Abstract
Background:
Psoriasis is a prevalent skin disease affecting approximately 1%–3% of the population and imposes significant medical, social and economic burdens. Psoriasis involves multiple organs and is often complicated with obesity, diabetes, dyslipidemia, and hypertension. Because of the benefits of lipid-lowering agents and antidiabetic medications for psoriasis, metabolic abnormalities possibly play a pathogenic role in psoriasis.
Objective:
This review focuses on the impacts of a variety of metabolic disorders on psoriasis and the underlying mechanisms.
Results:
In psoriasis, enhanced glycolysis, glutamine metabolism and altered fatty acid composition in the psoriatic lesion and plasma result in the excessive proliferation of keratinocytes and secretion of inflammatory cytokines. Altered metabolism is associated with the activation of MTORC signaling pathway and transcription factors such as HIF and S6K1. Therefore, MTORC1 can be a target for the treatment of psoriasis. Additionally, there are diabetes drugs and lipid-lowering drugs including TZDs, GLP-1 RAs, Metformin, statins and fibrates, which improve both metabolic levels and psoriasis symptoms.
1. Introduction
Psoriasis is an inflammatory skin disease, with a worldwide prevalence of approximately 1%–3%. Psoriasis is characterized by over-proliferation of keratinocytes and infiltration of immune cells, including T cells, macrophages, and neutrophils (Citation1). Although activated T cells play a crucial role in its pathogenesis, other skin cells, including keratinocytes, are also involved in the pathogenesis of psoriasis (Citation2). Many factors, such as inflammatory cytokines, oxidative stress and miRNA, can influence the development of psoriasis. In addition to immune cells, release of chemokines by keratinocytes can attract T cells. The latter secrete inflammatory cytokines, such as IL-22, (IFN) -γ, IL-17A, and IL-8, to promote the proliferation of epidermal keratinocytes (Citation3). Moreover, oxidative stress also induces epidermal hyperkeratosis (Citation4). The levels of biomarkers of oxidative stress are higher in the psoriatic skin (Citation5). Oxidative stress can stimulate the production of ROS in psoriasis (Citation6), while ROS can induce the proliferation and differentiation of Th22/Th1/Th17 cells, reduces the anti-inflammatory activity of regulatory T cells, and promotes the secretion of IL-17, IL-22, TNF-α, IFN-γ, and VEGF, as well as keratinocyte proliferation (Citation7). Moreover, aberrant expressions of small non-protein-coding RNAs (miRNA), which affect gene expression (Citation8), are also involved in the pathogenesis of psoriasis (Citation9). Keratinocyte proliferation is positively regulated by, at least, miR-17-92, miR-548a–3p, miR-223, miR-126, miR-744-3p, and miR-210 (Citation10–15), while negatively regulated by miR-20a-3p, miR-125b-5p, miR-181b-5p, miR124-3p, miR-125b, miR-187, miR-145-5p, and miR-320b (Citation16–23). Hence, psoriasis is a multifactorial disease.
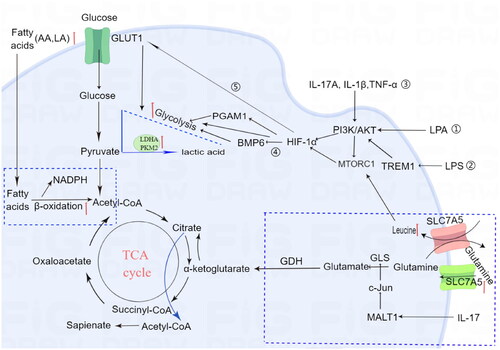
Psoriasis is not solely confined to the skin disease, but rather a systemic disease, along with metabolic diseases, such as obesity, diabetes and metabolic syndrome (Citation24). The inflammatory cascade in psoriasis affects metabolism of the entire body, and the persistent effects of inflammation may trigger profound metabolic changes (Citation25). Metabolic abnormalities can affect the occurrence, development, efficacy, and prognosis of diseases, the mechanisms by which metabolic dysregulation affects psoriasis provide great potential for developing effective clinical diagnostics, treatment monitoring, and further discovery of new metabolic-based therapeutic targets (Citation26). Previous studies have shown metabolic disorders manifest abnormal levels of glucose, amino acids and lipids in the circulation and the involved skin of psoriatic patients (Citation27). These metabolic changes can occur independently and dependently. The link between metabolism and psoriasis keratinocytes is illustrated in . Although metabolomic changes in psoriasis are well known, the underlying mechanisms are unclear. This paper comprehensively discusses the metabolic alterations and the underlying mechanisms in psoriasis.
Figure 1. Altered metabolic pathways in keratinocytes for psoriasis.
Glucose, fatty acids and amino acids are required for maintenance of metabolic homoeostasis and the generation of energy.
