Abstract
Background
Understanding the economic value of deucravacitinib and apremilast could assist treatment decision-making for patients with moderate to severe plaque psoriasis.
Objective
This study compared the cost per response (CPR) for US patients initiating deucravacitinib versus apremilast for moderate to severe plaque psoriasis.
Methods
A CPR model using pharmacy and administration costs was developed from a US payer perspective. Response was defined as a 75% reduction from baseline in Psoriasis Area and Severity Index (PASI 75) at weeks 16 and 24. Long-term response was defined as the cumulative benefit over 52 weeks, measured as area under the curve; subsequent treatment was included. Scenario analyses explored varying the efficacy measure or choices of subsequent treatments and limiting discontinuation.
Results
The CPR for deucravacitinib versus apremilast was lower at 16 weeks (difference: –$3796 [95% confidence interval (CI): –$6140 to –$1659]) and 24 weeks (difference: –$12,784 [95% CI: –$16,674 to –$9369]). At 52 weeks, the cost per cumulative benefit was lower for patients who initiated deucravacitinib, regardless of initial treatment period duration (16 or 24 weeks).
Conclusions
Scenario analyses found mainly consistent results. This study showed that the CPR is lower when initiating deucravacitinib versus apremilast in moderate to severe plaque psoriasis.
Introduction
Psoriasis is a chronic, immune-mediated dermatologic disease condition characterized by dysfunction of the immune system and systemic inflammation of the skin (Citation1–3). Plaque psoriasis is the most common form of psoriasis and affects between 58% and 97% of all patients; it can involve various parts of the body including the scalp, behind the ears, forearms, and shins (Citation2). In the United States, psoriasis has a prevalence rate of approximately 3.0%, and approximately 20% of patients with plaque psoriasis have moderate to severe disease, which is typically defined as disease coverage of more than 10% of the body surface area (Citation1,Citation4,Citation5). The Psoriasis Area and Severity Index (PASI) is often employed as the gold standard in clinical trials as an objective measurement of disease reduction in psoriasis, with patients achieving 50%, 75%, or 90% reduction in PASI score (Citation5,Citation6). Moderate to severe plaque psoriasis is associated with substantial economic and clinical burden in the US, through high direct medical costs and significant impairment to a patient’s quality of life (Citation7,Citation8).
Systemic treatments approved by the US Food and Drug Administration (FDA) for treatment of moderate to severe plaque psoriasis include the phosphodiesterase 4 inhibitor apremilast, the antitumor necrosis factor-α inhibitors adalimumab, infliximab, and etanercept, and biologic therapies that selectively target certain interleukin (IL) pathways (IL-17, IL-23, and/or IL-12), such as brodalumab, guselkumab, ixekizumab, risankizumab, secukinumab, and ustekinumab (Citation3,Citation9). Recent network meta-analyses (NMAs) that explored the effectiveness of targeted immunomodulating treatments showed that brodalumab, guselkumab, ixekizumab, and risankizumab were the most effective treatments in terms of PASI response (Citation10,Citation11). Although these biologic treatments have demonstrated significant therapeutic success, the costs are considerably higher compared with non-targeted and/or nonbiologic treatment options, thereby generating socioeconomic barriers to treatment for patients with psoriasis in the United States (Citation11). Cost-effectiveness should therefore be an important component of decision-making for patients with moderate to severe plaque psoriasis.
Deucravacitinib is a first-in-class oral, selective, allosteric tyrosine kinase 2 (TYK2) inhibitor that was approved in September 2022 by the US FDA for the treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy (Citation12). It is distinct from Janus kinase 1,2,3 inhibitors because deucravacitinib selectively binds to the regulatory domain of TYK2, thereby leading to targeted inhibition of the IL-12, IL-23, and type 1 interferon pathways (Citation13). Two phase 3, multicenter, randomized, double-blind, placebo-controlled trials (POETYK PSO-1 and POETYK PSO-2) demonstrated the efficacy and safety of once-daily deucravacitinib 6 mg versus that of placebo and apremilast as a therapy for moderate to severe plaque psoriasis by achieving a reduction of at least 75% on the PASI total score (PASI 75) (Citation14,Citation15). Results from a matching-adjusted indirect comparison suggest higher long-term response rates at 2 years with deucravacitinib compared with adalimumab (Citation16). However, the economic impact of adopting deucravacitinib after approval to US health care payers has yet to be fully understood.
