?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
The presence of wheals or hives has been viewed as a hallmark symptom of urticaria, a highly debilitating disease. This study explores our experience with omalizumab in patients with apparent mast-cell mediated pruritus in the absence of hives.
Materials and Methods
This is a retrospective case series examining all patients with mast cell-mediated pruritus in the absence of hives from April 2022 to May 2024 at a tertiary referral clinic at Icahn School of Medicine at Mount Sinai in New York. Peak pruritus-numerical rating scale (PP-NRS) itch score changes over time were recorded and analyzed.
Results
Six patients (67% women; mean [SD] age, 47.67 [13.52] years) were included in the analysis. The median [IQR] pruritus PP-NRS itch score before omalizumab injection was 9 [6 – 10] and the final median [IQR] PP-NRS itch score was 2.5 [0 – 5]. The mean [SD] reduction in the PP-NRS itch score was 6 [3.16].
Conclusions
This study suggests that patients with evidence of mast cell-mediated pruritus can be identified based on clinical features and may benefit from omalizumab therapy.
Introduction
Classically, chronic urticaria (CU) is diagnosed by the occurrence of recurrent hives or wheals, angioedema, or both for a period of six weeks or longer (Citation1). CU can be further classified by whether there is an identifiable physical trigger such as cold, heat, exercise, or pressure, in which case the term chronic inducible urticaria (CIndU) is used. The precise mechanisms underlying CIndU remain poorly defined. In cases in which lesions occur spontaneously without an identifiable external cause, CU is defined as chronic spontaneous urticaria (CSU). It is well understood that activation of the high affinity IgE receptor (FcεRI) on the cell surface of mast cells results in degranulation, release of mediators such as histamine, and the characteristic hive and itch. CSU comprises the majority (60–90%) of CU cases and can occur via a number of mechanisms including the generation of autoantibodies to FcεRI or IgE or IgE reactivity to autoallergens/antigens (Citation2–4). The presence of wheals or hives has been viewed as a hallmark symptom of urticaria. However, the Itch Clinic at Mount Sinai identified a series of patients who exhibit pruritus rather than hives, wheals, or angioedema as their predominant symptom. A number of patients presented with fluctuant pruritus and generalized skin flushing for over 6 weeks, seemingly triggered by environmental or seasonal factors and with a partial response to antihistamines. This combination of symptoms suggested that these patients may have mast cell-mediated itching akin to what occurs in urticaria in absence of overt hives.
Antihistamines and mast cell targeting therapies such as omalizumab, an anti-IgE monoclonal antibody (mAb), are mainstay treatments in CSU (Citation1). Here, we report our experience with omalizumab in patients with apparent mast cell-mediated pruritus in the absence of hives in a retrospective observational fashion.
Methods
The present cohort includes 6 patients, having fluctuant pruritus over 6 weeks in the absence of any wheals or angioedema and with partial response to antihistamines. All patients were young to middle aged (age < 65 years), and itch symptoms peaked seasonally in spring and summer months. During the course of routine care, patients underwent investigations such as complete blood count (CBC) with differential, complete metabolic panel (CMP), IgE, tryptase, and CU Index. As a measure of basophil histamine release, a positive CU Index suggests an autoimmune basis of urticarial symptoms or the potential presence of other serum histamine-releasing factors (Citation5).
The peak pruritus-numerical rating scale (PP-NRS) itch score is a single-question assessment tool to assess worst itch intensity over the prior 24 h, ranging from 0 (no itch) to 10 (worst possible itch). The scale allows for the stratification of itch intensity between 0–3 (mild), 4–6 (moderate), and 7 (severe).
This retrospective case series had the following inclusion criteria: (1) chronic pruritus > 6 weeks duration, (2) baseline PP-NRS itch score of 5 (moderate to severe), (3) age < 65 years old, (4) partial response to antihistamine treatment, characterized by
2 point reduction in PP-NRS, and
1 of the following: (a) seasonal itching, peaking in spring or summer months, (b) IgE > 1000, (c) tryptase > 11.5 ng/mL, or (d) positive CU Index. Patients with a prior diagnosis of CU, but no active symptoms of wheals or angioedema were included. Exclusion criteria included: any primary dermatologic disorder potentially responsible for the pruritus and the presence of any wheals or skin lesions.
All patients that were analyzed met the above inclusion criteria and were seen between April 2022 and May 2024 and underwent treatment with omalizumab (300 mg subcutaneous [SC] injection monthly).
Since this study constitutes a case series, concurrent medications were not discontinued, and the use of emollients, topical steroids, and antihistamines were permitted. Treatment response was evaluated by comparing the change in PP-NRS itch score from before starting omalizumab to the most recent PP-NRS itch score at the time of data analysis. Statistical significance was determined by a two-tailed p-value < 0.05. This study was approved by the Institutional Review Board of Icahn School of Medicine at Mount Sinai in New York and followed the tenets of the Declaration of Helsinki.
Results
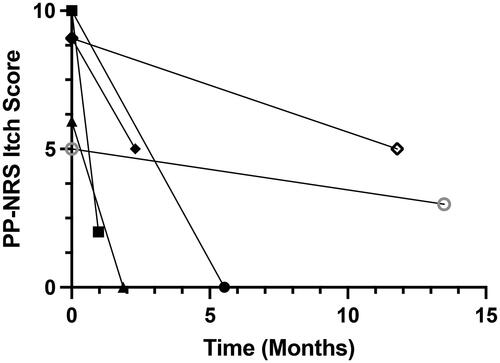
A total of 6 patients were analyzed based on our inclusion/exclusion criteria. The mean [SD] age was 47.67 [13.52] years and 4/6 were female. The mean [SD] duration of itch prior to presentation was 20.17 [21.78] years. The median [interquartile range, IQR] time from baseline PP-NRS itch score to the primary endpoint PP-NRS itch analysis was 8.52 [2.30-13.35] months. All baseline characteristics are presented in .