Glycolysis:
①LPA signaling induces PGAM1 expression via AKT/mTOR/HIF-1α pathway and increases aerobic glycolysis, contributing to keratinocyte proliferation.
②LPS-induced TREM-1 increases HIF-1a expression in HaCaT cells through the PI3 K/AKT pathway
③IL-1β, IL-17A, and TNF-α stimulate the mTOR pathway through the PI3K signaling pathway, resulting in enhanced proliferation of psoriatic keratinocytes
④ HIF-1α-mediated BMP6 down-regulation leads to hyperproliferation
⑤hypoxia-inducible factor 1α (HIF-1α) promotes glycolysis in psoriasis vulgaris by up-regulating GLUT1 expression.
Glutamine metabolism: The levels of leucine and SLC7A5 are increased in the plasma of psoriatic patients. Leucine can activate the MTORC pathway, leading to excessive proliferation of keratinocytes. Additionally, IL-17A enhances GLS1 expression via the MALT1/c-Jun pathway in keratinocytes, resulting in hyperproliferation of keratinocytes.
Fatty acid metabolism: Lipidomic analysis reveals significant alterations in plasma glycerophospholipid, such as LPA increased in psoriatic patients. Psoriatic lesions exhibit high levels of arachidonic acid (AA) and linoleic acid (LA), which both contribute to excessive fatty acid oxidation-induced inflammation and keratinocyte proliferation.
2. Glucose metabolism
Glucose metabolism not only provides ATP for cell functions, but also serves as an important carbon source for the synthesis of lipids and non-essential amino acids, thereby supporting the continuous growth of cells. Several studies have demonstrated an association of psoriasis with abnormal glucose metabolism.
2.1. Changes in glucose levels in circulation
Large number of epidemiological studies has shown that psoriasis predisposes to the development of type 2 diabetes, with a positive relationship between the severity of psoriasis and the risk of type 2 diabetes (Citation28). In addition, the blood glucose levels are significantly higher in psoriasis than healthy (Citation29). A large cohort study showed that psoriasis increases susceptibility to developing type 2 diabetes, possibly mediated by pro-inflammatory factors and this distinction is not restricted to the elderly, but also seen in the younger age groups (Citation30). Similarly, a meta-analysis revealed a higher risk of diabetes mellitus, insulin resistance, and metabolic syndrome especially in severe psoriasis (Citation31,Citation32). Interestingly, treatment of psoriasis with acitretin lowers both blood glucose levels in Chinese patients (Citation29), supporting the link between psoriasis and metabolic disorders.
The primary pathogenic factor underlying the development of type 2 diabetes is insulin resistance. Evidence suggests the contribution of inflammatory factors to the development of insulin resistance (Citation33). In psoriasis, activated bone marrow dendritic cells produce IL-23 and IL-12, promoting the proliferation and differentiation of Th22, Th17, and Th1 cells, resulting in an overproduction of pro-inflammatory cytokines, including interferon-γ (IFN-γ), IL-1, IL-6, IL-12, IL-17, IL-22, IL-23, and TNF-α (Citation34). These proinflammatory cytokines enter the circulation, a phenomenon referred to as the ‘inflammatory skin march’. The release of these pro-inflammatory factors can trigger chronic systemic inflammation, thereby causing obesity, hypertension, insulin resistance, and Metabolic Syndrome (Citation35).
2.2. Glucose metabolism in psoriatic skin
In psoriatic lesion, keratinocytes are characterized by high levels of glucose metabolism and lactate production (Citation36). In addition to UV-induced hyperplastic skin or during wound healing, psoriatic lesions also exhibit elevated expression levels of glucose transporter (GLUT1) (Citation37, Citation38). In mouse models of psoriasis-like disease, inhibition of GLUT1 decreases inflammation and epidermal proliferation (Citation39). In addition, hypoxia-inducible factor 1α (HIF-1α) promotes glycolysis in psoriasis vulgaris by up-regulating GLUT1 expression (Citation40). Glycolysis is a major source of ATP generation, supporting rapid proliferation in many cells (Citation41). Both single-cell transcriptome profile and RNA-seq data reveal higher expression levels of hexokinase 2 (HK2), lactate dehydrogenase (LDH) and pyruvate kinase isoform (PKM2), the enzymes required for aerobic glycolysis (Citation42–44), in the psoriatic epidermis and their expression levels are positively correlated with the extent of keratinocyte proliferation (Citation45, Citation46). The CD147 gene has been identified as a psoriasis susceptibility gene for. Recent studies have revealed that CD147 upregulates glucose uptake through its interaction with GLUT1, thereby enhancing glycolytic activity and exacerbating inflammation in psoriasis (Citation47).
