Abstract
Purpose: This noninterventional, cross-sectional survey estimated the prevalence and consequences of residual disease in apremilast-treated US adults with moderate to severe psoriasis. Materials and Methods: Residual disease was defined as experiencing moderate, severe, or very severe psoriasis over the past week or having ≥3% body surface area affected, despite treatment. Factors associated with residual disease and its effects on flare-ups, humanistic burden, and health care resource utilization (HCRU) were evaluated. Results: Of the 344 apremilast users (mean age, 44.9 years; female, 65.4%), 174 (50.6%) had residual disease. It was more prevalent in Black versus White participants (OR, 4.5; 95% CI, 1.6–12.2), those receiving apremilast for ≥1 versus <1 year (OR, 16.5; 95% CI, 7.9–34.4), those reporting ≥2 versus 0 to 1 flare-ups during the past 3 months (OR, 10.0; 95% CI, 5.0–20.1), and those with ≥4 versus 1 to 3 body regions affected at time of survey (OR, 8.6; 95% CI, 3.8–19.8). Participants with versus without residual disease self-reported more psoriasis flare-ups over the past 3 months (mean, 4.7 vs 0.9; p < .001) and more anxiety (89.7% vs 50.0%; p < .001) and depression (69.0% vs 23.6%; p < .001) over the past 30 days. Conclusion: Generally, participants with versus without residual disease also had significantly more comorbidities and greater HCRU.
Introduction
Psoriasis, a chronic immune-mediated dermatologic condition characterized by scaly epidermal plaques that can cause severe itching, affects about 3.2% of the US population (Citation1). This multisystem inflammatory disease can have negative physical, emotional, and social impacts on patients, and may contribute to comorbidities such as psoriatic arthritis, cardiovascular diseases, depression, and metabolic syndrome (Citation1–5).
The treatment of patients with psoriasis is based on disease severity and may include topical therapies, phototherapy, and systemic therapies, including biologic therapies (Citation1). Apremilast, an orally administered small molecule inhibitor that works by blocking phosphodiesterase-4 (Citation6), and targeted biologic therapies (e.g. interleukin [IL]-17, IL-12/23, and IL-23 inhibitors; tumor necrosis factor alpha inhibitors), which are typically administered by injection, are recommended to treat moderate to severe psoriasis (Citation1). However, patient preferences are also an important consideration in psoriasis treatment decision making (Citation1) and process-related treatment attributes, such as route of administration, are important to many people with psoriasis (Citation7,Citation8). In previous patient preference studies in the United States, anxiety about injections and preparation of injections were the most common reasons for biologic therapy being burdensome (Citation9), and people were willing to trade off some improvements in efficacy to avoid injections in favor of oral or topical treatment (Citation10).
Residual disease while on treatment is problematic for some people with psoriasis (Citation11) and/or psoriatic arthritis (Citation12). Evidence from studies in the United States suggests that people with psoriasis are dissatisfied with available treatments (Citation13,Citation14). However, there appears to be hesitancy to initiate and continue systemic therapies because of safety and tolerability concerns or loss of or lack of efficacy (Citation9,Citation13,Citation15). As the psoriasis treatment landscape continues to evolve, elucidation of the extent and nature of residual burden owing to inadequate skin clearance and systemic effects of psoriasis may help to highlight the unmet need in this population, despite the availability of existing systemic treatments.
To better understand the patient experience, this survey study aims to assess and quantify the prevalence of and factors associated with residual disease, as well as the impact of residual disease on the humanistic burden of adults with moderate to severe psoriasis treated with apremilast. Secondarily, the impact of residual disease in apremilast users with moderate to severe psoriasis on comorbidities and on all-cause and psoriasis-related health care resource utilization (HCRU) will be determined.
Methods
Study design
Patient-reported data obtained as part of a noninterventional, cross-sectional survey of adults in the United States with moderate to severe psoriasis were analyzed. Relevant survey concepts were identified in a targeted literature review and evaluated by clinical experts on the research team. The survey was programmed and hosted on an encrypted, Health Insurance Portability and Accountability Act (HIPAA)–compliant web-based data collection platform. Survey data were de-identified prior to analysis. The RTI Institutional Review Board (RTI Health Solutions; Research Triangle Park, NC) reviewed the study design, survey, and relevant recruitment materials and deemed them exempt. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki. All participants provided informed consent to participate in the study.