Table 1. Patient demographics, PP-NRS scores, and follow-up time of improvement of patients.
All 6 subjects experienced marked reduction in pruritus symptoms as measured by the PP-NRS itch score from baseline (). The median [IQR] baseline 24-h PP-NRS itch score was 9 [6-10] with a final PP-NRS itch score of 2.5 [0–5]. The mean [SD] reduction in the PP-NRS itch score was 6 [3.16]. Since these patients were evaluated during the course of routine care, systematic daily assessment of itch improvement over time was not acquired from all subjects. Nonetheless, 2 of the subjects reported remarkable improvement of their itch within one month after the first omalizumab injection.
Discussion
The occurrence of itch, wheals, and angioedema in CSU is mediated, in large part, by factors released from mast cells such as histamine. Indeed, histamine is known to directly stimulate sensory neurons to trigger itch, but also acts directly on endothelial cells to trigger capillary leak which underlies the edema that causes wheals and angioedema. However, it is increasingly appreciated that many factors can trigger pruritus independently of any effect on immune cells or endothelial cells. This provokes the hypothesis that, just as CSU patients experience itch associated with hives, it is possible to experience mast cell-mediated itch even in the absence of hives.
All subjects in our cohort experienced generalized pruritus and flushing in the absence of wheals, suggesting a distinct phenotype from classical CSU. Furthermore, all of our patients experienced partial but incomplete response to H1 antihistamines. Strikingly, all subjects experienced marked reduction in itch after omalizumab therapy, indicating that the pruritic symptoms are likely mediated by similar IgE-mediated mast cell pathways as in CSU. Hence, focusing primarily on the lesional manifestations of wheals or angioedema such as in the diagnosis of CSU would likely not capture this pruritic patient population. The current study lends further support for pharmacologic disruption of FcεR1-mediated mast cell activation as a potential treatment strategy for pruritus in patients with mast cell-mediated itch phenotypes.
Another condition that manifests as itch in the absence of skin lesions is chronic pruritus of unknown origin (CPUO). However, these patients tend to be more elderly, experience itch worsening in the winter, and are typically unresponsive to antihistamines. Therefore, to avoid this population in our current study, we excluded patients who were 65 years old and older, and included those who exhibited seasonal itching in the spring and/or summer and responded partially to antihistamines. Finally, in our experience and, to our knowledge, there are no reports of patients with CPUO who respond therapeutically to omalizumab. Therefore, we suspect that patients with mast cell-mediated itch in the absence of hives, as reported here, experience pruritus that is similar to that in CSU, rather than CPUO. Future studies unveiling biomarkers and response to treatment in patients with mast cell-mediated (e.g., urticarial) itch in the absence of hives and CPUO, respectively, will likely further differentiate these conditions.
Given that our study is a consecutive case series and these patients were undergoing routine care, there are limitations. The study was not randomized, unblinded, and some variables could not be controlled including concomitant medications and assessment timepoints. Notwithstanding these limitations, our findings suggest that patients may experience itch in the absence of hives that is mast cell mediated.
Conclusion
Patients with evidence of mast cell-mediated pruritus in the absence of hives can be identified based on clinical features and may benefit from omalizumab therapy.
Disclosure statement
B.S.K. is founder of Alys Pharmaceuticals; he has served as a consultant for 23andMe, ABRAX Japan, AbbVie, Almirall, Amgen, Attovia Therapeutics, Cara Therapeutics, Clexio Biosciences, Eli Lilly and Company, Escient Pharmaceuticals, Evommune, Galderma, Genentech, GlaxoSmithKline, Granular Therapeutics, Incyte Corporation, LEO Pharma, Novartis, Pfizer, Recens Medical, Regeneron Pharmaceuticals, Sanofi, Septerna, and Triveni Bio; he has stock in ABRAX Japan, Alys Pharmaceuticals, Attovia Therapeutics, Locus Biosciences, Recens Medical, and Triveni Bio; he holds a patent for the use of JAK1 inhibitors for chronic pruritus.
Additional information
Funding
References
- Zuberbier T, Abdul Latiff AH, Abuzakouk M, et al. The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy. 2022;77(3):1–4. doi:10.1111/all.15090.
- Kolkhir P, Giménez-Arnau AM, Kulthanan K, et al. Urticaria. Nat Rev Dis Primers. 2022;8(1):61. doi:10.1038/s41572-022-00389-z.
- Asero R, Ferrer M, Kocaturk E, et al. Chronic spontaneous Urticaria: the role and relevance of autoreactivity, autoimmunity, and autoallergy. J Allergy Clin Immunol Pract. 2023;11(8):2302–2308. doi:10.1016/j.jaip.2023.02.022.
- Kolkhir P, Muñoz M, Asero R, et al. Autoimmune chronic spontaneous Urticaria. J Allergy Clin Immunol. 2022;149(6):1819–1831. doi:10.1016/j.jaci.2022.04.010.
- Biagtan MJ, Viswanathan RK, Mathur SK. The chronic Urticaria index as a predictor of responsiveness to therapy. J Allergy Clin Immunol. 2011;127(2):AB104–AB104. doi:10.1016/j.jaci.2010.12.417.