Dear Editor,
The development of biological drugs and small molecules has dramatically changed the therapeutic landscape of moderate-to-severe atopic dermatitis (AD) [Citation1,Citation2]. In particular, Janus Kinase (JAK) inhibitors, including abrocitinib, baricitinib and upadacitinib, are currently approved in Europe for the treatment of these patients after showing efficacy and safety in phase-III clinical trials [Citation3,Citation4]. Upadacitinib is a selective inhibitor of JAK-1, and it is indicated for the treatment of moderate-to-severe AD across two dosages, 15 and 30 mg daily [Citation5]. Real-world data on the effectiveness and safety profile of upadacitinib are currently limited, in particular regarding upadacitinib 30 mg [Citation6–8]. We conducted a real-world single-center retrospective study on 31 patients affected by moderate-to-severe AD, all treated with upadacitinib 30 mg daily in a clinical setting.
We included adult patients with severe AD eligible for systemic treatment with upadacitinib 30 mg according to EuroGuiDerm guidelines [Citation2]. During the study period, patients could apply topical treatment as needed, while none of the patients used other systemic treatments.
We selected optimal effectiveness endpoints according to a recent recommendation from Silverberg et al. [Citation9]. The effectiveness of upadacitinib 30 mg was assessed at weeks 16, 32, and 52 in terms of EASI90 (at least 90% reduction from baseline Eczema Area and Severity Index), EASI100 (complete skin clearance) and absolute EASI ≤ 3. The impact of upadacitinib on itch and sleep was evaluated at the same time points as percentages of patients achieving an itch-NRS (numerical rating scale) and a sleep NRS of 0/1 (out of 10).
Twenty patients (64.5%) were male, with a mean age of 42.90 (standard deviation [SD] 14.85). At baseline, the mean EASI was 19.69 (6.18), while the itch-NRS and sleep-NRS scores were 8.06 (1.57) and 6.81 (1.89), respectively.
Complete demographic characteristics of our population at baseline are shown in .
Table 1. Demographic characteristics of our cohort of patients at baseline.
Table 2. Reported adverse events during the treatment with upadacitinib 30 mg in our cohort.
At week 16, EASI90, EASI100 and an absolute EASI≤ 3 were reached by 21 (67.7%), 17 (54.8%) and 23 patients (74.2%), respectively (). At the same time point, 20 (64.5%) and 23 (74.2%) reported an itch-NRS score and sleep-NRS score of 0/1, respectively (). Twenty-five patients reached one year of follow-up. Among them, 21 (84%) achieved an absolute EASI ≤ 3, 21 (84%) reached EASI90 and 17 (68%) achieved a complete skin clearance (). In terms of patient-reported outcomes at week 52, an itch-NRS score and a sleep-NRS score of 0/1 were achieved by 18 (72%) and 22 patients (88%), respectively (). Adverse events (AEs) were reported from 9 patients (29%) (). Only three patients discontinued upadacitinib 30 mg because of AEs such as lymphopenia, herpes zoster, and severe hyperlipidemia. Two patients were switched to upadacitinib 15 mg due to elevated liver enzymes and moderate papulopustular acne.
Figure 1. Objective (a) and subjective (b) outcomes of our population throughout 52 weeks of treatment with upadacitinib 30 mg. EASI: eczema area and severity index; NRS: Numerical Rating Scale.
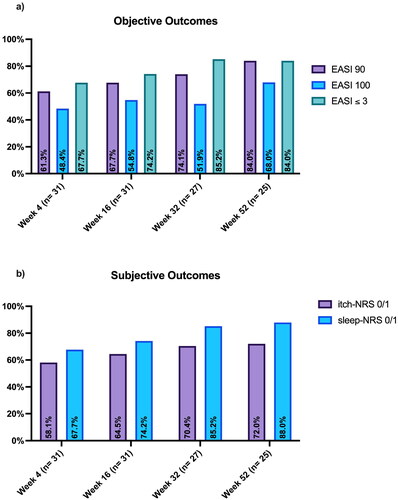
In our experience, upadacitinib 30 mg showed comparable or higher effectiveness and safety compared to phase-III clinical trials [Citation3,Citation4]. In particular, in our study, at week 16, most of the patients achieved optimal clinical outcomes. Evidence suggests that current treat-to-target outcomes are insufficient to assess optimal treatment effectiveness. For this reason, we followed recent recommendations from Silverberg et al. [Citation9], which include the achievement of clinical and subjective endpoints as optimal treatment targets, such as EASI90 or EASI ≤ 3, itch-NRS 0/1 and sleep-NRS 0/1. In our study, the percentage of patients who achieved all outcomes is higher than that observed in other real-life experiences with upadacitinib 30 mg [Citation10,Citation11]. Our findings support the role of upadacitinib 30 mg as a very effective option for the treatment of moderate-to-severe AD in order to achieve optimal disease control. Upadacitinib 30 mg was well-tolerated in the absence of new significant safety findings. Despite a few limitations, due to the retrospective nature of the study, the relatively short follow-up and the limited sample size, our experience could provide more information on the use of upadacitinib 30 mg in clinical practice. Further studies are needed to confirm our findings and to better explore the effectiveness and safety of this drug in patients with AD.