From the perspective of the health care payer, the cost per response (CPR) is a useful metric for health care decision-making to evaluate the costs and outcomes associated with deucravacitinib and apremilast, as well as with other currently available targeted therapies indicated for treatment of moderate to severe plaque psoriasis. The primary objective of this study was to evaluate the economic value of deucravacitinib compared with apremilast for the treatment of moderate to severe plaque psoriasis in the United States over a short-term (16–24 weeks) time horizon, using a CPR framework and a long-term (52 weeks) time horizon utilizing a cost per cumulative benefit framework. The CPR of deucravacitinib compared with first-line (1L) biologic treatments in this population was evaluated as a secondary objective.
Methods
Model structure and analyses
The CPR model was developed in Microsoft Excel (Redmond, WA) to compare deucravacitinib with comparators (apremilast and 1L biologics) in the 1L treatment of patients with moderate to severe plaque psoriasis in the United States. The sources for efficacy response rates are described below. The modeled patient population was adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy, aligned with the FDA-approved indication for deucravacitinib (Citation12). A hypothetical 1000-patient population was assumed. The treated population remained constant over the modeled time horizon for each analysis; no patients left the model because of mortality or other causes.
The CPR was calculated as the total treatment cost per patient (including drug acquisition and administration costs) divided by the clinical response rate in responders over the specified time horizon. The difference in the CPRs between deucravacitinib and comparators was computed, and 95% confidence intervals (CIs) for the difference in CPR were estimated via bootstrapping.
The primary endpoint assessed costs and treatment response at 16 and 24 weeks in line with the time points used to assess treatment response for deucravacitinib and apremilast during the POETYK-PSO trials (Citation14,Citation15). The secondary endpoint used a 52-week time horizon that included treatment switching. Long-term response was defined as the cumulative benefit over 52 weeks. Treatment response at weeks 16 or 24 (interim response assessment) was used to identify patients as responders (i.e., those who remained on their initial therapy and continued to maintenance) or nonresponders (those who switched to a second-line (2L) biologic therapy as described below). In addition, a proportion of patients in the 52-week model discontinued 1L treatment in each cycle and moved onto subsequent treatment, where they remained for the duration of the time horizon. The cumulative clinical benefit was defined as the area under the curve (AUC) over the 52-week treatment period, which was calculated using response rates at all available time points (Citation17,Citation18). Cost per cumulative benefit was calculated for each treatment by dividing the total cost over 52 weeks by the normalized percentage of the maximum AUC, which is calculated as the cumulative AUC multiplied by 100 divided by the maximum AUC available. Further explanation of how AUC estimates are generated from the clinical response data using the trapezoidal rule is provided in the Supplemental Materials and Supplementary Figure 1. For deucravacitinib, the number needed to treat (NNT) to achieve one additional PASI 75 response relative to comparators was calculated as the inverse of the difference in estimated PASI 75 response rates.
An overview of the 52-week model is provided in .
Model inputs
Primary objective: deucravacitinib compared with apremilast
Treatments
Apremilast was the main comparator in the model. Patients who failed to achieve efficacy response at the interim response point were switched to a subsequent treatment with one of the following biologic therapies that are currently reimbursed in the United States: adalimumab, brodalumab, certolizumab, etanercept, guselkumab, ixekizumab, risankizumab, secukinumab, tildrakizumab, or ustekinumab. When calculating certain inputs, such as the weighted annual treatment cost, each treatment was weighted by real-world 2L market shares obtained from anonymized patient-level data provided by Symphony Health (ICON plc, Dublin, Ireland) () (Citation19).
Table 1. Input values for efficacy, discontinuation, and market share.
Costs
Total pharmacy costs were estimated from a US health care payer perspective. Only drug acquisition and administration costs were considered in the model. Where possible, all costs were sourced in 2023 US dollars; costs that were not available in 2023 US dollars were adjusted based on the US consumer price index for medical care services for urban consumers (Citation21).
