Abstract
Background
Psoriasis significantly impacts patients’ quality of life (QoL). Dissatisfaction and non-adherence are major barriers associated with topical treatments. A cream based on the polyaphron dispersion (PAD) Technology containing a fixed-dose of calcipotriol (CAL) and betamethasone dipropionate (BDP) was designed for a patient-friendly psoriasis management. The CAL/BDP PAD-cream demonstrated efficacy, convenience, and safety/tolerability in clinical trials.
Objectives
This research assesses the real-world use, perception, satisfaction, and adherence of CAL/BDP PAD-cream among plaque psoriasis patients.
Methods
Between September–November 2023, psoriasis patients from Spain and Germany using or having used CAL/BDP PAD-cream for >2 weeks were recruited via Wefight network to complete a 30-questions online survey. Anonymized results were pooled for descriptive statistical analysis.
Results
The survey was completed by 129 patients (mean age: 43 years; 66% females; mean psoriasis duration: 12 years). Most patients (93%) were satisfied with CAL/BDP PAD-cream. The 66% reported high adherence (visual analogue scale 80-100) and 91% preferred CAL/BDP PAD-cream to their previous topical(s). Patients highlighted its ease/convenience of application, tolerability, and lack of itching/burning.
Conclusions
Psoriasis patients treated with CAL/BDP PAD-cream in a real-world setting show high satisfaction, good adherence, and a positive perception of the product, suggesting that favorable outcomes observed in clinical trials translate to real clinical practice.
Introduction
Psoriasis is a chronic immune-mediated skin disease that affects approximately 125 million people worldwide. Plaque psoriasis is the most common form of the disease, representing between 80% and 90% of all cases (Citation1). It is characterized by the formation of sharply demarcated, erythematous, scaly plaques, typically in a symmetric distribution (Citation1,Citation2). The involvement of certain body regions together with its associated comorbidities have a significant impact on patients’ quality of life, even on those with a mild form of the disease (Citation3).
The management of psoriasis is based on three different types of treatments, which can be used in combination or as monotherapy: topical therapy, phototherapy and systemic therapy (Citation4). Topical therapies are the keystone of the management of psoriasis, since most mild-to-moderate forms can be successfully controlled with topical treatments (Citation3). Main advantages of topicals include the availability of a wide array of topical agents, the adaptability to different body areas and the possible combination with systemics and phototherapy if needed (Citation3).
Despite numerous active ingredients and formulations are available for the topical treatment of psoriasis, some unmet needs still exist (Citation5). Patient adherence is recognized as a major barrier to treatment success with topicals (Citation6,Citation7). A poor adherence can be caused by factors related to the topical treatment, such as patient dissatisfaction with the product, fear of side effects, complicated or inconvenient regimens, and drug vehicles with unpleasant cosmetic and galenical properties (Citation6). Thus, there is a need to improve the patient satisfaction with the characteristics of existing topical treatments in order to enhance adherence and, therefore, clinical outcomes and the quality of life for patients with psoriasis (Citation8,Citation9). An improvement in the vehicle can result in benefits on both clinical outcomes and patient-reported outcomes (Citation10). There is no one topical drug formulation that suits the preferences of all patients, which highlights the importance of individualized topical treatment (Citation11). Patients should play an essential role in the pharmaceutical design of drug products (Citation12) and also in the choice of treatment strategy (Citation11). Through shared decision-making and tailoring vehicles to patients’ preferences, rejection and non-adherence to medicines may be counteracted (Citation12).
The fixed-dose combination of calcipotriol (CAL) and betamethasone dipropionate (BDP) is recommended as the first-line choice in topical psoriasis treatment (Citation13). A novel cream formulation based on the polyaphron dispersion (PAD) Technology containing the fixed-dose combination of CAL/BDP was designed for a more convenient topical treatment of plaque psoriasis of the body and scalp, in adults (Citation14). The CAL/BDP PAD-cream was developed with the aim of obtaining a topical product with the desirable cosmetic properties by patients and to generate consequently greater acceptance and adherence (Citation15). Compared with other existing formulations, the CAL/BDP PAD-cream vehicle has better sensory properties than ointment and non-aqueous foam and easier manipulation than oleogel (Citation15,Citation16). The CAL/BDP PAD-cream has demonstrated a similar safety and tolerability profile compared to CAL/BDP gel, but with greater efficacy, satisfaction and convenience in two phase III studies (Citation14,Citation17). Although high patient acceptability and treatment satisfaction have been observed with CAL/BDP PAD-cream under controlled clinical trials conditions, there is a lack of evidence in a real-world setting. The objective of this research was to assess through a patient survey the real-world use, perception, satisfaction, and adherence of CAL/BDP PAD-cream among patients with plaque psoriasis who were treated with the product for more than 2 weeks.