Participants
A convenience sample of individuals with moderate or severe psoriasis was recruited from an established panel via email by Global Perspectives, a global patient engagement consultancy. Potential survey participants were provided a link to the survey and completed screening questions to determine eligibility. Participants were eligible for inclusion if they self-reported a physician’s diagnosis of moderate or severe psoriasis, were at least 18 years of age, resided in the United States, and were able to read and understand English. Respondents deemed eligible for participation provided electronic consent and were subsequently directed to the online survey.
As part of the survey, participants were assigned to predefined groups based on their self-reported primary psoriasis treatment at the time of survey participation. For this analysis, apremilast was the exposure of interest; therefore, this article focuses on participants who self-reported apremilast as their primary psoriasis treatment when the survey was conducted.
Variables
The survey included questions on sociodemographics, clinical characteristics, and treatment preferences and experiences. Sociodemographic data collected included participant-specific demographics as well as information on type of insurance, income level, and type of treating physician. Clinical characteristics included self-reported psoriasis severity over the past week, total body surface area (BSA) affected by psoriasis, psoriasis flare-up frequency over the past 3 months, and body regions affected by psoriasis. Psoriasis severity over the past week was self-assessed using a 6-point scale (none [no psoriasis activity experienced], very mild, mild, moderate, severe, and very severe). Total BSA affected by psoriasis was estimated by participants using the 1% hand test (Citation16). Participants were asked to count the number of handprints that would cover all bodily areas affected by psoriasis. One handprint was assumed to be equivalent to approximately 1% of an individual’s BSA. Psoriasis severity based on the 1% hand test was classified as mild (<3% BSA), moderate (3%–10% BSA), or severe (>10% BSA). For the base case analysis, participants were considered to have residual disease if they reported experiencing moderate, severe, or very severe psoriasis over the past week based on the 6-point severity scale, or had 3% or greater affected BSA using the 1% hand test, as measured by the participant. Participants also self-reported the frequency of psoriasis flare-ups over the preceding 3 months and the bodily areas (scalp, face, neck, ears, hands, fingers, fingernails, arms, elbows, chest, abdomen, back, shoulders, genital area, buttocks, thighs, knees, lower legs, ankles, toes, or toenails) affected by psoriasis at the time of the survey. Flare-ups were defined in the survey as “a worsening of your psoriasis symptoms, such as plaques returning or worsening, or itch worsening.”
Humanistic burden was assessed using the Dermatology Life Quality Index (DLQI) (Citation17) and the Work Productivity and Activity Impairment Questionnaire–Psoriasis (WPAI-PSO) (Citation18). Anxiety and depression related to psoriasis were assessed over the past 30 days or since the start of psoriasis treatment. Severity of anxiety was determined using a 5-point scale (very anxious, anxious, somewhat anxious, a little anxious, or not at all anxious), and the frequency of anxiety was determined using a 4-point scale (nearly every day, more than half the days, several days, or not at all) over the past 30 days. Reduction in anxiety was determined from the start of a participant’s current psoriasis treatment, using a 5-point scale (no, definitely not; probably not; neither yes nor no; probably yes; or yes, definitely). Severity of depression was determined using a 5-point scale (very depressed, depressed, somewhat depressed, a little depressed, or not at all depressed) and frequency of depression was determined using a 4-point scale (nearly every day, more than half the days, several days, or not at all) over the past 30 days. Reduction in depression was determined from the start of a participant’s current psoriasis treatment on a 5-point scale (no, definitely not; probably not; neither yes nor no; probably yes; or yes, definitely). Finally, participants were presented with a hypothetical new oral psoriasis treatment (Figure S1) and were asked if they felt that this new oral treatment would give them less anxiety than a psoriasis treatment given as an injection or infusion.
Health care resource utilization was assessed using participants’ self-reported frequency of different types of health care–related visits, both psoriasis-related and all-cause visits, over the preceding 3 months. Participants were asked to include the following types of visits: physician’s office visits, office-based tests/procedures, office-based phototherapy sessions, emergency room/urgent care clinic visits, and hospital admissions.
Sensitivity analysis
To understand the effect of the definition used for residual disease on study results, a sensitivity analysis was performed in which residual disease was defined as a participant-reported response of 3% or greater on the BSA scale.