Drug acquisition costs were based on the wholesale acquisition cost obtained from the Micromedex Red Book (Merative, Ann Arbor, MI) in February 2023 () (Citation22). Dosing for each treatment was sourced from US prescribing information and reflected higher or more frequent doses associated with induction periods for treatments, as appropriate (Citation23–30). Costs were calculated by multiplying the estimated number of doses required per relevant time horizon by the treatment cost per dose. The mean body weight and percentages of patients from the POETYK-PSO trials weighing more than 90 kg and more than 100 kg were used to estimate costs for subsequent treatments of ustekinumab and infliximab. For subsequent biologic therapies, the annual treatment cost was first calculated for the 16-week induction period, split over four 4-week cycles, to account for the higher dosing frequency required for some treatments in the induction phase. For the maintenance phase, the remaining number of doses was multiplied by the cost per treatment dose and split over nine 4-week cycles. It was assumed that patients receiving deucravacitinib and apremilast would go through the induction phase required when initiating biologic therapies because they were not treated with a biologic therapy in the 1L setting. After interim response, an average cycle cost was calculated for 1L initiators by multiplying the proportion of patients on 1L treatment in that cycle by the 4-week average 1L treatment cost, plus the proportion of patients on subsequent treatment in that cycle, multiplied by the average cycle cost for 2L treatment. The annual average cost was therefore summed over all 13 cycles.
Table 2. Treatment costs and doses.
Treatment administration costs were incurred based on regimen and administration method. For treatments administered via subcutaneous injection, it was assumed that the cost would be incurred for a clinic visit for the first administration only, and the remaining doses were assumed to be administered at home, as per previous models in moderate to severe plaque psoriasis (Citation31). Both deucravacitinib and apremilast are administered orally and, therefore, incurred no cost per administration. An administration cost of $63.58 was used for all subcutaneous drugs (Current Procedural Terminology code: 96372) (Citation32).
Efficacy
Treatment response was assessed using PASI 75 response rates sourced from pooled efficacy analyses of the POETYK PSO-1 and POETYK PSO-2 clinical trials (Citation14,Citation15) (). For the secondary endpoint, the probability of achieving PASI 75 observed in the POETYK PSO clinical trials was used to define the proportion of patients who went on to receive maintenance therapy (responders) and those who switched to subsequent therapy (nonresponders). The pooled trial results demonstrated that deucravacitinib had a higher probability of achieving PASI 75 compared with apremilast (). To calculate the cumulative benefit over weeks 16–52, the efficacy of subsequent biologic therapies for nonresponders was sourced from a published NMA of therapies for the treatment of moderate to severe plaque psoriasis (Citation20). The PASI 75 values from the NMA were then weighted by the 2L market share values () to generate a weighted efficacy value for the subsequent biologic options.
Treatment discontinuation
Treatment discontinuation was only considered in the 52-week endpoint. Annual discontinuation rates for deucravacitinib and apremilast were derived directly from the POETYK PSO-1 trial and then transformed into a 4-week rate that was applied in each cycle after the interim assessment time point to week 52 (i.e., weeks 20–52 or weeks 28–52) ().
Secondary objective: deucravacitinib compared with 1L biologics
Additional model inputs were needed for the 1L biologic comparators. The composition of the group of 1L biologics was based on market share distributions from a 3-month rolling average for the total population that were obtained from Symphony Health APLD data (Citation19). The market share inputs that were applied as weights for the 1L biologic treatment group are presented in . The response rates for 1L biologics were calculated as a weighted average of the response rates of the treatments included in the group, which were obtained from an NMA (Citation20), and multiplied by the respective market shares (). The discontinuation rate inputs for the deucravacitinib versus 1L biologics comparison were derived from a real-world claims study in the United States comparing treatment patterns and healthcare costs for apremilast versus biologics (Citation33). The discontinuation rate for 1L biologics was 25.3%; the rate for deucravacitinib, 16.5%, was assumed to be the same as that reported for apremilast in the real-world study.
Table 3. Input values for market share and efficacy for 1L biologics.
