s40257-019-00483-1.
- Kennedy PGE, Mogensen TH, Cohrs RJ. Recent issues in varicella-zoster virus latency. Viruses. 2021;13(10):2018. doi: 10.3390/v13102018.
- Gruver C, Guthmiller KB. Postherpetic neuralgia. Treasure Island (FL): StatPearls Publishing LLC; 2023.
- Tayyar R, Ho D. Herpes simplex virus and varicella zoster virus infections in cancer patients. Viruses. 2023;15(2):439. doi: 10.3390/v15020439.
- MacDougall C, Guglielmo BJ. Pharmacokinetics of valaciclovir. J Antimicrob Chemother. 2004;53(6):899–901. doi: 10.1093/jac/dkh244.
- De Clercq E. The development of BVDU: an odyssey. Antivir Chem Chemother. 2023;31:20402066231152971. doi: 10.1177/20402066231152971.
- Kaikai SM, Dowling Evans D. Presentation, management, and prevention of herpes zoster. Adv Emerg Nurs J. 2022;44(1):3–10. doi: 10.1097/tme.0000000000000395.
- Vogel C, Wetzel L, Wutzler P, et al. Treatment with brivudine in immunocompromised pediatric patients with herpes zoster. Chemotherapy. 2023;68(4):222–227. doi: 10.1159/000531034.
- Kim SH. Current scenario and future applicability of antivirals against herpes zoster. Korean J Pain. 2023;36(1):4–10. doi: 10.3344/kjp.22391.
- Wassilew S. Brivudin compared with famciclovir in the treatment of herpes zoster: effects in acute disease and chronic pain in immunocompetent patients. A randomized, double-blind, multinational study. J Eur Acad Dermatol Venereol. 2005;19(1):47–55. doi: 10.1111/j.1468-3083.2004.01119.x.
- Yaldiz M, Solak B, Kara RO, et al. Comparison of famciclovir, valaciclovir, and brivudine treatments in adult immunocompetent patients with herpes zoster. Am J Ther. 2018;25(6):e626–e634. doi: 10.1097/mjt.0000000000000436.
- Zeng X, Jiang J, Liu S, et al. Bidirectional effects of geniposide in liver injury: preclinical evidence construction based on meta-analysis. J Ethnopharmacol. 2024;319(Pt 1):117061. doi: 10.1016/j.jep.2023.117061.
- Yang M, Shen C, Zhu S-J, et al. Chinese patent medicine Aidi injection for cancer care: an overview of systematic reviews and meta-analyses. J Ethnopharmacol. 2022;282:114656. doi: 10.1016/j.jep.2021.114656.
- Lee YH. An overview of meta-analysis for clinicians. Korean J Intern Med. 2018;33(2):277–283. doi: 10.3904/kjim.2016.195.
- Tufanaru C, Munn Z, Stephenson M, et al. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid Based Healthc. 2015;13(3):196–207. doi: 10.1097/xeb.0000000000000065.
- Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions. 2nd ed. Chichester (UK): John Wiley & Sons; 2019.
- Wassilew SW, Wutzler P. Oral brivudin in comparison with acyclovir for herpes zoster: a survey study on postherpetic neuralgia. Antiviral Res. 2003;59(1):57–60. doi: 10.1016/s0166-3542(03)00064-0.
- Wutzler P, De Clercq E, Wutke K, et al. Oral brivudin vs. intravenous acyclovir in the treatment of herpes zoster in immunocompromised patients: a randomized double-blind trial. J Med Virol. 1995;46(3):252–257. doi: 10.1002/jmv.1890460315.
- Wassilew SW, Wutzler P. Oral brivudin in comparison with acyclovir for improved therapy of herpes zoster in immunocompetent patients: results of a randomized, double-blind, multicentered study. Antiviral Res. 2003;59(1):49–56. doi: 10.1016/s0166-3542(03)00065-2.
- Mao F, Wu Z, Zhang L, et al. Therapeutic effect of brivudine on 37 cases of herpes zoster. Mod Pract Med. 2018;30(04):535–536.
- Zhang X, Su B, Wang L. Observation of curative effect of brivudine on herpes zoster. Eval Anal Drug Use Hosp China. 2013;13(03):255–256. doi: 10.14009/j.issn.1672-2124.2013.03.003.
- Cheng H, Zhang D, Song T, et al. Efficacy observation and safety evaluation of brivudine in the treatment of herpes zoster. J Med Aesthet Cosmetol. 2021;30(14):80–81.
- Patil A, Goldust M, Wollina U. Herpes zoster: a review of clinical manifestations and management. Viruses. 2022;14(2):192. doi: 10.3390/v14020192.
- Gross GE, Eisert L, Doerr HW, et al. S2k guidelines for the diagnosis and treatment of herpes zoster and postherpetic neuralgia. J Dtsch Dermatol Ges. 2020;18(1):55–78. doi: 10.1111/ddg.14013.
- Hassan STS, Šudomová M, Mazurakova A, et al. Insights into antiviral properties and molecular mechanisms of non-flavonoid polyphenols against human herpesviruses. Int J Mol Sci. 2022;23(22):2022. doi: 10.3390/ijms232213891.
- Kenzaka T, Sugimoto K, Goda K, et al. Acute kidney injury and acyclovir-associated encephalopathy after administration of valacyclovir in an elderly person with normal renal function: a case report and literature review. Medicine. 2021;100(21):e26147. doi: 10.1097/md.0000000000026147.
- Shiraki K, Takemoto M, Daikoku T. Emergence of varicella-zoster virus resistance to acyclovir: epidemiology, prevention, and treatment. Expert Rev Anti Infect Ther. 2021;19(11):1415–1425. doi: 10.1080/14787210.2021.1917992.
- Werner RN, Nikkels AF, Marinović B, et al. European consensus-based (S2k) guideline on the management of herpes zoster - guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV), part 2: treatment. J Eur Acad Dermatol Venereol. 2017;31(1):20–29. doi: 10.1111/jdv.13957.
- García Herrera AN, Moncayola Vicén JC. [Lethal interaction between 5-fluorouracil and brivudine]. An Sist Sanit Navar. 2018;41(2):277–278. doi: 10.23938/assn.0297.
- Andrei G, Snoeck R, Reymen D, et al. Comparative activity of selected antiviral compounds against clinical isolates of varicella-zoster virus. Eur J Clin Microbiol Infect Dis. 1995;14(4):318–329. doi: 10.1007/bf02116525.
- Laskin OL, Longstreth JA, Saral R, et al. Pharmacokinetics and tolerance of acyclovir, a new anti-herpesvirus agent, in humans. Antimicrob Agents Chemother. 1982;21(3):393–398. doi: 10.1128/aac.21.3.393.
- Rabasseda X. Brivudine: a herpes virostatic with rapid antiviral activity and once-daily dosing. Drugs Today. 2003;39(5):359–371. doi: 10.1358/dot.2003.39.5.740221.
- McDonald EM, de Kock J, Ram FS. Antivirals for management of herpes zoster including ophthalmicus: a systematic review of high-quality randomized controlled trials. Antivir Ther. 2012;17(2):255–264. doi: 10.3851/imp2011.
- Wilhelmus KR, Gee L, Hauck WW, et al. Herpetic eye disease study: a controlled trial of topical corticosteroids for herpes simplex stromal keratitis. Ophthalmology. 2020;127(4s):S5–S18. doi: 10.1016/j.ophtha.2020.01.037.