Abstract
Purpose
To evaluate the efficacy of Mohs micrographic surgery (MMS) combined with photodynamic therapy (PDT) in treating non-invasive extramammary Paget’s disease (EMPD).
Materials and methods
A 77-year-old male patient with non-invasive EMPD was treated with MMS followed by PDT. Preoperative fluorescence localization using 5-aminolevulinic acid (ALA) was performed to determine the surgical scope. MMS was conducted under lumbar anesthesia with intraoperative frozen-section pathology. Postoperative PDT was administered weekly for three sessions.
Results
The patient achieved negative surgical margins after two rounds of intraoperative pathology. Postoperative follow-up over two years showed no recurrence, and the patient did not experience significant adverse reactions.
Conclusion
The combination of MMS and PDT was effective in treating non-invasive EMPD, demonstrating favorable clinical outcomes and no recurrence over the two-year follow-up period.
Introduction
Extramammary Paget’s disease (EMPD) is a rare intraepithelial tumor that primarily affects the vulva, genitals, and anus in older adults [Citation1]. It typically manifests as carcinoma in situ, characterized by slow progression and a generally favorable prognosis, although invasive growth occurs in 15–40% of cases [Citation2–4]. For noninvasive lesions without distant metastases, wide local excision is frequently performed; however, owing to the unique clinical presentation and growth pattern of EMPD, recurrence rates remain high (20–60%) with these treatment modalities [Citation5–7]. Mohs micrographic surgery (MMS) is the gold standard surgical approach for EMPD, significantly reducing recurrence rates and preserving healthy tissues.
The general condition of older patients, who are often comorbid various diseases, can result in difficulties in tolerating the extended operative time associated with MMS. Consequently, there is a need to explore and discuss strategies that balance the pursuit of negative intraoperative margins to ensure the surgical safety of these patients.
Photodynamic therapy (PDT) is a noninvasive alternative therapy with minimal side effects, as evidenced by various studies [Citation8–10]. It is well-tolerated by patients and preserves their normal anatomy and function [Citation8]. Several investigations have demonstrated that combining surgery with PDT significantly decreases the recurrence rate of EMPD compared with surgery or PDT alone [Citation11]. However, not all studies agree on the usefulness of PDT in the treatment of EMPD; some authors also reported discouraging results regarding PDT following fractional CO2 laser treatment [Citation12].
Previous studies have primarily investigated the effectiveness of wide local excision combined with PDT, with a limited focus on the efficacy of MMS combined with PDT. Therefore, this report aimed to address this gap by reporting a case of nonmetastatic, noninvasive EMPD treated with MMS combined with PDT. The treatment efficacy, recurrence rates, and adverse effects were assessed. This case report has obtained informed consent from the patient for the publication of relevant photos and medical records.
Case presentation
A 77-year-old male presented with an erythematous eruption of the scrotum, with itching persisting for >8 months. Eight months prior, erythema and erosion were noted on the right scrotum, accompanied by mild itching. He had been previously diagnosed with eczema at a local hospital, and topical medication (specifically unknown) temporarily alleviated the symptoms. However, the condition recurred upon discontinuation of the medication and the erythema gradually spread. One month prior to the consultation, the patient underwent pathological biopsy at another hospital, which revealed EMPD. Additionally, the patient had a history of poorly controlled hypertension and had undergone right inguinal hernia repair surgery.
A physical examination revealed a blood pressure of 161/89 mmHg. Additionally, red scaly plaques with scattered vesicles were observed on the right scrotum and groin.
The laboratory test results, including complete blood counts, metabolic panels, coagulation studies, and other preoperative screenings, were unremarkable. The CEA (carcinoembryonic antigen) level was within the normal range. Bacterial culture of skin scrapings yielded Escherichia coli. Electrocardiography revealed complete right bundle branch block. Cardiac ultrasonography revealed a normal ejection fraction (72%). Systemic positron emission tomography–computed tomography revealed no evidence of distant metastases. Ultrasonography revealed an enlarged right inguinal lymph node with uneven internal echogenicity, indicating a reactive lymph node. The diagnoses of EMPD was made based on pathological results.