Materials and methods
Patient recruitment
Between 12th September and 14th November 2023, patients were recruited from two European countries (Spain and Germany) via the Wefight network. Wefight is a digital health company that creates and develops virtual health assistants (named Vik) to support patients with specific chronic conditions, such as psoriasis. To be eligible for the study, patients had to be diagnosed with psoriasis (with or without psoriatic arthritis) and had to be current users or past users (i.e., within the past three months) of CAL/BDP PAD-cream for >2 weeks.
Survey design
Patients were offered to participate in a 10-minute online survey containing 30 questions on their demographic and clinical disease characteristics, and CAL/BDP PAD-cream use, perception, satisfaction, and adherence. Per design, this online survey did not affect the treatment or health outcomes of the participants. The survey was provided to the patients through Vik, which is the mobile app of Wefight and was also advertised in the Wefight social networks.
Ethics
This research follows the European Pharmaceutical Market Research Association (EPHMRA) Code of Conduct. Since this was a one-time online survey on patients with plaque psoriasis, without any intervention, it did not require ethical approval from a local ethics committee or Institutional Review Board.
According to the General Data Protection Regulation (GDPR) guidelines, all participating patients provided consent for the collection and processing of their personal health data before starting to complete the survey. They were clearly explained the purpose of the data collection and that data would be always shared in an anonymous and aggregated manner. All patients agreed to voluntarily participate in the survey.
Statistical analyses
The survey results were anonymized, pooled, and processed for statistical analysis. Since the survey was not controlled and took place only once, the data collected only underwent descriptive analysis. Results are described by summary statistics. No formal statistical hypotheses were planned, and no inferential tests were performed.
Results
Demographics and clinical characteristics
A total of 129 patients with psoriasis from Spain and Germany (67% and 33%, respectively) completed the survey. Most patients were females (66%). The mean (standard deviation [SD]) age was 43.1 (12.9) years old, with the majority of patients being into the 18–34 years (31%) and 35-44 years (32%) age ranges. Patients had psoriasis for a mean (SD) duration of 12.2 (11.3) years and 20% of them had also psoriatic arthritis. Among all respondents, the mean body surface area (BSA) affected by psoriasis was 11%, mostly scalp (64% of patients) and elbows (58% of patients). The 98% of patients considered that their QoL had been impacted, from slightly to very much, by the disease. In addition to using a topical, 30% of patients were also currently using an oral treatment and 24% were using an injectable treatment ().
Table 1. Demographic and clinical characteristics of patients who completed the survey.
Real-world use of CAL/BDP PAD-cream
This survey was completed by current users and past users (i.e., within the past three months) of CAL/BDP PAD-cream for >2 weeks. Among survey respondents, the 78% of patients were current users of CAL/BDP PAD-cream and the 33% of patients had used it in the past. It has to be highlighted, that 11% of patients reported to use CAL/BDP PAD-cream both currently and in the past.
Regarding prescriptions, CAL/BDP PAD-cream is mainly prescribed by dermatologists (78%), followed by general practitioners (22%). The initial doctor’s prescription did not specify the number of weeks to use CAL/BDP PAD-cream for 40% of the users, was renewed by the doctor in 37% of the cases and was not renewed for 20% of them.
