Abstract
Aim: Bullous pemphigoid induced by secukinumab in treatment of psoriasis is rare.
Methods: We report a 49-year-old man with psoriasis who developed bullous pemphigoid during treatment with secukinumab.
Results: Scattered tense vesicles with itching appeared all over the body after the fourth treatment. Bullous pemphigoid was confirmed by pathological examination and direct immunofluorescence. The patient was treated with topical corticosteroids, oral nicotinamide and minocycline hydrochloride. The lesions of bullous pemphigoid improved significantly after 7 days of treatment.
Conclusions: Bullous pemphigoid is a rare adverse event following administration of secukinumab.
Keywords:
Psoriasis is a chronic, relapsing, immune-mediated inflammatory skin disease. In recent years, increasing biologics have been approved for the treatment of moderate-to-severe psoriasis. Although relatively safe, there have been reports of bullous pemphigoid(BP) induced by biologic agents, including ustekinumab, adalimumab, and etanercept (Citation1). Here, we report a case of bullous pemphigoid caused by secukinumab in treatment of psoriasis.
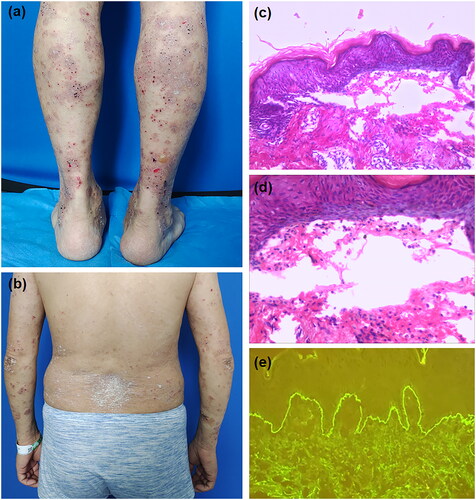
A 49-year-old man, with a 6-years history of recurrent whole-body erythema, scaling and itching, was diagnosed as severe plaque psoriasis based on clinical features and histopathology. He had been unsuccessfully treated with NB-UVB and topical halomethasone cream. After excluding tuberculosis, tumors and hepatitis virus, secukinumab was administered from May 4, 2022 (300 mg/week Subcutaneous injection). Upon the second dose, the erythema and scales around the body gradually relieved. However, one day after the fourth treatment, scattered tense vesicles with itching appeared all over the body. Dermatological examination showed scattered thick-wall vesicles based on the erythema (Nikolsky sign is negative), mainly on the extremities, with some vesicles breaking to form superficial erosions and crusts (). A few red plaques with silvery white scales were seen on the trunk and extremities (). Histopathology examination of the vesicle on the forearm showed subepidermal blisters, eosinophils within the blisters and around the dermal vessels (). Direct immunofluorescence showed linear deposition of IgG at the basement membrane zone (). Eosinophils in serum (1.66*10^9/L; normal: 0.02–0.52*10^9/L) and anti-BP180 autoantibody levels (>200 RU/mL; normal <20) were elevated. Based on these findings, the patient was diagnosed as bullous pemphigoid and psoriasis vulgaris. After discontinueing secukinumab, the patient was treated with topical halomethasone cream, oral nicotinamide(600 mg/d) and minocycline hydrochloride(200 mg/d). The lesions of bullous pemphigoid improved significantly after 7 days of treatment, and no recurrence was observed during the follow-up.
Figure 1. (a) Scattered thick-walls vesicles based on erythematous on the lower limbs, with some small superficial erosions and crusts. (b) a few scattered red plaques covered with silvery white scales are seen on the back and upper limbs. (c) Histopathology examination shows subepidermal blisters. (d) Small number of eosinophils were seen within the blisters. (e) Direct immunofluorescence studies reveal linear deposition of IgG at the basement membrane zone.
Secukinumab is a fully human monoclonal antibody of IL-17A that selectively binds to IL-17A and prevents its binding to receptors, thereby inhibiting the release of associated inflammatory cytokines and chemokines. It has been approved for the treatment of moderate to severe psoriasis (Citation2). Although side effects have been reported about this agent, reports of BP as the side effect were rare. The patient in our article received no other systemic immunosuppressants prior to application of secukinumab. BP recovered rapidly after discontinuation of secukinumab and treatment with topical glucocorticoids. Thus, we considered secukinumab as the primary cause of BP. Ho et al. reported a case of BP that was considered to be caused by secukinumab. However, the patient did not experience a recurrence of BP on reuse of secukinumab after her BP symptoms have resolved (Citation3). In addition, a case of BP after the treatment of Psoriatic Arthritis with secukinumab has been reported (Citation4). Psoriasis is considered to be a disorder driven predominantly by the cytokine products of Th17 cells. However, Bullous pemphigoid is an autoimmune blistering disease. T-helper 2 (Th2)-associated cytokines are involved in the pathogenesis of BP (Citation5). Many studies have reported that the use of IL-17A blockers leads to the same Th2-dominated atopic eczema phenotype (Citation6). We speculate that IL-17A blockers could induce a shift to a Th2-dominated immune response, therefore leading to the development of bullous pemphigoid. The specific relationship between secukinumab and BP needs to be further investigated.
Previous studies have shown that patients with BP triggered by biologic agents recovered quickly after discontinuing the biologic agent and applying topical steroids (Citation1). This is consistent with the case here we report. It is essential to monitor the patient’s skin condition during the application of biologics in treatment of psoriasis. Careful screening of past medical history and immune status should be performed to minimize the occurrence of similar adverse reactions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Husein-ElAhmed H, Steinhoff M. Bullous pemphigoid induced by biologic drugs in psoriasis: a systematic review. J Dermatolog Treat. 2022;33(7):1–4. doi:10.1080/09546634.2022.2089331.
- Krueger JG, Wharton KA, Schlitt T, et al. IL-17A inhibition by secukinumab induces early clinical, histopathologic, and molecular resolution of psoriasis. J Allergy Clin Immunol. 2019;144(3):750–763. doi:10.1016/j.jaci.2019.04.029.
- Ho PH, Tsai TF. Development of bullous pemphigoid during secukinumab treatment for psoriasis. J Dermatol. 2017;44(9):e220–e1. doi:10.1111/1346-8138.13909.
- Fatima R, Altorok N. Secukinumab-induced bullous pemphigoid in a patient with psoriatic arthritis. Am J Ther. 2023. doi:10.1097/MJT.0000000000001642.
- Tabatabaei-Panah P-S, Moravvej H, Alirajab M, et al. Association between TH2 cytokine gene polymorphisms and risk of bullous pemphigoid. Immunol Invest. 2022;51(2):343–356. doi:10.1080/08820139.2020.1832113.
- Al-Janabi A, Foulkes AC, Mason K, et al. Phenotypic switch to eczema in patients receiving biologics for plaque psoriasis: a systematic review. Acad Dermatol Venereol. 2020;34(7):1440–1448. doi:10.1111/jdv.16246.