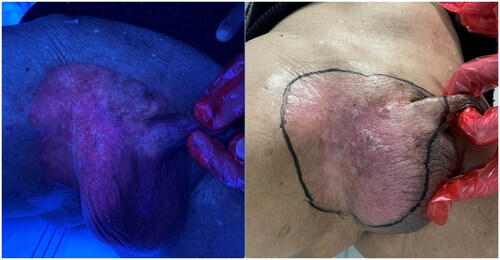
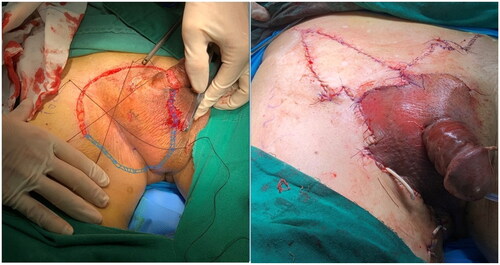
Subsequently, the patient underwent the following course of treatment. 1) Preoperative 5-aminolevulinic acid (ALA) fluorescence localization was performed to determine the extent of the procedure, as illustrated in . Systemic and local prophylactic antibiotics were administered to prevent and combat infections. 2) MMS () was performed for tumor excision; the specific procedure was tumor excision under lumbar anesthesia with the use of a skin flap, and right inguinal lymph node biopsy. Intraoperative frozen-section pathology was performed twice, and the wound was closed after the examination results suggested negative margins. 3) Postoperative PDT was administered once a week for a total of three sessions. Each session involved applying 590 mg of 20% 5-aminolevulinic acid (ALA) dressing, with a dressing time of 4 h. Illumination was performed using LED-IBS equipment with the following parameters: power density of 80 mW/cm2; energy density of 192 J/cm2; distance, 20 cm between the skin to the LED-IBS; and an illumination time of 40 min. The patient experienced localized discomfort during the initial stages of treatment. Discomfort was mitigated by applying a chiller to the affected area and gradually reducing the sensation of pain; the patient’s tolerance increased over time.
Postoperative pathological results revealed that the thickest part of the tumor was consistent with EMPD, with an infiltration depth of 5 mm. No metastasis was observed in the right inguinal lymph node. Immunohistochemical findings of the tumor showed positivity for CK7 and CK, with Ki-67 staining of approximately 15%. In contrast, immunohistochemistry results for the lymph nodes showed negative staining for CK7 and CK, with Ki-67 staining in approximately 90% of germinal centers and approximately 15% of non-germinal centers.
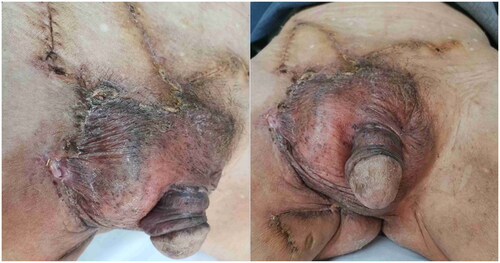
Postoperative follow-up () included regular examinations every 3 months, including clinical evaluation, lymph node ultrasound, and serum CEA monitoring. Encouragingly, no recurrence was observed during the 2-year follow-up period. There were no adverse reactions such as infection, pain, paresthesia, or lymphatic leakage.
Discussion
Conventionally, wide local excision often results in higher recurrence rates than MMS, which is correlated with the extension pattern of EMPD. EMPD exhibits at least two subclinical extension patterns, continuous and satellite extension, with significant differences in extension rates [Citation13]. Studies have reported that subclinical lesions >3 cm account for 47% of biopsy sites [Citation14], and a histopathologically clear margin of 5 cm is required in 97% of cases to eradicate EMPD cells [Citation6]. However, a wide margin of 5 cm in the rectal area may significantly affect the local structure and function, impairing the quality of life. With the help of intraoperative frozen sections, the recurrence rate of MMS is notably lower than that of the traditional wide local excision. The overall EMPD recurrence rate following treatment with MMS was 12.2% [Citation5]. Moreover, most studies have demonstrated that a 2 cm margin with MMS is sufficient for treating EMPD [Citation15]. Therefore, MMS is currently the most highly regarded and recommended surgical approach for managing EMPD.