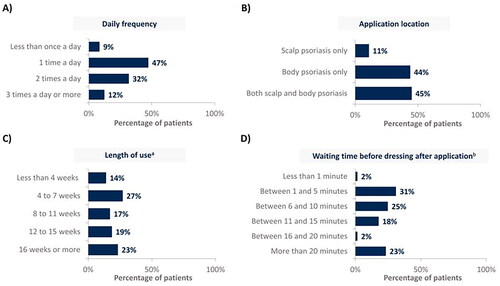
shows real-world treatment patterns of CAL/BDP PAD-cream. The 79% of patients use CAL/BDP PAD-cream between once and twice a day (). Almost half of them (45%) use CAL/BDP PAD-cream for treating both their scalp and body, and 11% for their scalp only (). A prolonged use of CAL/BDP PAD-cream was observed, beyond the recommended 8 weeks. The mean length of CAL/BDP PAD-cream use was 14 weeks (). The 58% of patients reported waiting ≤10 minutes before getting dressed after application, with an average waiting time of 15 minutes before dressing ().
Figure 1. CAL/BDP PAD-cream use in real-world according to (A) daily frequency, (B) application location, (C) length of use and (D) waiting time before dressing after application.
Rounded percentages calculated based on the total number of patients who completed the survey (n = 129).
aMean length of use was 14 weeks; bMean waiting time before dressing after application was 15 minutes.
CAL: calcipotriol; BDP: betamethasone dipropionate; PAD: polyaphron dispersion.
Satisfaction with CAL/BDP PAD-cream
When asking patients for their overall satisfaction with the treatment, 120 out of the 129 survey respondents (93%) answered to be satisfied with CAL/BDP PAD-cream, with 63 (49%) being very satisfied and 57 (44%) being somewhat satisfied. Only nine patients (7%) were somewhat unsatisfied with the CAL/BDP PAD-cream. No patients reported to be very unsatisfied with CAL/BDP PAD-cream.
Perception of CAL/BDP PAD-cream
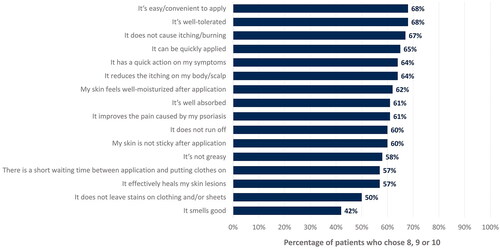
Patients rated from 0 (‘do not agree at all’) to 10 (‘completely agree’) different criteria related to the perception of CAL/BDP PAD-cream. shows the percentage of patients who gave a score of 8 to 10 for each criterion. The top three criteria better rated were that CAL/BDP PAD-cream is ‘easy/convenient to apply’, ‘well tolerated’, and ‘does not cause itching/burning’.
Figure 2. Percentage of patients who rated high agreement or complete agreement with each perception criterion, depending on whether it applied to CAL/BDP PAD-cream.
In the survey, each criterion could be rated by patients from 0 (‘do not agree at all’) to 10 (‘completely agree’). This figure only displays the percentage of patients who chose 8, 9, or 10 for each criterion. Rounded percentages calculated based on the total number of patients who completed the survey (n = 129).
CAL: calcipotriol; BDP: betamethasone dipropionate; PAD: polyaphron dispersion.
From the 46 patients who use CAL/BDP PAD-cream for scalp, 30 patients (65%) highlighted that CAL/BDP PAD-cream was easy to apply and spread on the scalp and 24 patients (52%) that it was easy to remove when washing their hair.
Regarding impact of CAL/BDP PAD-cream on daily activities and routine, 81 patients (63%) felt that the use of CAL/BDP PAD-cream did not affect at all or did not greatly affect their ability to take part in daily activities and routine. Although 47 patients (36%) thought that CAL/BDP PAD-cream could be noticeable to others, only 12 of them felt stigmatized and discriminated against.
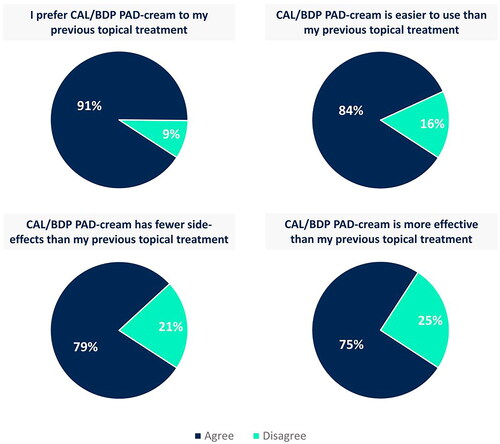
CAL/BDP PAD-cream generally has a positive impact on its users, with most patients feeling better about their appearance and seeing their self-esteem improved (). Despite the inability to wear the clothes and adopt the hair style they desire still persists (), the 64% of patients stated that the good things about CAL/BDP PAD-cream outweigh the bad and the 67% of patients would be willing to use it again in the future (). Furthermore, 91% of patients preferred CAL/BDP PAD-cream compared to their previous topical treatment. Most patients also found CAL/BDP PAD-cream easier to use (84%), more effective (75%), and with fewer side effects (79%) than their previous topical treatment ().