Ethical approval
Institutional review board approval was exempted, as the study procedures did not deviate from standard clinical practice. For some of the patients, AbbVie provided the drug upadacitinib through a Compassionate Use Program activated according to the DM 7/9/2017. All included patients had provided written informed consent for the retrospective analysis of their clinical data. The study was conducted in accordance with the Helsinki Declaration of 1964 and its later amendments.
Disclosure statement
L. Gargiulo has been a consultant for Almirall, UCB and Pfizer. L. Ibba has been a consultant for Almirall. P. Facheris has served as a consultant for Eli Lilly. M. Valenti has been a consultant and/or speaker for Sanofi, Leo Pharma, Eli Lilly, Novartis, Janssen, AbbVie and Boehringer Ingelheim. A. Costanzo has served as an advisory board member, consultant and has received fees and speaker’s honoraria or has participated in clinical trials for Abbvie, Almirall, Biogen, LEO Pharma, Lilly, Janssen, Novartis, Pfizer, Sanofi Genzyme, and UCB-Pharma. A. Narcisi has served on advisory boards, received honoraria for lectures and research grants from Almirall, Abbvie, Leo Pharma, Celgene, Eli Lilly, Janssen, Novartis, Sanofi‐Genzyme, Amgen and Boehringer Ingelheim. A. Alfano, R. Cascio Ingurgio, M. Bianco, S. Di Giulio and C. Perugini have nothing to declare.
Data availability statement
Additional data supporting the findings of this manuscript are available on reasonable request to the corresponding author.
Additional information
Funding
References
- Gargiulo L, Ibba L, Malagoli P, et al. Management of patients affected by moderate-to-severe atopic dermatitis with JAK inhibitors in real-world clinical practice: an Italian Delphi consensus. Dermatol Ther. 2024;14(4):1–4. doi: 10.1007/s13555-024-01135-x.
- Wollenberg A, Kinberger M, Arents B, et al. European guideline (EuroGuiDerm) on atopic eczema: part I – systemic therapy. J Eur Acad Dermatol Venereol. 2022;36(9):1409–1431. doi: 10.1111/jdv.18345.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (measure up 1 and measure up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397(10290):2151–2168. doi: 10.1016/S0140-6736(21)00588-2.
- Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo controlled, phase 3 trial. Lancet. 2021;397(10290):2169–2181. doi: 10.1016/S0140-6736(21)00589-4.
- European Medicines Agency. Rinvoq (upadacitinib): summary of product characteristics; 2019 [cited 2023 April 21]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/rinvoq.
- Gargiulo L, Ibba L, Piscazzi F, et al. Effectiveness and safety of upadacitinib for moderate-to-severe atopic dermatitis in a real-world setting: a 52-week retrospective study. J Eur Acad Dermatol Venereol. 2024;38(2):e152–e154. doi: 10.1111/jdv.19507.
- Ibba L, Gargiulo L, Vignoli CA, et al. Practical use of upadacitinib in patients with severe atopic dermatitis in a real-world setting: a systematic review. Clin Cosmet Investig Dermatol. 2024;17:593–604. doi: 10.2147/CCID.S329442.
- Hagino T, Saeki H, Kanda N. The efficacy and safety of upadacitinib treatment for moderate to severe atopic dermatitis in real-world practice in Japan. J Dermatol. 2022;49(11):1158–1167. doi: 10.1111/1346-8138.16549.
- Silverberg JI, Gooderham M, Katoh N, et al. 327 Optimizing the management of atopic dermatitis with a new minimal disease activity concept and criteria and consensus-based recommendations for systemic therapy. British J Dermatol. 2023;188(Supplement_2). doi: 10.1093/bjd/ljac140.022.
- Hagino T, Saeki H, Fujimoto E, et al. Long-term effectiveness and safety of upadacitinib for Japanese patients with moderate-to-severe atopic dermatitis: a real-world clinical study. J Dermatolog Treat. 2024;35(1):2344591. doi: 10.1080/09546634.2024.2344591.
- Hagino T, Hamada R, Yoshida M, et al. Sustained effectiveness of upadacitinib in moderate-to-severe atopic dermatitis: a 48-week real-world study. Pharmaceuticals. 2024;17(4):519. doi: 10.3390/ph17040519.