Scenario analyses
Several scenario analyses were conducted for the primary objective. For the primary short-term endpoint, the scenario analysis used a score of 0 or 1 on the static Physician Global Assessment (sPGA) for efficacy rather than PASI 75, with sPGA response sourced from the pooled efficacy analyses of the POETYK PSO-1 and POETYK PSO-2 clinical trials. For the secondary long-term endpoint, scenario analyses included removing discontinuation from the maintenance phase and changing the subsequent treatment to only one biologic treatment (adalimumab, guselkumab, ixekizumab, risankizumab, or secukinumab). The same scenario analyses for the long-term endpoint were conducted for the secondary objective.
Results
Primary objective: deucravacitinib compared with apremilast
Short-term CPR
The primary endpoint at weeks 16 and 24 demonstrated that total costs per patient were higher for deucravacitinib compared with apremilast. Only drug acquisition contributed to the total cost in the primary analyses since deucravacitinib and apremilast are both oral treatments and therefore incur no administration costs. In the pooled efficacy analysis of the POETYK-PSO trials, deucravacitinib had a higher probability of achieving PASI 75 response than apremilast at both 16 and 24 weeks (). At the initial treatment period of 16 weeks, the CPR for deucravacitinib was $41,856 compared with $45,651 for apremilast, which represented a statistically significant difference in CPR of –$3796 (95% CI: –$6140 to –$1659). A similar trend was observed at 24 weeks (), with deucravacitinib demonstrating a significantly greater difference in CPR over apremilast (–$12,784 [95% CI: –$16,674 to –$9369]). The NNT to achieve an additional PASI 75 responder for deucravacitinib relative to apremilast was smaller in magnitude at week 24 (NNT: 4.1) than at week 16 (NNT: 5.8).
Figure 2. Cost per PASI 75 response outcomes at 16 and 24 weeks, and cost per cumulative benefit at 52 weeks with biologic therapies as subsequent treatment. CI: confidence interval; CPR: cost per response; PASI: Psoriasis Area and Severity Index.
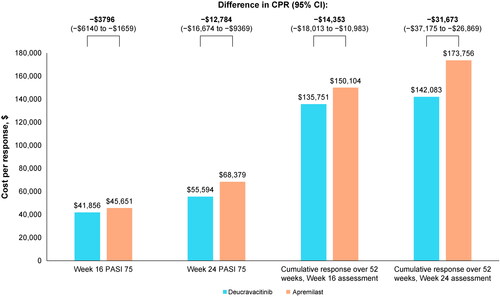
Table 4. Cost per response and cost per cumulative benefit outcomes for PASI 75: deucravacitinib versus apremilast.Table Footnotea
Long-term cumulative benefit
Over 52 weeks, deucravacitinib demonstrated significantly greater differences in cost per cumulative benefit for PASI 75 response compared with apremilast (). When the interim response assessment was set at 16 weeks, the AUC was 66% for deucravacitinib versus 57% for apremilast. Although the average pharmacy cost per patient was slightly higher for deucravacitinib compared with apremilast ($89,711 vs. $86,266, respectively), patients who initiated deucravacitinib had a lower cost per cumulative benefit compared with apremilast ($135,751 vs. $150,104, respectively). This led to a significant difference in the cost per cumulative benefit of –$14,353 (95% CI: –$18,013 to –$10,983) in favor of deucravacitinib for PASI 75 response ( and ).
Similar trends for deucravacitinib versus apremilast were observed when the interim response assessment was set at 24 weeks (). However, treatment costs per patient for both deucravacitinib ($86,056) and apremilast ($82,493) were slightly lower at 24 weeks compared with 16 weeks, a resulting outcome for delaying the switch to the costly biologic treatments. The AUC was 61% for deucravacitinib versus 47% for apremilast, and the cost per cumulative benefit was $142,083 and $173,756, respectively, leading to a significant difference in cost per cumulative benefit of –$31,673 (95% CI: –$37,175 to –$26,869) in favor of deucravacitinib (). Consistent with the primary endpoint, the NNT was smaller for deucravacitinib relative to apremilast when considering interim response at week 24 (NNT: 7.6) over week 16 (NNT: 11.6) ().