Reports on MMS indicate that multiple stages are required to attain negative margins in a significant percentage of cases, ranging from 75% to 100% [Citation16,Citation17]. However, there are concerns regarding the clinical predominance of EMPD in elderly individuals, typically those >65 years of age. In the present case, the patient was 77 years old and preoperative evaluation suggested potential challenges in tolerating prolonged surgery, such as hypertension, cardiac issues, and other health concerns. This raised concerns about the patient’s ability to undergo multiple intraoperative frozen section pathology to achieve negative margins. Consequently, the treatment plan prioritized MMS as the initial approach, aiming to achieve negative margins intraoperatively and subsequently provide adjuvant therapy post-surgery. Fortunately, the patient achieved negative margins after two rounds of frozen-section pathology.
PDT is a compelling option for postoperative adjuvant therapy. PDT is a noninvasive alternative with minimal side effects, as reported in several studies [Citation9,Citation10]. It is well tolerated by patients and preserves normal anatomy and function. Given the multicentric nature of EMPD lesions, PDT also aids in targeting small lesions missed during surgery, thereby improving patient prognosis [Citation18]. Numerous studies have demonstrated that combining surgery with PDT significantly reduces disease recurrence rates compared with either modality alone, with some reporting up to a three-fold reduction [Citation19,Citation20]. However, the reported efficacy of PDT in the treatment of EMPD varies. Previous studies have predominantly focused on combining wide local excision with PDT, with a limited exploration of MMS in combination with PDT. Theoretically, MMS combined with PDT may further eliminate subclinical lesions, potentially leading to improved therapeutic outcomes.
The development of surgical site infections is a concern in genital surgery. E. coli was found in the bacterial culture of skin scrapings at the tumor site; however, we considered it a colonizing flora because of the lack of manifestations of skin infection, such as abscess, pain, or fever [Citation21]. PDT not only exerts antitumor effects, but also possesses anti-infective properties against microorganisms [Citation22]. Research has indicated that PDT can modulate the bacterial growth microenvironment, stimulate the immune system, and induce cytotoxicity via reactive oxygen species, thereby exhibiting antibacterial effects [Citation23]. Additionally, PDT promotes wound healing by reducing inflammation, stimulating fibroblast proliferation, and increasing levels of transforming growth factor-β and metalloproteinases [Citation24]. Consequently, this treatment regimen was deemed safe, well-tolerated, and conducive for operative wound recovery.
Overall, MMS combined with PDT is the recommended treatment modality for EMPD. Some studies have reported an estimated 5-year Kaplan Meier tumor-free rate was 81.8% after MMS [Citation25]. The present case did not experience recurrence within 2 years; however, it is essential to continue follow-up for long-term observation. Our team is currently collecting data from all patients treated with this approach over the past 5 years for further study and analysis. This study provides additional insights into the efficacy and long-term outcomes of MMS combined with PDT for the treatment of EMPD.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing and Ming Hu for his technical and moral support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Goldsmith LA, Fitzpatrick TB. Fitzpatrick’s dermatology in general medicine. New York McGraw-Hill Medical; 2012.
- Kanitakis J. Mammary and extramammary Paget’s disease. J Eur Acad Dermatol Venereol. 2007;21(5):1–5. doi:10.1111/j.1468-3083.2007.02154.x.
- Sopracordevole F, Di Giuseppe J, De Piero G, et al. Surgical treatment of Paget disease of the vulva: prognostic significance of stromal invasion and surgical margin status. J Low Genit Tract Dis. 2016;20(2):184–188. doi:10.1097/LGT.0000000000000191.
- Ito T, Kaku Y, Nagae K, et al. Tumor thickness as a prognostic factor in extramammary Paget’s disease. J Dermatol. 2015;42(3):269–275. doi:10.1111/1346-8138.12764.
- Bae JM, Choi YY, Kim H, et al. Mohs micrographic surgery for extramammary Paget disease: a pooled analysis of individual patient data. J Am Acad Dermatol. 2013;68(4):632–637. doi:10.1016/j.jaad.2012.12.960.
- Hendi A, Brodland DG, Zitelli JA. Extramammary Paget’s disease: surgical treatment with Mohs micrographic surgery. J Am Acad Dermatol. 2004;51(5):767–773. doi:10.1016/j.jaad.2004.07.004.
- O’Connor WJ, Lim KK, Zalla MJ, et al. Comparison of Mohs micrographic surgery and wide excision for extramammary Paget’s disease. Dermatol Surg. 2003;29(7):723–727. doi:10.1111/j.1524-4725.2008.34380.x.
- Wang X, Wang H, Guo M, et al. Treatment of skin cancer and precancer using topical ALA-PDT—a single hospital experience. Photodiagn Photodyn Ther. 2008;5(2):127–133. doi:10.1016/j.pdpdt.2008.05.003.