2.3. Benefit of antidiabetic drugs for psoriasis
2.3.1. Thiazolidinediones (TZDs)
Several double-blind randomized controlled trials (RCTs) have demonstrated the benefit of TZDs for psoriasis, particularly pioglitazone. TZD is an important class of peroxisome proliferator-activated receptor-gamma (PPAR-γ) agonists, which are widely employed as insulin sensitizers for the management of diabetes (Citation48, Citation49). Oral administration of pioglitazone () results in ≥75% improvement compared to baseline (PASI 75) in the Psoriasis Area and Severity Index (PASI) score (Citation50–52). In terms of safety, pioglitazone treatment does not increase the incidence of adverse reactions, such as elevated hepatic transaminase levels, weight increased, fatigue and nausea. This evidence suggests that pioglitazone is safe and effective for psoriasis. The underlying mechanisms accounting for PPARγ activator-induced improvement in psoriasis can be attributed to inhibition of keratinocyte proliferation and cutaneous inflammation, stimulation of keratinocyte differentiation (Citation53, Citation54), and improvement in the lipid profile by reducing triglycerides, fatty acids, and LDL levels while increasing HDL levels (Citation55) ().
Table 1. Antidiabetic drugs for psoriasis.
2.3.2. GLP-1-based therapies
Glucagon-like peptide 1 (GLP-1) receptor agonists and dipeptidyl peptidase-4 (DPP-4) inhibitors are effective hypoglycemic agents. GLP-1 is an incretin hormone that regulates glucose metabolism, while DPP-4 is a degrading enzyme of GLP-1 and reduces blood glucose levels via the activation of GLP-1 (Citation56). Studies have demonstrated that GLP-1 receptor agonists (GLP-RA) can ameliorate psoriasis symptoms by direct or indirect immunomodulatory effects, leading to anti-inflammatory responses. GLP-1RA is effective for the treatment of psoriasis in patients with T2DM (). The underlying mechanisms of GLP-1RA action include the reductions in γ, δ, and T cells within psoriatic lesions as well as the suppression of IL-17 secretion, thereby attenuating inflammatory responses (Citation57) (). Moreover, GLP-1RA exhibits the ability to augment circulating levels of constant natural killer T cells, while simultaneously decreasing psoriasis plaque area (Citation57). Furthermore, a series of small-scale randomized controlled trials have demonstrated the potential of GLP-1RA in reducing body weight, improving visceral fat distribution, and inhibiting adipokine activity (Citation58), thereby offering promising adjunctive treatment for psoriasis through weight loss (). In addition, GLP-1RA can effectively suppress the inflammatory response by elevating plasma adiponectin levels. Thus, synergistic hypoglycemic and weight-reducing effects are additional mechanisms whereby GLP-1RA improves psoriasis with T2DM. GLP-1 analogues can also mitigate the severity of psoriasis by interplay with the innate immune system, particularly natural killer T cells (Citation59–61).
2.3.3. Metformin
Metformin, a synthetic biguanide, is widely prescribed as an oral glucose-lowering medication for the treatment of type 2 diabetes (Citation62). Clinical data showed that metformin alleviates psoriasis by modulating the immune response. Metformin exerts insulin-sensitizing effects and anti-inflammatory properties, and inhibits keratinocyte over proliferation through the activation of adenosine monophosphate-activated protein kinase (AMPK) (). The dose-response analysis has confirmed the safety profile of metformin in psoriasis with diabetes (Citation63, Citation64). One randomized controlled trial (RCT) has been conducted metformin are effective for treatment of psoriatic patients with metabolic syndrome (Citation65). A statistically significant improvement was observed in the mean percentage change of erythema, scaling, and induration (ESI) (p = 0.048), as well as in the mean weight, body mass index (BMI) (), waist circumference (WC), fasting plasma glucose (FPG), serum triglycerides, total cholesterol, and low-density lipoprotein cholesterol (LDL-C) levels in psoriasis treated with metformin compared to placebo.
2.4. Other antidiabetic drugs
Sodium-glucose co-transporter 2 inhibitor (SGLT2i) is a novel class of antidiabetic drug that enhances urinary glucose excretion by inhibiting glucose reabsorption in the proximal renal tubule (Citation66). Wu et al. first performed a nationwide population-based cohort study to elucidate between SGLT2i treatment and psoriasis. This study found that people with kidney disease who were treatment with SGLT2i have an increased risk of developing psoriasis (Citation67) (). The administration of SGLT2i therapy may elevate the susceptibility to psoriasis via two distinct mechanisms (). Firstly, considering the effects of sodium and water balance, this therapeutic approach might potentially induce dehydrated skin, dryness, pruritus, and inflammation (Citation68). However, an alternative study suggested that SGLT2i therapy may induce psoriasis by impairing uric acid excretion and resulting in its skin accumulation.