Statistical methods
Descriptive univariate analyses were conducted to estimate the prevalence of residual disease among participants with moderate to severe psoriasis being treated with apremilast at the time of the survey. Full and stepwise multivariable logistic regression was conducted to identify factors associated with residual disease among participants with moderate to severe psoriasis who received apremilast treatment, controlling for relevant participant sociodemographics (e.g. age, sex, race, ethnicity) and clinical characteristics (number of comorbidities, disease duration, treatment duration, psoriatic arthritis at baseline, number of flare-ups, and number of body regions affected). Bivariate comparisons were conducted to compare the prevalence of flare-ups, the extent of humanistic burden, the incidence of comorbidities, and psoriasis-related and all-cause HCRU in participants treated with apremilast who were classified as having or not having residual disease.
For univariate analyses, frequency and percentage distributions are reported for categorical variables, and mean and standard deviation (SD) are reported for continuous and count variables. For bivariate analyses, statistical comparisons were conducted using Pearson chi-square tests or Fisher’s exact test for categorical variables. For continuous variables, t tests were used for normally distributed variables, and Mann–Whitney tests were used for non-normally distributed variables. For normally distributed variables, the pooled test P-value was used unless the pooled P-value of variance equality was <.05; in that case, the Satterthwaite P-value was used. When multiple group comparisons were needed, an analysis of variance was conducted.
All analyses were conducted using SAS version 9.4 (SAS Institute; Cary, NC, USA).
Results
In total, 344 of 882 eligible participants who accessed the survey were treated with apremilast at the time of the survey (). The mean (SD) age of participants who were receiving apremilast at the time of the survey was 44.9 (12.7) years, with most of these participants being female (65.4%) and White (72.4%; ; Supplementary Table S1). The mean (SD) disease duration was 15.0 (SD, 11.0) years, and participants self-reported taking apremilast for a mean (SD) of 1.6 (3.7) years. Participants receiving apremilast at the time of the survey self-reported a mean (SD) of 2.8 (5.8) symptom flare-ups over the past 3 months, including worsening of skin plaques and itching. The most common body regions affected were the scalp (45.1%), elbows (45.1%), and hands (42.2%; Supplementary Table S1).
Figure 1. Study sample attrition flow chart.aRespondents could have failed the screen for multiple reasons.
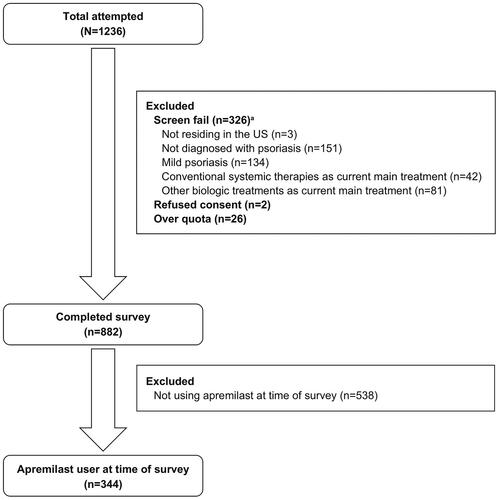
Table 1. Participants’ sociodemographics and clinical characteristics.
Prevalence of and factors associated with residual disease
Among the 344 participants receiving apremilast at the time of the survey, 174 (50.6%) were classified as having residual disease. Results from the multivariable logistic regression analysis identified a significantly higher prevalence of residual disease in Black versus White participants (odds ratio [OR], 4.5; 95% CI, 1.6–12.2); participants who were receiving apremilast for at least 1 year versus less than 1 year (OR, 16.5; 95% CI, 7.9–34.4); those who self-reported 2 or more flare-ups versus 0 to 1 flare-ups in the past 3 months (OR, 10.0; 95% CI, 5.0–20.1); and those who had at least 4 body regions affected versus 1 to 3 body regions affected at the time of the survey (OR, 8.6; 95% CI, 3.8–19.8; ).
Table 2. Factors associated with residual disease among participants treated with apremilast.Table Footnotea
Humanistic burden of residual disease
The mean (SD) number of self-reported psoriasis flare-ups in the 3 months prior to survey participation was significantly higher in participants classified as having versus not having residual disease (4.7 [7.6] vs 0.9 [1.1]; p < .001). A significantly greater proportion of participants classified as having versus not having residual disease self-reported having anxiety (89.7% vs 50.0%; p < .001; ). Among participants reporting anxiety, a significantly greater proportion classified as having versus not having residual disease reported anxiety lasting several days or more (94.9% vs 78.8%; p = .001). A significantly greater proportion of participants classified as having versus not having residual disease self-reported depression (69.0% vs 23.6%; p < .001; ). Among participants who self-reported depression, a significantly greater proportion of participants classified as having compared with not having residual disease reported depression lasting several days or more (95.0% vs 75.0%; p < .001). When participants were provided information about a new hypothetical oral psoriasis treatment, 71.8% of apremilast users with residual disease said this hypothetical oral treatment would cause less anxiety than a treatment given by injection or infusion.