- Szpringer E, Lutnicki K, Marciniak A. Photodynamic therapy- mechanism and employment. Ann Univ Mariae Curie Sklodowska Med. 2004;59(2):498–502.
- Kalisiak MS, Rao J. Photodynamic therapy for actinic keratoses. Dermatol Clin. 2007;25(1):15–23. doi:10.1016/j.det.2006.09.006.
- Gao Y, Zhang XC, Wang WS, et al. Efficacy and safety of topical ALA-PDT in the treatment of EMPD. Photodiagnosis Photodyn Ther. 2015;12(1):92–97. doi:10.1016/j.pdpdt.2014.11.004.
- Ferrara F, Bardazzi F, Messori S, et al. Photodynamic therapy following fractional CO2 laser for treatment of primary vulvar Paget’s disease: does it really work? J Dermatolog Treat. 2021;32(7):800–802. doi:10.1080/09546634.2019.1707755.
- Murata T, Honda T, Egawa G, et al. Three-dimensional evaluation of subclinical extension of extramammary Paget disease: visualization of the histological border and its comparison to the clinical border. Br J Dermatol. 2017;177(1):229–237. doi:10.1111/bjd.15282.
- Gunn RA, Gallager HS. Vulvar Paget’s disease. A topographic study. Cancer. 1980;46(3):590–594. doi:10.1002/1097-|0142(19800801)46:3<590::aid-cncr2820460327>3.0.co;2-q.
- Hatta N, Yamada M, Hirano T, et al. Extramammary Paget’s disease: treatment, prognostic factors and outcome in 76 patients. Br J Dermatol. 2008;158(2):313–318. doi:10.1111/j.1365-2133.2007.08314.x.
- Boehringer A, Leiter U, Metzler G, et al. Extramammary Paget’s disease: extended subclinical growth detected using three-dimensional histology in routine paraffin procedure and course of the disease. Dermatol Surg. 2011;37(10):1417–1426. doi:10.1111/j.1524-4725.2011.02091.x.
- Lee K-Y, Roh MR, Chung WG, et al. Comparison of Mohs micrographic surgery and wide excision for extramammary Paget’s disease: korean experience. Dermatol Surg. 2009;35(1):34–40. doi:10.1111/j.1524-4725.2008.34380.x.
- Xu H, Li YM, Ma H, et al. Photodynamic therapy combined with dermabrasion in cutaneous squamous cell carcinoma concomitant with psoriasis. Photobiomodul Photomed Laser Surg. 2019;37(3):191–193. doi:10.1089/photob.2018.4552.
- Wang HW, Lv T, Zhang LL, et al. A prospective pilot study to evaluate combined topical photodynamic therapy and surgery for extramammary paget’s disease. Lasers Surg Med. 2013;45(5):296–301. doi:10.1002/lsm.22142.
- Simonds RM, Segal RJ, Sharma A. Extramammary Paget’s disease: a review of the literature. Int J Dermatol. 2019;58(8):871–879. doi:10.1111/ijd.14328.
- Moghissi K. Can Surgical Site Infection (SSI) be treated by photodynamic therapy (PDT)? Photodiagnosis Photodyn Ther. 2010;7(1):1–2. doi:10.1016/j.pdpdt.2010.01.005.
- Maleki A, He J, Bochani S, et al. Multifunctional Photoactive hydrogels for wound healing acceleration. ACS Nano. 2021;15(12):18895–18930. doi:10.1021/acsnano.1c08334.
- Kim M, Jung HY, Park HJ. Topical PDT in the treatment of benign skin diseases: principles and new applications. Int J Mol Sci. 2015;16(10):23259–23278. doi:10.3390/ijms161023259.
- Nesi-Reis V, Lera-Nonose DSSL, Oyama J, et al. Contribution of photodynamic therapy in wound healing: a systematic review. Photodiagnosis Photodyn Ther. 2018;21:294–305. doi:10.1016/j.pdpdt.2017.12.015.
- Bellew S, Del Rosso JQ. Comparison of Mohs micrographic surgery and wide excision for extramammary Paget’s disease: Korean experience. Yearbook of Dermatology and Dermatologic Surgery. 2010;2010:431–432. doi:10.1016/S0093-3619(09)79334-7.