Secondary objective: deucravacitinib compared with 1L biologics
Over 16 weeks, the total costs per patient were higher with 1L biologics compared with deucravacitinib, although biologics had a higher probability of achieving PASI 75 response (). At the initial treatment period of 16 weeks, the CPR for deucravacitinib was $42,685 compared with $60,184 for 1L biologics, which represented a significant difference in CPR of −$17,499 (95% CI: −$19,386 to −$15,602). A similar trend was observed at 24 weeks, with deucravacitinib demonstrating a significantly greater difference in CPR over 1L biologics (−$19,589 [95% CI: −$21,575 to −$17,698]). The NNT to achieve an additional PASI 75 responder for deucravacitinib relative to 1L biologics was smaller in magnitude at week 16 (NNT: 3.6) than at week 24 (NNT: 4.8) ().
Table 5. Cost per response and cost per cumulative benefit outcomes for PASI 75: deucravacitinib versus 1L biologics.Table Footnotea
Scenario analyses
Primary objective: deucravacitinib compared with apremilast
The magnitude of the difference in CPR was larger when a score of 0 or 1 in the sPGA was used as the response metric rather than PASI 75 (base case) (). In the pooled efficacy analysis of the POETYK PSO trials, patients receiving deucravacitinib had a higher probability than those receiving apremilast of achieving sPGA 0/1 at both 16 weeks (51.3% vs. 33.2%) and 24 weeks (52.9% vs. 30.1%). In this scenario analysis, the difference in CPR for sPGA 0/1 response was –$7112 at 16 weeks and –$20,682 at 24 weeks in favor of deucravacitinib versus apremilast ().
Figure 3. Scenario analysis results for the difference in cost per response and cost per cumulative benefit: deucravacitinib vs. apremilast. (A) Interim response assessment at 16 weeks and (B) interim response assessment at 24 weeks. 2L: second line; sPGA: Static Physicians Global Assessment.
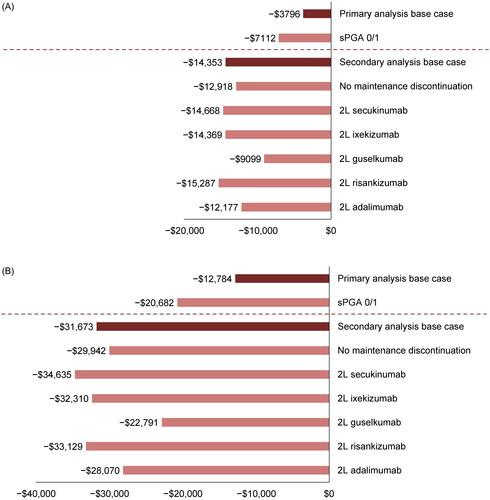
Several scenario analyses were conducted for the 52-week endpoint, as presented in . Results largely demonstrated consistent trends with that of the base case, regardless of the interim response point used (i.e., 16 or 24 weeks). When discontinuation of 1L agents throughout the maintenance phase was not considered, deucravacitinib still demonstrated a significantly lower cost per cumulative benefit compared with apremilast (). When subsequent treatment for nonresponders was based on one specific biologic therapy, patients who initiated deucravacitinib continued to demonstrate a significantly lower cost per cumulative benefit than those who initiated apremilast ().
Secondary objective: deucravacitinib compared with 1L biologics
Scenario analysis (Supplementary Table 1) results for deucravacitinib versus 1L biologics are presented in the Supplementary Materials. The results were consistent with the base case results: regardless of the interim response point used (i.e., 16 or 24 weeks), deucravacitinib demonstrated a significantly lower cost per cumulative benefit compared with 1L biologics when discontinuation was not considered, and when subsequent treatment for nonresponders was based on one specific biologic therapy.
Discussion
In this study, a CPR analysis was conducted to evaluate the economic value of deucravacitinib for treatment of moderate to severe plaque psoriasis in the United States. We examined the CPR in two different time horizons: in the short-term (16 and 24 weeks) over the initial treatment period, then over the long-term (52 weeks) where the cumulative benefit for PASI 75 was used and subsequent treatment for nonresponders was considered. In comparison to apremilast, deucravacitinib had a lower cost per PASI 75 response over both 16 and 24 weeks of the initial treatment period. At 52 weeks, similar trends were observed to the short-term when assessing the cost per cumulative benefit; deucravacitinib demonstrated a lower cost per cumulative benefit than apremilast when considering PASI 75 responses. The results therefore demonstrate a favorable CPR for deucravacitinib compared with apremilast, suggesting that deucravacitinib is a more cost-effective therapy choice.