Therefore, not all diabetes drugs may have beneficial adjunctive effects on psoriasis, and clinical trials are needed to validate and tailor treatment to individual patient circumstances (Citation69).
3. Lipid metabolism
Lipids not only serve as the primary constituents of cell membrane bilayers, but also play a crucial role in regulating diverse biological processes, including cell proliferation, apoptosis, and inflammation (Citation70). The prevalence of dyslipidemia is higher psoriatic patients than in the controls. A review of several cross-sectional and a case-control study revealed that psoriasis is significantly associated with dyslipidemia, evidenced by a positive correlation between these two conditions (Odds ratio of 1.04 to 5.55). Moreover, the rates of dyslipidemia are related to the severity of psoriasis (odds ratio of 1.10 to 3.38 for mild psoriasis and 1.36–5.55 for severe psoriasis) (Citation71). Current evidence suggests that the inflammatory mediators from psoriatic skin lesions can enter the circulatory system, thereby inducing dyslipidemia through perturbation of fat metabolism (Citation72). Accumulating evidence indicates that dyslipidemia is a significant risk factor for the development of psoriasis, exacerbates existing cases, and underscores the potential benefits of weight reduction in ameliorating its severity among overweight individuals (Citation73).
3.1. Altered lipid profile in the circulation
The levels of total cholesterol, LDL, and VLDL in the plasma of psoriatic patients are significantly higher than healthy (Citation74, Citation75). A study on 70 psoriatic patients with an average PASI score of 16.65 revealed that the prevalence of dyslipidemia was 62.85% in psoriatic patients. The common lipid abnormalities are hypertriglyceridemia and combination of hypertriglyceridemia and reduced HDL levels (Citation76). Similarly, glycerophospholipid metabolism is altered in psoriatic patients, manifested by the levels of lysophosphatidylcholine (LysoPC), lysophosphatidic acid (LPA), and phosphatidic acid (PA) were elevated, while the levels of phosphatidylcholine (PC) and phosphatidylinositol (PI) were reduced (Citation77). The LPC class of lipids constitutes the major part of human plasma lipids. LPC can act as ligands for specific G protein-coupled signaling receptors and is also a precursor for lysophosphatidic acid (LPA). LPC exerts proinflammatory effects through various signaling pathways (nuclear factor kappa B, protein kinase C, and extracellular-signal-regulated kinase pathways) (Citation78, Citation79). Induction of LPC can enhance glycolytic activity in psoriasis. The LPC/G2A/STAT1 axis significantly induces glycolytic activity and promotes the production of IL-1b in keratinocytes, thereby indirectly promoting Th17 differentiation (Citation80). The transcription factor STAT1 plays a crucial role in the regulation of key glycolytic genes, including ENO1, PDK3, and HK2 (Citation80,Citation81). In addition, LPA serves as an intermediate in the biosynthetic pathway of cardiolipin, a crucial dimeric phospholipid. The binding of LPA to its specific receptors participates in cell differentiation and proliferation (Citation82). In psoriasis, LPA enhances aerobic glycolysis by upregulating the expression of glycolytic enzyme, PGAM1, through AKT/MTOR/HIF-1𝛼. In HaCaT cells, knockdown of PGAM1 inhibits LPA-induced cell proliferation and aerobic glycolysis (Citation83).
Studies have shown a consistent downward trend in unsaturated fatty acids in the serum lipid metabolites of patients with psoriasis. These fatty acids positively impact the lipid profile by increasing high-density lipoprotein cholesterol levels while simultaneously reducing serum triglycerides, total cholesterol, and low-density lipoprotein cholesterol. Moreover, clinical studies have shown that intravenous infusions in conjunction with oral supplementation of omega-3 fatty acids significantly improve psoriasis (Citation84). Linolenic acid, a member of the omega-3 family, is classified as a polyunsaturated fatty acid. It exhibits remarkable anti-inflammatory properties attributable to its ability to inhibit TNF-alpha, inducible nitric oxide synthase, and cyclooxygenase-2 (Citation85).
3.2. Lipid profile in the skin lesion
The levels of unsaturated fatty acids in psoriatic lesions exhibit significant alterations. Lipoxygenases (LOX) are abundant and produce monohydroxy derivatives from arachidonic acid and linoleic acid (9-and13-hydroxyoctadecadienoic acid [HODE]) (Citation86). Camp et al. reported that psoriatic lesion had higher 13(S)- HODE than healthy skin (Citation87). Moreover, psoriatic skin overexpresses E-FABP, while deletion of E-FABP decreases cellular linoleic acid derivative 13(S)-HODE mediated by NF-kB pathway, and consequently decreasing K1 expression (Citation88).