Figure 3. (A) Positive impact, (B) negative impact and (C) evaluation of CAL/BDP PAD-cream in a real-world setting.
Rounded percentages calculated based on the total number of patients who completed the survey (n = 129).
CAL: calcipotriol; BDP: betamethasone dipropionate; HCP: healthcare practitioner; PAD: polyaphron dispersion.
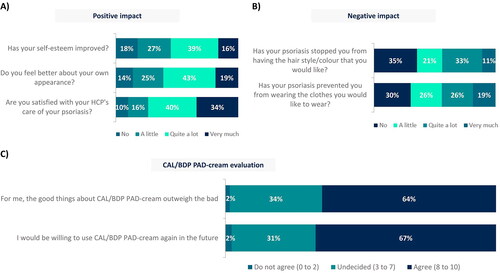
Figure 4. Comparison of CAL/BDP PAD-cream to the previous topical treatment used by patients.
Agree corresponds to overall positive responses (i.e., ‘agree’ and ‘strongly agree’). Disagree corresponds to overall negative responses (i.e., ‘disagree’ and ‘strongly disagree’). Rounded percentages calculated based on the total number of patients who had used a topical in the past (n = 67).
CAL: calcipotriol; BDP: betamethasone dipropionate; PAD: polyaphron dispersion.
Adherence with CAL/BDP PAD-cream
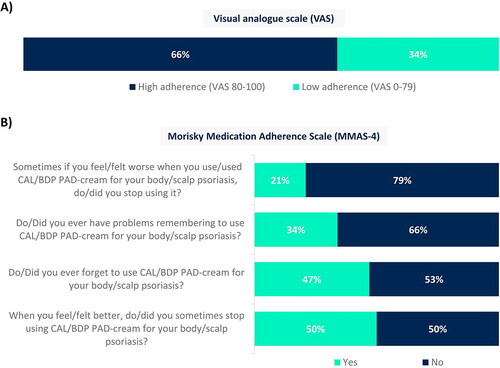
The 66% of patients reported high adherence through a visual analogue scale (VAS), i.e., VAS 80-100 (). However, when focusing on responses corresponding to the Morisky Medication Adherence Scale (MMAS-4), the 50% and 47% of patients have stopped using CAL/BDP PAD-cream when they felt better and have forgotten to use it, respectively (). Patients with higher adherence to CAL/BDP PAD-cream showed better results on all metrics of the MMAS-4, while lower adherence to CAL/BDP PAD-cream (VAS < 80) was generally associated with a less positive impact of the cream, especially on self-esteem, and significantly lower willingness to use CAL/BDP PAD-cream again in the future. Compared with the subgroup of patients with higher adherence (VAS 80-100), patients with lower adherence (VAS < 80) were more likely to be men (45% vs 28%), had been diagnosed with psoriasis longer ago (mean of 15 years vs 11 years since diagnosis), had less involvement of scalp (52% vs 69% of patients) and knees (30% vs 46% of patients), and their mean CAL/BDP PAD-cream length of treatment was higher (18 weeks vs 12 weeks).
Figure 5. Adherence with CAL/BDP PAD-cream assessed through (A) a visual analogue scale (VAS) and (B) the Morisky Medication Adherence Scale (MMAS-4).
Rounded percentages calculated based on the total number of patients who completed the survey (n = 129).
CAL: calcipotriol; BDP: betamethasone dipropionate; MMAS-4: Morisky Medication Adherence Scale; PAD: polyaphron dispersion; VAS: visual analogue scale.