Noticeably, the CPR was higher for both deucravacitinib and apremilast when interim response was assessed at 24 versus 16 weeks, although pharmacy costs per patient were lower at 24 weeks. This is likely reflective of the delay in switching to more efficacious, yet more costly, targeted therapies, leading to nonresponders spending less time accumulating cumulative benefit from subsequent treatment, as shown by the differences in AUC. However, the lower treatment costs shown with an initial treatment period of 24 weeks compared with 16 weeks demonstrate the potential economic value of using oral treatments prior to the initiation of biologics.
In this study, deucravacitinib was associated with a lower CPR than 1L biologic therapies at 16 weeks and 24 weeks when using PASI 75 as the response measure. The results indicate that the cost is lower with deucravacitinib to achieve clinical response. Recent studies have assessed the cost per cumulative benefits of biologic therapies. An NMA and cost per cumulative benefit analysis by Blauvelt et al. reported that the maximum AUC for PASI 75 at 16 weeks varied from 40.9% to 66.2% across biologics, and the cost per cumulative benefit ranged from $62,778 to $203,597 (Citation34). However, comparisons between the Blauvelt analysis and our study are challenging because we did not analyze individual 1L biologics. First-line biologic therapies have proven to be highly effective in rapidly achieving treatment goals via symptom relief, clearer skin, and improved health-related quality of life, but they are significantly more costly compared with older therapies (Citation2). The higher economic burden of biologic versus nonbiologic therapy is likely to generate socioeconomic barriers in the treatment and long-term management of psoriasis (Citation2). It is, therefore, important to assess the economic value of new nonbiologic therapies, such as deucravacitinib, as both an effective and affordable treatment option for patients. While recent studies have examined the CPR or cost per cumulative benefit for other targeted therapies in this population (Citation17,Citation34–38), to our knowledge, no prior analysis has assessed and demonstrated the potential economic value of deucravacitinib as a treatment option for moderate to severe plaque psoriasis in the United States.
This study has several strengths. First, it quantified the CPR of deucravacitinib and apremilast for moderate to severe psoriasis and adds to the current understanding of the comparative cost-effectiveness of deucravacitinib and apremilast as oral systemic treatments. The 52-week analyses allowed us to explore the economic benefit of deucravacitinib as a 1L treatment option before switching to a subsequent therapy and, therefore, may reflect real-world treatment switching patterns. Scenario analyses also demonstrated the impact of switching to different subsequent treatments as some biologic therapies are more expensive than their competitors. Second, the response rates of deucravacitinib versus apremilast were based on pooled efficacy analyses from the head-to-head POETYK PSO-1 and POETYK PSO-2 phase 3 trials to be representative of both trials rather than selecting one trial over the other. In addition, the study considered both the short-term CPR as well as the long-term economic value using the cost per cumulative benefit over 52 weeks, which also assumed a treatment switch reflective of real-world treatment patterns. The long-term 52-week model also included discontinuation of 1L agents throughout the maintenance phase and not just at the interim switch point by applying trial-level annual discontinuation rates across each cycle.
This study is subject to the following limitations. First, only drug-related costs were included in the model. Neither deucravacitinib nor apremilast incur administration costs, so drug acquisition costs comprised the total costs in the short-term primary analyses. Although the actual prices to payers may vary from the wholesale acquisition costs used in the model calculations, other options for estimating costs in the United States are limited and wholesale acquisition costs have been used in other studies (Citation34,Citation39). Further cost components that may be incurred by real-world patients, such as the cost of adverse events and health care resource use (e.g., additional laboratory tests), could be considered in the analysis. However, in our opinion, it is unlikely the addition of these cost components to our model would have a significant impact on the outcomes of the analysis. Second, patients were assumed to be 100% adherent to the treatment, yet this may not be reflective of the real world. A further limitation for the 52-week endpoint was that the trapezoidal rule was used, per Blauvelt et al. (Citation34); therefore, a linear increase was assumed in the probability of achieving the clinical endpoint of PASI 75 from week 0 to week 16 in the initial treatment period, as data were unavailable at weeks 4, 8, and 12. Finally, the model results are reliant on the accuracy and completeness of data in the databases used to source real-world model inputs, such as wholesale acquisition costs and market share at the time the model was constructed; the data in these real-world sources can also change over time, so the results would vary if different inputs were used.