Choline not only involved in the synthesis of bioactive lipids but also provide the energy for cell growth and division. Elevated choline levels in psoriatic lesions promoted keratinocyte hyperproliferation (Citation89).
3.3. Benefit of lipid-lowering drugs for psoriasis
Several studies showed the benefit of lipid-lowering agents for psoriasis (see below). Statins and fibrates are the most frequently prescribed pharmacological agents for managing dyslipidemia in clinical practice.
3.3.1. Statin
Statin is an inhibitor of cholesterol synthesiss. Several studies have demonstrated the potential of statins in reducing LDL levels and lowering PASI score (Citation90) (). The therapeutic effects of statins on psoriasis are by reducing the expression levels of inflammatory mediators. Fluvastatin and simvastatin exert anti-inflammatory effects by inhibiting the release of chemokine CCL20 in psoriasis, thereby further suppressing the interaction between CCL20 and CCR6 (Citation91) (). Both mevastatin and atorvastatin exhibit inhibitory effects on NF-kB activation, thereby suppressing the release of inflammatory factors such as TNF-a and chemokines (Citation92) ().
Table 2. Benefit of lipid-lowering drugs for psoriasis.
3.3.2. Fibrates
Fibrates, activators of PPARα, can decrease circulating levels of triglyceride, LDL, and VLDL, while increasing HDL. In addition, fibrates can reduce inflammation through transcriptional regulation (Citation93) (). The benefits of fibrates for psoriasis have not been well studied yet. One study on two psoriatic patients with hypertriglyceridemia showed that administration of clofibrate at a daily dose of 750 mg lowered circulating triglyceride levels and attenuated endothelial cell swelling and dermal capillary hyperplasia (Citation94). Further clinical studies are needed to assess the benefits of fibrates for psoriasis.
3.4. Other treatment
3.4.1. Adjust eating habits
Research has shown that adjusting dietary habits can improve symptoms of psoriasis. Zhou et al. found that a high-fat diet can cause an increase in IL-1, IL-6, and TNF-α, promoting the development of psoriasis. A study on mice models induced by imiquimod has found that a high-fat diet activates T cells to produce IL-17, especially affecting intestinal permeability, and can activate monocytes, macrophages, and dendritic cells to excessively proliferate, even inducing keratinocyte activation to exacerbate the progression of psoriasis (Citation95). Therefore, adopting a healthy diet can be beneficial for obese patients with psoriasis in terms of ameliorating both obesity and psoriasis symptoms. The healthy eating habits encompass low-calorie diets, mediterranean diets, and gluten-free diets (Citation96). Furthermore, it is strongly advised that individuals with psoriasis cease smoking and alcohol consumption, while actively engaging in regular physical exercise. Above lifestyle can enhance the efficacy of pharmacological interventions and promote long-term remission of this condition.
3.4.2. Bariatric surgery
Research findings indicated that bariatric surgery can ameliorate symptoms in psoriasis patients, as it effectively reduces the inflammatory activity of T cells, and improved insulin sensitivity thereby mitigating the systemic inflammatory response associated with psoriasis (Citation97). Egeberg et al. study demonstrated that undergone bariatric surgery have a significantly reduced risk of developing psoriasis (Citation98). However, further clinical trials are still necessary to validate this therapeutic approach more comprehensively.
4. Amino acid metabolism
4.1. Altered circulating levels of amino acids
In comparison to healthy, psoriatic patients display 23 up-regulated (including essential amino acids and branched-chain amino acids) and 14 down-regulated amino acids (including glutamine, cysteine and asparagine) in the plasma. Branched-chain amino acids (BCAAs), including leucine, isoleucine and valine, can enhance oxidative stress and inflammation via activation of mTORC1 (Citation99).
4.2. Altered amino acid content in the skin lesions
In addition to the circulation, amino acid levels in the skin also differ between psoriatic patients and the healthy. Glutamine levels are lower in the psoriasis-involved skin than in the skin of normal healthy individuals. In contrast, the levels of LAT1 (L-type amino acid transporter1) are increased in both keratinocytes and lymphocytes from the psoriasis-involved skin. The low glutamine levels could be due to increased consumption, resulting from increased proliferation of immune cells and keratinocytes. Interestingly, cutaneous production of alpha-ketoglutarate is higher in patients with psoriasis vulgaris, but lower in psoriatic arthritis (Citation100). The alpha-ketoglutarate is involved in both proline synthesis, which is a substrate for collagen synthesis, and the supply of oxidative energy to the tricarboxylic acid cycle. Thus, the differential expression of alpha-ketoglutarate in the psoriasis-involved skin and psoriatic arthritis can contribute to increased keratinocyte proliferation and decreased collagen synthesis (Citation101).