Discussion
Patients’ feedback regarding treatment satisfaction and adherence is crucial for a successful management of psoriasis with topical therapies (Citation5). The present research assessed for the first time the use, perception, satisfaction and adherence of CAL/BDP PAD-cream in a real-world setting among patients with plaque psoriasis. All patients voluntarily completed a one-time online survey assessing their experience with the CAL/BDP PAD-cream. Our population consisted of 129 patients with psoriasis from Spain and Germany who were part of the Wefight network. Demographics and clinical characteristics of survey respondents generally align with those of patients who received CAL/BDP PAD-cream in the phase III trials. However, survey respondents tended to be younger and there was a higher proportion of females among them (Citation17).
Regarding the satisfaction with the CAL/BDP PAD-cream, almost all users (93%) reported to be satisfied with the product. This high satisfaction observed in clinical practice agrees with the high convenience obtained in the phase III clinical trials, where a high patient acceptability of the CAL/BDP PAD-cream was demonstrated by improvements in the Psoriasis Treatment Convenience Scale (PTCS) (Citation17). In a post-hoc analysis from the two phase III clinical trials, high levels of treatment satisfaction were also reported based on individual items of the Dermatology Life Quality Index (DLQI) questionnaire (Citation18). In contrast to the survey conducted by van Cranenburgh et al., where the patients receiving different types of topical treatments were less satisfied (Citation19), in the present survey nearly half of users (49%) are very satisfied, and 44% somewhat satisfied with the CAL/BDP PAD-cream under real-world conditions. The CAL/BDP PAD-cream was developed incorporating patient’s perspectives and preferences into the design of the product, in order to develop a more patient-friendly topical treatment for psoriasis with the CAL/BDP fixed-dose combination (Citation14). The elevated satisfaction expressed by patients supports that consideration should be given not only to the usual focus on safety and efficacy but also to convenience factors (Citation20).
Prescribing therapy in line with patient preference for treatment vehicle may be a key factor for maximizing patient adherence (Citation21). In our survey, CAL/BDP PAD-cream is well-perceived, with most patients willing to use it again in the future. This positive perception among patients with body and/or scalp psoriasis may be attributed to its formulation as an aqueous cream based on PAD Technology (Citation22). A recently published survey demonstrated that water-based creams are the most preferred vehicle for the topical treatment of psoriasis (Citation23). As assessed in a sensory properties analysis, the vehicle used to deliver CAL/BDP PAD-cream has the desirable requirements for a topical product for the treatment of psoriasis. Specifically, it exhibits low stickiness, low grease behavior, good wetness, and good spreadability (Citation15). These vehicle characteristics aligning with patient preferences may indeed contribute to explain the positive perception of the CAL/BDP PAD-cream and the patients’ reported willingness to use the treatment again in the future (Citation23).
When focusing on the perception of specific formulation attributes of the CAL/BDP PAD-cream, ≥50% of patients rated the CAL/BDP PAD-cream with a high score (8 to 10) on different formulation criteria which have been previously identified as the most important from the patients’ point of view (Citation11,Citation23,Citation24). Findings from Bewley et al. suggest that the most important criteria when choosing a topical are the reduction in itching and good tolerability (Citation20). CAL/BDP PAD-cream is well perceived on these two most important criteria. Additionally, the product stands out for its ease and convenience of application. In fact, 58% of patients wait ≤10 minutes before getting dressed after application. This is really important considering that the time needed for treatment has been identified as a major predictor of quality of life in psoriasis (Citation25). Furthermore, the benefits of the CAL/BDP PAD-cream are accentuated when patients compare this positive experience with their previous topical treatments. Most patients prefer the CAL/BDP PAD-cream because it is easier to use, has fewer side effects and is more effective.