To conclude, the CPR and cost per cumulative benefit were consistently lower for patients with moderate to severe plaque psoriasis who initiated deucravacitinib versus apremilast, regardless of the initial treatment period length (16 or 24 weeks) or the response measure used (PASI 75 or sPGA 0/1). The favorable clinical efficacy of deucravacitinib outweighed its higher pharmacy costs per patient compared with apremilast. These results may provide valuable information for payers, and potentially for physicians and patients, to help optimize treatment decisions when considering oral systemic treatments in the United States.
Supplementary Material
Download PDF (204.5 KB)Acknowledgements
Medical writing and editorial assistance were provided by Catherine Mirvis of OPEN Health Evidence & Access, and funded by Bristol Myers Squibb.
Disclosure statement
SHP, KW, and VP are employees of Bristol Myers Squibb and may own stock/options in the company. SH was an employee of Bristol Myers Squibb at the time of the study. ML and JS are employees of OPEN Health Evidence & Access, which received financial support from Bristol Myers Squibb for consulting.
Data availability statement
The Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
Additional information
Funding
References
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157(8):1–10. doi: 10.1001/jamadermatol.2021.2007.
- Michalek IM, Loring B, John SM. Global report on psoriasis. Geneva, Switzerland: World Health Organization; 2016.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–1072. doi: 10.1016/j.jaad.2018.11.057.
- Helmick CG, Lee-Han H, Hirsch SC, et al. Prevalence of psoriasis among adults in the U.S.: 2003–2006 and 2009–2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47(1):37–45. doi: 10.1016/j.amepre.2014.02.012.
- Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303(1):1–10. doi: 10.1007/s00403-010-1080-1.
- Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis. 2005;64(Suppl. 2):ii65–ii68. doi: 10.1136/ard.2004.031237.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151(6):651–658. doi: 10.1001/jamadermatol.2014.3593.
- Gelfand JM, Feldman SR, Stern RS, et al. Determinants of quality of life in patients with psoriasis: a study from the US population. J Am Acad Dermatol. 2004;51(5):704–708. doi: 10.1016/j.jaad.2004.04.014.
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82(6):1445–1486. doi: 10.1016/j.jaad.2020.02.044.
- Armstrong AW, Puig L, Joshi A, et al. Comparison of biologics and oral treatments for plaque psoriasis: a meta-analysis. JAMA Dermatol. 2020;156(3):258–269. doi: 10.1001/jamadermatol.2019.4029.
- Institute for Clinical Economic Review. Targeted immunomodulators for the treatment of moderate-to-severe plaque psoriasis: effectiveness and value. Boston (MA): Institute for Clinical Economic Review; 2018.
- Sotyktu [package insert]; 2022. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214958s000lbl.pdf
- Catlett IM, Hu Y, Gao L, et al. Molecular and clinical effects of selective tyrosine kinase 2 inhibition with deucravacitinib in psoriasis. J Allergy Clin Immunol. 2022;149(6):2010–2020.e8. doi: 10.1016/j.jaci.2021.11.001.
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88(1):29–39.
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program for evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88(1):40–51.
- Armstrong AW, Park SH, Patel V, et al. Matching-adjusted indirect comparison of the long-term efficacy of deucravacitinib versus adalimumab for moderate to severe plaque psoriasis. Dermatol Ther. 2023;13(11):2589–2603. doi: 10.1007/s13555-023-00977-1.
- Armstrong AW, Feldman SR, Korman NJ, et al. Assessing the overall benefit of a medication: cumulative benefit of secukinumab over time in patients with moderate-to-severe plaque psoriasis. J Dermatol Treat. 2017;28(3):200–205.