In psoriasis, abnormal activation of glutaminase 1–mediated (GLS1) induces overconsumption of glutamine. IL-17A enhances GLS1 expression in keratinocytes through the MALT1/cJun pathway, resulting in keratinocyte hyperproliferation and increased chemokine production. Inhibition of either GLS1 or MALT1 protease suppresses Th17 and γδ T17 cell differentiation and epidermal hyperplasia in a mouse model of psoriasis-like dermatitis (Citation102). In addition, increased arginase 1 in PP6-deficient keratinocytes drives the production of polyamines in the urea cycle. Polyamines enhance toll-like receptor 7 (TLR7) dependent RNA sensing and IL-6 production. Arginase inhibitors alleviate skin inflammation in psoriasis mouse model (Citation103).
5. Interconnection between metabolomics
Rapamycin (mTOR), a serine/threonine kinase, regulates cell proliferation, differentiation and apoptosis, as well as metabolism. Its upstream pathway includes PI3K-Akt, LKB1/CD73-AMPK and others. There are two distinct types of mTOR, mTORC1 and mTORC2 (Citation104). The mTORC1, comprising mTOR, mLST8, Raptor, PRAS40, and Deptor, is sensitive to rapamycin. The targets of MTORC1 encompass key pathway proteins, including eukaryotic translation initiation factor 4E-binding protein 1 (4EBP1), ribosomal protein S6 kinase 1 (S6K1/P70S6K) and autophagy precursor protein UNC-51-like kinase 1 (ULK1) (Citation105). As a central regulator of metabolic and inflammatory signaling pathways, mTORC1 is intricately involved in the modulation of gene expression in various cell types and mediators, including cyclin D1 (CyclinD1), hypoxia-inducible factor 1𝛼 (HIF-1𝛼) and c-myc. Thus, it plays a pivotal role in governing cellular processes, such as proliferation, differentiation, apoptosis, angiogenesis, and other vital biological functions (Citation106). The mTORC2 complex primarily comprises mTOR, Rictor, mSIN1, Protor, mLST8, and Deptor. It is insensitive to rapamycin and actively participates in the regulation of apoptosis and migration through signaling pathway proteins, such as phosphorylated protein kinase B (Akt), protein kinase C (PKC), and serum/glucocorticoid-regulated kinase 1 (SGK1) (Citation107).
In chronic inflammatory dermatoses, such as psoriasis and AD, metabolic pathways can regulate the activation and differentiation of both immune cells and keratinocytes mainly through the mTOR pathway. Both S6K1 (a downstream signaling molecule of mTORC1) and total mTOR are overexpressed in psoriasis-involved skin (Citation108). Likewise, psoriasis-involved skin displays higher expression levels of Ser2448 and Ser2481, which are mTORC1 and mTORC2 agonists, respectively (Citation109). In addition, expression levels of mTORC1, S6K1, and P-S6K1 are increased in both the epidermis and the dermis in a model of the IMQ-induced psoriasis-like dermatitis. The pathogenic role of mTORC2 in psoriasis is also evidenced by alleviation of IMQ-induced psoriasis-like dermatitis in mTORC2-ko mice (Citation110). Thus, the mTOR pathway is highly enriched in psoriasis and may represent a therapeutic target for psoriasis.
Hypoxia inducible factor (HIF) transcription factor family is integrated by 3 a molecules and one b molecule, called HIF-1𝛼, HIF-2𝛼, HIF-3𝛼, and HIF-1𝛽 (Citation111). MTORC1 can activate HIF-1α. The latter upregulates expression levels of glycolysis-related genes, such as GLUT1, HK, and LDHA, resulting in M1 polarization of Macrophages (Citation112). The expression levels of HDAC-1, HIF-1𝛼 and VEGF are increased in psoriasis-involved skin (Citation113–115), while HIF-1α-mediated BMP6 suppression is associated keratinocyte hyperproliferation and abnormal differentiation (Citation116). Under hypoxic conditions, miR-150 regulates keratinocyte proliferation by targeting HIF-1α and VEGFA (Citation117).
A study showed that IL-1β, IL-17A, and TNF-α stimulate the mTOR pathway through the PI3K signaling pathway, resulting in enhanced proliferation of psoriatic keratinocytes (Citation118). Another study showed that mTOR causes serine/threonine phosphorylation of IRS by activating S6K and reduces it’s phosphorylate ability at tyrosine residues, leading to damaged insulin signaling and insulin resistance (Citation119). MTORC1 can activate HIF-1α.The latter upregulates expression levels of glycolysis-related genes, such as GLUT1, HK, and LDHA (Citation112). In psoriasis, LPA enhances aerobic glycolysis by upregulating the expression of glycolytic enzyme, PGAM1, through AKT/mTOR/HIF-1α/LPAR1 axis. Knockout of HIF-1α inhibits LPA-induced PGAM1 expression and aerobic glycolysis in HaCaT cells (Citation83). LPS-induced TREM-1 increases HIF-1a expression in HaCaT cells through the PI3 K/Akt pathway (Citation120). The aforementioned findings suggest that the link between psoriasis and enhanced glycolytic activity is likely mediated by MTORC1. MTORC1 can be activated by a variety of factors, including elevated levels of leucine, LPA and LPS in the plasma of psoriatic patients, and upregulation of amino acid transporter (SLC7A5) in skin lesions. Therefore, MTORC1 can be another target for the treatment of psoriasis.