The above-mentioned attributes, together with the overall favorable perception of the product, may elucidate the high levels of treatment satisfaction and adherence observed with the CAL/BDP PAD-cream. It is well known that adherence is a major issue in the topical treatment of psoriasis (Citation21). It is also well known that nearly half of topical prescriptions are not being filled by patients with dermatological conditions (i.e., primary adherence) and up to 95% of patients suffering skin diseases are not using the medications as prescribed (i.e., secondary adherence) (Citation26–28). Among patients who fill their prescriptions, only a median of 35% of the expected dose is used (Citation28). Studies using a dichotomous classification of adherence tend to report suboptimal adherence, with as few as 22% patients classed as adherent (Citation29). However, in this survey 66% of patients reported high-adherence with the CAL/BDP PAD-cream, which is in contrast with the poor adherence rates to topicals reported in previous studies. Only a third of users reported low adherence (i.e., VAS <80), which were more likely to be men, had been diagnosed with psoriasis longer ago and exhibited a higher mean duration of treatment with CAL/BDP PAD-cream. Our findings support that greater treatment satisfaction is associated with better compliance (Citation30). These results highlight that it is necessary to improve communication between patients and healthcare professionals in order to increase adherence and satisfaction in patients treated with topicals for their plaque psoriasis (Citation9). Moreover, healthcare professionals should also properly explain the correct use of the topical therapy (Citation31).
Real-world treatment pattern results revealed that the mean length use of CAL/BDP PAD-cream is above the recommended treatment period (14 weeks vs 8 weeks). This prolonged use of CAL/BDP PAD-cream observed in the real clinical practice may be explained by the fact that, for 40% of CAL/BDP PAD-cream users, their prescription did not indicate the number of weeks of usage. Thus, a good communication between patients and healthcare professionals is deemed necessary, as it has been demonstrated that after a personalized medication training, patients learn how to correctly apply the medication and therefore obtain better clinical outcomes (Citation31).
This survey shows for the first time CAL/BDP PAD-cream results in a real-world setting. Findings from clinical trials performed under controlled conditions seem to be translated to the real clinical practice, as supported by the high treatment satisfaction and good adherence reported by patients. Patients who completed the survey were forming part of the Wefight network, a large well-established psoriasis patient network in Europe. Moreover, the inclusion of past and current users who were treated with CAL/BDP PAD-cream for more than 2 weeks, ensured that survey respondents had enough familiarity with the product to provide substantive feedback. However, this research has some limitations. Voluntary participation in online surveys may result in selection bias, as for example older patients are less likely to participate and only patients sufficiently interested in the disease may respond the survey (Citation32,Citation33). Inclusion of patients from other sources, rather than Wefight, would have increased the generalizability of the results. Another constraint associated with the use of surveys to gather data is the time limitation. Patients may consider of low priority to carry out a survey because of competing urgent tasks or may feel fatigued with responding the survey, which may lead to incorrect answers to the research questions (Citation34,Citation35). Finally, self-administered surveys may yield significantly different results when compared to face-to-face or telephone surveys. In this case, as results were entirely reported by patients, disease characteristics may be less reliable than if reported by a healthcare professional. However, responses regarding treatment patterns, adherence and satisfaction may mirror more accurately real-world clinical practices, as patients may feel less pressured in the absence of an interviewer (Citation36,Citation37).
In conclusion, plaque psoriasis patients treated with CAL/BDP PAD-cream in a real-world setting show high treatment satisfaction and good adherence. The product is well perceived by users, who highlight its ease/convenience of use, tolerability, rapid onset of action, and positive impact on their appearance and self-esteem. The results of this online survey confirm that the high patient acceptability and treatment satisfaction shown by the CAL/BDP PAD-cream in clinical trials are also observed in the real clinical practice, together with good adherence results.
Acknowledgements
Medical writing and editorial assistance in the preparation of this article was provided by Paula Casajust, MSc of TFS HealthScience, with financial support provided by Almirall S.A.
Disclosure statement
José Luis López Estebaranz served as a consultant, participated in clinical trials and/or received speaking fees from Almirall, Janssen, Leo-Pharma, Lilly, Abbvie, Bioderma, Galderma, UCB, Novartis, Pierre-Fabre, Invasix, Isdin and Incyte. Hjalmar Kurzen received travel funds and speaker honoraria from Almirall in the past two years. Jordi Galván is an employee of Almirall S.A.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1–9. doi: 10.1001/jama.2020.4006.
- Raharja A, Mahil SK, Barker JN. Psoriasis: a brief overview. Clin Med (Lond). 2021;21(3):170–173. doi: 10.7861/clinmed.2021-0257.