- Warren RB, Gooderham M, Burge R, et al. Comparison of cumulative clinical benefits of biologics for the treatment of psoriasis over 16 weeks: results from a network meta-analysis. J Am Acad Dermatol. 2020;82(5):1138–1149. doi: 10.1016/j.jaad.2019.12.038.
- Symphony Health Anonymized Patient Level Data (APLD). Based on a 3 month rolling average for the total population. Dublin (Ireland): Icon; 2022.
- Armstrong AW, Warren RB, Zhong Y, et al. Short-, mid-, and long-term efficacy of deucravacitinib versus biologics and nonbiologics for plaque psoriasis: a network meta-analysis. Dermatol Ther. 2023;13(11):2839–2857. doi: 10.1007/s13555-023-01034-7.
- U.S. Bureau of Labor Statistics. CPI for all urban consumers. Medical care in U.S. city average, all urban consumers, seasonally adjusted; 2021 [cited 2021 Apr]. Available from: https://data.bls.gov/
- IBM Watson. Merative™ Micromedex®. RED BOOK®; 2023 [cited 2023 Feb]. Available from: https://www.micromedexsolutions.com/micromedex2/librarian/
- Skyrizi [package insert]; 2019 [cited 2022 Nov]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761105s000lbl.pdf
- Humira [package insert]; 2018 [cited 2022 Nov]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125057s406lbl.pdf
- Siliq [package insert]; 2017 [cited 2022 Nov]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf
- Tremfya [package insert]; 2017 [cited 2022 Nov]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761061s000lbl.pdf
- Enbrel [package insert]; 2021 [cited 2022 Nov]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/103795s5582lbl.pdf
- Taltz [package insert]; 2016 [cited 2022 Nov]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125521s000lbl.pdf
- Ilumya [package insert]; 2018 [cited 2022 Nov]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761067s000lbl.pdf
- Stelara [package insert]; 2016 [cited 2022 Nov]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761044lbl.pdf
- Carrico J, Zhao Y, Jia X, et al. The budget impact of introducing tildrakizumab to a United States health plan for managing moderate-to-severe plaque psoriasis. Pharmacoecon Open. 2020;4(4):669–677. doi: 10.1007/s41669-020-00208-9.
- Practice Management Information Corporation (PMIC). Medical fees 2021 eBook. Los Angeles (CA): Practice Management Information Corporation; 2020. Available from: https://www.pmiconline.com/
- Feldman SR, Zhang J, Martinez DJ, et al. Real-world biologic and apremilast treatment patterns and healthcare costs in moderate-to-severe plaque psoriasis. Dermatol Online J. 2021;27(1):2.
- Blauvelt A, Burge R, Malatestinic W, et al. Cost per cumulative clinical benefit of biologic therapies for patients with plaque psoriasis: a systematic review. J Manag Care Spec Pharm. 2021;27(1):84–94. doi: 10.18553/jmcp.2021.27.1.084.
- Teeple A, Fitzgerald T. Cost per responder for guselkumab versus secukinumab in the United States based on a head-to-head trial of moderate to severe plaque psoriasis. J Dermatol Treat. 2022;33(1):518–524.
- Teeple A, Muser E. Cost per response for guselkumab versus adalimumab in the United States using data from a head-to-head trial in moderate-to-severe plaque psoriasis. J Med Econ. 2019;22(12):1268–1273. doi: 10.1080/13696998.2019.1632204.
- Al Sawah S, Foster SA, Burge R, et al. Cost per additional responder for ixekizumab and other FDA-approved biologics in moderate-to-severe plaque psoriasis. J Med Econ. 2017;20(12):1224–1230. doi: 10.1080/13696998.2017.1362413.
- Feldman SR, Foster SA, Zhu B, et al. Cost per additional responder associated with ixekizumab and etanercept in the treatment of moderate-to-severe psoriasis. J Drugs Dermatol. 2017;16(12):1246–1252.
- Mattingly TJ, Levy JF, Slejko JF, et al. Estimating drug costs: how do manufacturer net prices compare with other common US price references? Pharmacoeconomics. 2018;36(9):1093–1099. doi: 10.1007/s40273-018-0667-9.