6. Discussion
A number of basic and clinical studies have shown that psoriasis is a metabolism-related disease characterized by abnormal glucose, amino acid, and lipid metabolisms. Enhanced glycolysis, glutamine metabolism, and altered fatty acid composition in the psoriatic lesion and plasma result in the excessive proliferation of keratinocytes and secretion of inflammatory cytokines. Altered metabolism is associated with the activation of MTORC signaling pathway and transcription factors such as HIF and S6K1. Therefore, MTORC1 can be a target for the treatment of psoriasis.
The coexistence of psoriasis and metabolism-related disease can lead to complex interactions, posing a significant therapeutic challenge. Common metabolism-related diseases include diabetes, hyperlipidemia, hypertension, obesity, and metabolic syndrome. Methotrexate () is the most frequently used traditional systemic medication for psoriasis. A study showed that, regardless of whether the patient has a metabolic disorder; there is no significant change in glycated hemoglobin (Hb1Ac) levels after 12 weeks of methotrexate treatment (Citation121). Cyclosporine (), another pharmacological agent employed in the management of systemic psoriasis, had been found to potentially exacerbate lipid and glucose levels in the bloodstream based on research findings (Citation122). Acitretin (), an anti-psoriatic medication, exhibits significant metabolic interactions, particularly in relation to hyperlipidemia where it may induce hypertriglyceridemia (Citation123). Otherwise, it was reported that the Acitretin can reduce blood glucose levels (Citation29).
Table 3. Classic oral drugs and biologicals for psoriasis.
However, with the advancement of research on the mechanisms of the immune system and the development of biologics, there has been a fundamental transformation in the management of psoriasis. Biological agents have demonstrated remarkable efficacy in the management of psoriasis, leading to complete resolution of cutaneous lesions. But, a part of psoriasis patients exhibit resistance or inadequate response to treatment. In a recent study, 290 Chinese patients with moderate to severe plaque psoriasis were administered biological therapy, including adalimumab, ixekizumab, secukinumab, ustekinumab, and guselkumab. Iinsulin resistance levels were evaluated using the triglyceride-glucose-body mass index (TyG-BMI) index (Citation24). The results revealed a significantly higher incidence of diabetes, BMI, fasting blood sugar, and triglyceride levels in the high insulin resistance (IR) group compared to the low IR group (p < 0.05), and a low ration patient in the high insulin resistance group achieved a 75% or more improvement in PASI (PASI 75), a 90% or more improvement in PASI (PASI 90) and a PGA score of ‘clear’ or ‘almost clear’ (PGA 0/1) after 12 weeks of treatment. Therefore, they speculated that Insulin resistance is associated with lower biologics response in moderate to severe plaque psoriasis patients in China. Additionally, the study revealed that a 12-week treatment regimen of biological agents did not yield significant improvements in reducing insulin resistance. Otherwise, it was reported that TNF-α inhibitors may be associated with an increased risk of weight gain, while other studies have demonstrated their favorable impact on psoriasis-related lipid metabolism and insulin resistance (Citation124) (). Ustekinumab treatment for 24 weeks may increase body mass index, fasting blood sugar, and triglyceride levels (Citation125). Thus, the impact of biologics agents on metabolic parameters necessitates further validation.
For psoriasis who also presents insulin resistance diabetes, it is crucial to actively manage glycemic control. Patients may choose antidiabetic drugs that demonstrate efficacy in addressing both conditions. It contains classic diabetes medications (TZDs, GLP-1 RAs, and Metformin). The mechanisms of antidiabetic drugs that improve psoriasis may be by increasing insulin sensitivity or attenuating inflammatory factors. Additionally, patients with psoriasis-associated dyslipidemia should receive treatment with lipid-lowering medications and dietary modifications to effectively manage lipid metabolism. Clinically effective lipid-lowering agents for psoriasis include statins and fibrates. The mechanisms of lipid-lowering drugs may be related to decrease circulating levels of VLDL or LDL, inhibition of CCL20–CCR6 interaction, and reduction in the levels of inflammatory factors. However, large-scale, well-designed studies are imperative to further evaluate the efficacy and safety of intervention of metabolism for psoriasis.