Table 3. Humanistic burden in participants treated with apremilastTable Footnotea by residual disease classification.
In general, significantly lower quality of life (QoL) as measured by the DLQI and work productivity as measured by the WPAI-PsO were self-reported by participants classified as having versus not having residual disease ().
Comorbidities and HCRU
The prevalence of psoriatic arthritis (25.3% vs 7.1%; p < .001) and high blood pressure (26.4% vs 17.6%; p = .049) were significantly higher in participants classified as having versus not having residual disease (). In general, both psoriasis-related and all-cause HCRU were significantly higher in participants classified as having versus not having residual disease ().
Table 4. Comorbidities in participants treated with apremilastTable Footnotea by residual disease classification.
Table 5. HCRU in participants treated with apremilastTable Footnotea by residual disease classification.
Sensitivity analysis
Results from the sensitivity analysis support the base case analysis, with no significant differences identified between the base case and sensitivity analyses.
Discussion
Results from this study highlight the high prevalence of and factors associated with residual disease in adults with moderate to severe psoriasis who were treated with apremilast. Approximately, half of the participants (50.6%) were classified as having residual disease. Participants who were receiving apremilast and self-reported residual disease reported significantly more disease flare-ups and had significantly higher humanistic burden, comorbidities, and HCRU than participants without residual disease. Although previous studies assessed the effects of residual disease in patients with psoriasis and/or psoriatic arthritis (Citation11,Citation12) we are not aware of any studies that have analyzed patient factors that may affect residual disease, specifically in adults with psoriasis being treated with apremilast in the United States.
The sociodemographics and clinical characteristics of participants completing the survey were generally representative of the United States adult population with moderate to severe psoriasis. Approximately 65% of participants were female and the mean age was approximately 45 years, similar to that of the population in another study in this disease space that surveyed patients with psoriasis in the United States and Germany (Citation19).
These survey study results highlight the negative impact that residual disease has on QoL. Participants classified as having residual disease had a significantly higher DLQI global score (11.8), indicating a greater negative impact on their overall QoL, compared with participants not classified as having residual disease (4.4). This finding is in line with results from another psoriasis study that found that even minimal amounts of residual disease can negatively impact QoL, as measured by the DLQI (Citation11). The impairment seen in work productivity in our study was also reported in other survey studies (Citation20,Citation21). These studies, along with ours, show that people with psoriasis tend to miss work and are impaired while working because of their psoriasis.
Survey participants were asked to self-report their psoriasis-related and all-cause HCRU by indicating the number of days over the past 3 months they visited a physician’s office, had an office-based test/procedures, underwent a phototherapy session, visited the emergency room/urgent care clinic, or were admitted to a hospital. Our results are similar to those from a study in which people with psoriasis undergoing phototherapy tended to have a greater number of visits than those on other systemic treatments (Citation15).
This study provides evidence on the prevalence and impact of residual disease on QoL and HCRU, as well as factors which influence residual disease, from a large real-world sample of apremilast users with moderate to severe psoriasis in the United States. Large real-world studies such as this provide important evidence on long-term treatment outcomes in real-world practice.
Study participants were recruited via an online panel and were included if they fulfilled study eligibility criteria. Participants’ self-reported diagnosis, treatment history, and disease severity could not be verified, as there was no mechanism in place to obtain clinician-confirmed information. In addition, online panel participants may differ in their characteristics and experiences from people who do not participate in such panels; thus, participants in this survey may not be entirely representative of the population of patients with moderate to severe psoriasis in the United States who are treated with apremilast, which may affect the generalizability of these results. In addition, the study population did not include patients who started apremilast but discontinued it for any reason. Lastly, the definition of residual disease varies across studies in the literature, making comparisons between studies challenging. For example, Maliyar et al. 2020 used BSA to define residual disease in a sample of patients with moderate to severe psoriasis, with mild residual disease defined as a BSA of 2% to 3% and moderate residual psoriasis defined as a BSA greater than 3% (Citation22). For our survey study, a sensitivity analysis was conducted in which only participants reporting a BSA of 3% or greater over the past week were defined as having residual disease. Results from the sensitivity analysis supported the base case analysis.