- Pinter A, van de Kerkhof P. The role of topical therapies along the psoriasis patient journey: an overview from the symposium “Tailoring topical psoriasis treatments to patients’ needs and expectations" of the 30th EADV congress 2021. J Eur Acad Dermatol Venereol. 2023;37(Suppl 1):3–8. doi: 10.1111/jdv.18761.
- Menter A, Griffiths CE. Current and future management of psoriasis. Lancet. 2007;370(9583):272–284. doi: 10.1016/S0140-6736(07)61129-5.
- Gupta S, Garbarini S, Nazareth T, et al. Characterizing outcomes and unmet needs among patients in the United States with mild-to-moderate plaque psoriasis using prescription topicals. Dermatol Ther (Heidelb). 2021;11(6):2057–2075. doi: 10.1007/s13555-021-00620-x.
- Bewley A, van de Kerkhof P. Engaging psoriasis patients in adherence and outcomes to topical treatments: a summary from the symposium “Tailoring topical psoriasis treatments to patients’ needs and expectations" of the 30th EADV congress 2021. J Eur Acad Dermatol Venereol. 2023;37(Suppl 1):9–13. doi: 10.1111/jdv.18751.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176(3):759–764. doi: 10.1111/bjd.15085.
- Belinchón I, Rivera R, Blanch C, et al. Adherence, satisfaction and preferences for treatment in patients with psoriasis in the European Union: a systematic review of the literature. Patient Prefer Adherence. 2016;10:2357–2367. doi: 10.2147/PPA.S117006.
- Puig L, Carrascosa JM, Belinchón I, et al. Adherence and patient satisfaction with topical treatment in psoriasis, and the use, and organoleptic properties of such treatments: a Delphi study with an expert panel and members of the psoriasis group of the Spanish Academy of Dermatology and Venereology. Actas Dermosifiliogr. 2013;104(6):488–496.
- Iversen L, Dauden E, Segaert S, et al. Reformulations of well-known active ingredients in the topical treatment of psoriasis vulgaris can improve clinical outcomes for patients. J Eur Acad Dermatol Venereol. 2017;31(8):1271–1284. doi: 10.1111/jdv.14277.
- Svendsen MT, Feldman SR, Tiedemann SN, et al. Psoriasis patient preferences for topical drugs: a systematic review. J Dermatolog Treat. 2021;32(5):478–483. doi: 10.1080/09546634.2019.1675855.
- Oliveira R, Almeida IF. Patient-centric design of topical dermatological medicines. Pharmaceuticals (Basel). 2023;16(4):617. doi: 10.3390/ph16040617.
- Megna M, Cinelli E, Camela E, et al. Calcipotriol/betamethasone dipropionate formulations for psoriasis: an overview of the options and efficacy data. Expert Rev Clin Immunol. 2020;16(6):599–620. doi: 10.1080/1744666X.2020.1776116.
- Torres T, Galván J, Crutchley N, et al. Calcipotriol and betamethasone dipropionate cream based on PAD technology for the treatment of plaque psoriasis: a narrative review. Dermatol Ther (Heidelb). 2023;13(10):2153–2169. doi: 10.1007/s13555-023-01003-0.
- García N, Guiró P, Galván J, et al. Sensory properties analysis of a calcipotriol and betamethasone dipropionate cream vehicle formulated with an innovative PAD technology for the treatment of plaque psoriasis on the skin and scalp. Drugs Context. 2023;12:1–8.
- Guilà T, García N, Guiró P, et al. Sensory properties analysis of the main pharmaceutical forms used in the topical treatment of plaque psoriasis. Poster 2323 presented at the 32nd European Academy of Dermatology and Venereology Congress. Berlin, 11–14 October 2023.
- Pinter A, Green LJ, Selmer J, et al. A pooled analysis of randomized, controlled, phase 3 trials investigating the efficacy and safety of a novel, fixed dose calcipotriene and betamethasone dipropionate cream for the topical treatment of plaque psoriasis. J Eur Acad Dermatol Venereol. 2022;36(2):228–236. doi: 10.1111/jdv.17734.
- Halioua B, Caillet G, Taieb C, et al. A novel calcipotriol and betamethasone dipropionate (CAL/BDP) PAD-cream demonstrates greater improvements in daily activities and personal relationships than CAL/BDP gel/TS: a post-hoc analysis of DLQI outcomes from two phase 3 placebo-controlled randomized clinical trials in mild-to-moderate psoriasis. J Eur Acad Dermatol Venereol. 2023;38(4):e326–e328. doi: 10.1111/jdv.19600.