This study focused on the patient experience and used patients’ self-reported disease severity in the definition of residual disease. Previous studies have shown a potential disconnect between how patients and physicians perceive disease severity (Citation23,Citation24). While dermatologists’ assessment of residual disease is also important, it was beyond the scope of the present study, and future research is needed to explore this area. Further research is also needed to understand why some patients continue treatment despite experiencing residual disease.
In conclusion, the results of this analysis show that residual disease is highly prevalent in individuals using apremilast as their main psoriasis treatment. Apremilast users with residual disease self-reported more flare-ups; worse QoL, anxiety, depression, and work productivity; and greater HCRU than those without residual disease. Our survey results highlight that additional, effective treatment options are needed for people with moderate to severe psoriasis and that the presence of residual disease should be a focus for psoriasis care, to improve outcomes and reduce HCRU.
Author contributions
Tina Bhutani, Sayeli Jayade, Sanika Rege, Vardhaman Patel, Samaneh Kalirai, Daniel Wolin, and Lauren Seigel contributed to the study design. Sayeli Jayade, Sanika Rege, Daniel Wolin, and Kimberly Boyle participated in the collection and assembly of data. Sayeli Jayade, Sanika Rege, and Hannah Penton performed data analysis. All authors participated in data interpretation and collaborated in the preparation of the manuscript, supported by a professional medical writer funded by Bristol Myers Squibb. All authors critically reviewed and provided revisions to the manuscript. All authors had access to the data and assume responsibility for the completeness and accuracy of the data and data analyses. All authors granted approval of the final manuscript for submission.
Patient consent
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki. All participants provided informed consent to participate in the study.
Supplemental Material
Download MS Word (55.7 KB)Acknowledgments
Medical writing assistance was provided by Catherine Mirvis, BA, and Beth A. Lesher, PharmD, BCPS, of OPEN Health Evidence & Access. The authors thank Namita Joshi for her contributions to the study conception, execution, and analysis of study data, and David Davidson, MS, PA-C, formerly with Bristol Myers Squibb, for his contributions to study conception and data interpretation.
Disclosure statement
Tina Bhutani has served as an investigator for AbbVie, Castle Biosciences, CorEvitas, Dermavant, Galderma, Mindera Health, and Pfizer; has received research support from Novartis and Regeneron; and has served as an advisor for AbbVie, Arcutis, Boehringer Ingelheim, Bristol Myers Squibb, Janssen, Leo Pharma, Lilly, Novartis, Pfizer, Sun Pharma, and UCB. Sayeli Jayade and Hannah Penton are employees of OPEN Health Evidence & Access, which received consulting fees from Bristol Myers Squibb in connection with this study. Sanika Rege was an employee of OPEN Health Evidence & Access at the time of this study. Vardhaman Patel, Samaneh Kalirai, and Lauren Seigel are employees of Bristol Myers Squibb and may own stock/options in the company. Daniel Wolin and Kimberly Boyle are employees of RTI Health Solutions, which received consulting fees from Bristol Myers Squibb in connection with this study.
Data availability statement
The Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
Additional information
Funding
References
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–1072. doi: 10.1016/j.jaad.2018.11.057.
- Armstrong AW, Schupp C, Wu J, et al. Quality of life and work productivity impairment among psoriasis patients: findings from the National Psoriasis Foundation survey data 2003-2011. PLoS One. 2012;7(12):e52935. doi: 10.1371/journal.pone.0052935.
- Bu J, Ding R, Zhou L, et al. Epidemiology of psoriasis and comorbid diseases: a narrative review. Front Immunol. 2022;13:880201. doi: 10.3389/fimmu.2022.880201.
- Garshick MS, Ward NL, Krueger JG, et al. Cardiovascular risk in patients with psoriasis. J Am Coll Cardiol. 2021;77(13):1670–1680. doi: 10.1016/j.jacc.2021.02.009.
- Lowes MA, Suárez-Fariñas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol. 2014;32(1):227–255. doi: 10.1146/annurev-immunol-032713-120225.
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82(6):1445–1486. doi: 10.1016/j.jaad.2020.02.044.
- Florek AG, Wang CJ, Armstrong AW. Treatment preferences and treatment satisfaction among psoriasis patients: a systematic review. Arch Dermatol Res. 2018;310(4):271–319. doi: 10.1007/s00403-018-1808-x.
- Schaarschmidt M-L, Schmieder A, Umar N, et al. Patient preferences for psoriasis treatments: process characteristics can outweigh outcome attributes. Arch Dermatol. 2011;147(11):1285–1294. doi: 10.1001/archdermatol.2011.309.
- Lebwohl MG, Kavanaugh A, Armstrong AW, et al. US perspectives in the management of psoriasis and psoriatic arthritis: patient and physician results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) survey. Am J Clin Dermatol. 2016;17(1):87–97. doi: 10.1007/s40257-015-0169-x.
- Feldman SR, Holmen Moeller A, Erntoft Idemyr ST, et al. Relative importance of mode of administration in treatment preferences among plaque psoriasis patients in the United States. J Health Econ Outcomes Res. 2017;4(2):141–157. doi: 10.36469/9817.
- Viswanathan HN, Chau D, Milmont CE, et al. Total skin clearance results in improvements in health-related quality of life and reduced symptom severity among patients with moderate to severe psoriasis. J Dermatolog Treat. 2015;26(3):235–239. doi: 10.3109/09546634.2014.943687.
- Coates LC, de Wit M, Buchanan-Hughes A, et al. Residual disease associated with suboptimal treatment response in patients with psoriatic arthritis: a systematic review of real-world evidence. Rheumatol Ther. 2022;9(3):803–821. doi: 10.1007/s40744-022-00443-y.
- Armstrong AW, Robertson AD, Wu J, et al. Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: findings from the National Psoriasis Foundation surveys, 2003-2011. JAMA Dermatol. 2013;149(10):1180–1185. doi: 10.1001/jamadermatol.2013.5264.
- Teeple A, Villacorta R, Pharm DS, et al. Determinants of patient and physician treatment satisfaction in moderate-to-severe psoriasis: a multinational survey of psoriasis patients. Dermatol Online J. 2021;27(10). doi: 10.5070/D3271055618.
- Armstrong AW, Foster SA, Comer BS, et al. Real-world health outcomes in adults with moderate-to-severe psoriasis in the United States: a population study using electronic health records to examine patient-perceived treatment effectiveness, medication use, and healthcare resource utilization. BMC Dermatol. 2018;18(1):4. doi: 10.1186/s12895-018-0072-2.
- Alcusky M, Lee S, Lau G, et al. Dermatologist and patient preferences in choosing treatments for moderate to severe psoriasis. Dermatol Ther (Heidelb). 2017;7(4):463–483. doi: 10.1007/s13555-017-0205-2.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x.
- Reilly MC. WPAI General Information: 1. Development of the WPAI; 2024 [cited May 14]. Available from: http://www.reillyassociates.net/WPAI_General.html
- Feldman SR, Poulos C, Gilloteau I, et al. Exploring determinants of psoriasis patients’ treatment choices: a discrete-choice experiment study in the United States and Germany. J Dermatolog Treat. 2022;33(3):1511–1520.
- Bronckers I, van Geel MJ, van de Kerkhof PCM, et al. A cross-sectional study in young adults with psoriasis: potential determining factors in quality of life, life course and work productivity. J Dermatolog Treat. 2019;30(3):208–215.
- Saeki H, Kanai Y, Murotani K, et al. Work productivity in real-life employed patients with plaque psoriasis: results from the ProLOGUE study. J Dermatol. 2022;49(10):970–978. doi: 10.1111/1346-8138.16517.
- Maliyar K, O’Toole A, Gooderham MJ. Long-term single center experience in treating plaque psoriasis with guselkumab. J Cutan Med Surg. 2020;24(6):588–595. doi: 10.1177/1203475420932514.
- Griffiths CEM, Augustin M, Naldi L, et al. Patient–dermatologist agreement in psoriasis severity, symptoms and satisfaction: results from a real-world multinational survey. J Eur Acad Dermatol Venereol. 2018;32(9):1523–1529. doi: 10.1111/jdv.14937.
- Merola JF, Ogdie A, Gottlieb AB, et al. Patient and physician perceptions of psoriatic disease in the United States: results from the UPLIFT survey. Dermatol Ther (Heidelb). 2023;13(6):1329–1346. doi: 10.1007/s13555-023-00929-9.