- van Cranenburgh OD, de Korte J, Sprangers MaG, et al. Satisfaction with treatment among patients with psoriasis: a web-based survey study. Br J Dermatol. 2013;169(2):398–405. doi: 10.1111/bjd.12372.
- Bewley A, Hiribarne L, Galván J, et al. Burden of topical treatments in psoriasis and preferred criteria of choice: a survey-based evaluation of patients in Europe. Dermatol Ther (Heidelb). 2024. doi: 10.1007/s13555-024-01132-0. Online ahead of print.
- Bewley A, Page B. Maximizing patient adherence for optimal outcomes in psoriasis. J Eur Acad Dermatol Venereol. 2011;25(Suppl 4):9–14. doi: 10.1111/j.1468-3083.2011.04060.x.
- Praestegaard M, Steele F, Crutchley N. Polyaphron dispersion technology, a novel topical formulation and delivery system combining drug penetration, local tolerability and convenience of application. Dermatol Ther (Heidelb). 2022;12(10):2217–2231. doi: 10.1007/s13555-022-00794-y.
- Curcio A, Kontzias C, Gorodokin B, et al. Patient preferences in topical psoriasis treatment. J Drugs Dermatol. 2023;22(4):326–332. doi: 10.36849/JDD.7372.
- Vasconcelos V, Teixeira A, Almeida V, et al. Patient preferences for attributes of topical anti-psoriatic medicines. J Dermatolog Treat. 2019;30(7):659–663.
- Blome C, Simianer S, Purwins S, et al. Time needed for treatment is the major predictor of quality of life in psoriasis. Dermatology. 2010;221(2):154–159. doi: 10.1159/000313825.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59(1):27–33.
- Teixeira A, Teixeira M, Almeida V, et al. Methodologies for medication adherence evaluation: focus on psoriasis topical treatment. J Dermatol Sci. 2016;82(2):63–68. doi: 10.1016/j.jdermsci.2016.02.008.
- Storm A, Benfeldt E, Andersen SE, et al. A prospective study of patient adherence to topical treatments: 95% of patients underdose. J Am Acad Dermatol. 2008;59(6):975–980. doi: 10.1016/j.jaad.2008.07.039.
- Thorneloe RJ, Bundy C, Griffiths CEM, et al. Adherence to medication in patients with psoriasis: a systematic literature review. Br J Dermatol. 2013;168(1):20–31. doi: 10.1111/bjd.12039.
- Barbosa CD, Balp MM, Kulich K, et al. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence. 2012;6:39–48. doi: 10.2147/PPA.S24752.
- Caldarola G, De Simone C, Moretta G, et al. Role of personalized medication training in improving efficacy and adherence to a topical therapy in psoriatic patients. J Dermatolog Treat. 2017;28(8):722–725. doi: 10.1080/09546634.2017.1328100.
- Eysenbach G, Wyatt J. Using the internet for surveys and health research. J Med Internet Res. 2002;4(2):E13. doi: 10.2196/jmir.4.2.e13.
- Andrade C. The limitations of online surveys. Indian J Psychol Med. 2020;42(6):575–576. doi: 10.1177/0253717620957496.
- Shiyab W, Ferguson C, Rolls K, et al. Solutions to address low response rates in online surveys. Eur J Cardiovasc Nurs. 2023;22(4):441–444. doi: 10.1093/eurjcn/zvad030.
- Egleston BL, Miller SM, Meropol NJ. The impact of misclassification due to survey response fatigue on estimation and identifiability of treatment effects. Stat Med. 2011; 30(30):3560–3572. doi: 10.1002/sim.4377.
- Duffy B, Smith K, Terhanian G, et al. Comparing data from online and face-to-face surveys. IJAM. 2005;47(6):615–639.
- Milton AC, Ellis LA, Davenport TA, et al. Comparison of self-reported telephone interviewing and web-based survey responses: findings from the second Australian young and well national survey. JMIR Ment Health. 2017;4(3):e37. doi: 10.2196/mental.8222.