In clinical practice, control of metabolic diseases enhances the efficacy of psoriasis treatment, and in some cases, remarkable improvement in psoriasis condition can be achieved solely through topical treatment or local phototherapy even without the need for systemic intervention. Therefore, for patients with psoriasis accompanied by metabolic disorders, a comprehensive approach integrating metabolic therapy with the primary treatment for psoriasis should be implemented. Treatment of psoriasis is the main treatment, adjuvant Metabolic therapy and lifestyle modifications to achieve a holistic and all-encompassing resolution of psoriasis.
Authors’ contributions
Y.Y.L conceived the structure of manuscript and wrote the manuscript, J.Q.L contributed to conception, review and editing. Y.W and A.H.P collected materials. All authors contributed to manuscript revision, read, and approved the submitted version.
Acknowledgments
This work was done in Shanxi Key Laboratory of Stem Cells for Immunological Dermatosis, Institute of Dermatology, Taiyuan Central Hospital of Shanxi Medical University; Taiyuan, China.
Disclosure statement
The authors declare that the paper was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Funding
References
- Jarocka-Karpowicz I, Biernacki M, Wroński A, et al. Cannabidiol effects on phospholipid metabolism in keratinocytes from patients with psoriasis vulgaris. Biomolecules. 2020;10(3):367. doi: 10.3390/biom10030367.
- Rendon A, Schäkel K. Psoriasis pathogenesis and treatment. Int J Mol Sci. 2019;20(6):1475. doi: 10.3390/ijms20061475.
- Orlik C, Deibel D, Küblbeck J, et al. Keratinocytes costimulate naive human T cells via CD2: a potential target to prevent the development of proinflammatory Th1 cells in the skin. Cell Mol Immunol. 2020;17(4):380–394. doi: 10.1038/s41423-019-0261-x.
- Cannavò SP, Riso G, Casciaro M, et al. Oxidative stress involvement in psoriasis: a systematic review. Free Radic Res. 2019;53(8):829–840. doi: 10.1080/10715762.2019.1648800.
- Barygina V, Becatti M, Prignano F, et al. Fibroblasts to keratinocytes redox signaling: the possible role of ros in psoriatic plaque formation. Antioxidants (Basel). 2019;8(11). doi: 10.3390/antiox8110566.
- Xu F, Xu J, Xiong X, et al. Salidroside inhibits MAPK, NF-κB, and STAT3 pathways in psoriasis-associated oxidative stress via SIRT1 activation. Redox Rep. 2019;24(1):70–74. doi: 10.1080/13510002.2019.1658377.
- Lai R, Xian D, Xiong X, et al. Proanthocyanidins: novel treatment for psoriasis that reduces oxidative stress and modulates Th17 and Treg cells. Redox Rep. 2018;23(1):130–135. doi: 10.1080/13510002.2018.1462027.
- Suwanwongse K, Shabarek N. miRNA125b downregulation: a review of the novel paradigm of psoriasis epigenetic regulation. Cureus. 2020;12(1):e6798. doi: 10.7759/cureus.6798.
- Sonkoly E, Wei T, Janson PCJ, et al. MicroRNAs: novel regulators involved in the pathogenesis of psoriasis? PloS One. 2007;2(7):e610. doi: 10.1371/journal.pone.0000610.
- Zhang W, Yi X, An Y, et al. MicroRNA-17-92 cluster promotes the proliferation and the chemokine production of keratinocytes: implication for the pathogenesis of psoriasis. Cell Death Dis. 2018;9(5):567. doi: 10.1038/s41419-018-0621-y.
- Zhao X, Li R, Qiao M, et al. MiR-548a-3p Promotes Keratinocyte Proliferation Targeting PPP3R1 after Being Induced by IL-22. Inflammation. 2018;41(2):496–504. doi: 10.1007/s10753-017-0705-3.
- Wu R, Zeng J, Yuan J, et al. MicroRNA-210 overexpression promotes psoriasis-like inflammation by inducing Th1 and Th17 cell differentiation. J Clin Invest. 2018;128(6):2551–2568. doi: 10.1172/jci97426.
- Wang R, Wang F-F, Cao H-W, et al. MiR-223 regulates proliferation and apoptosis of IL-22-stimulated HaCat human keratinocyte cell lines via the PTEN/Akt pathway. Life Sci. 2019;230:28–34. doi: 10.1016/j.lfs.2019.05.045.
- Wang C, Zong J, Li Y, et al. MiR-744-3p regulates keratinocyte proliferation and differentiation via targeting KLLN in psoriasis. Exp Dermatol. 2019;28(3):283–291. doi: 10.1111/exd.13888.
- Feng S, Wang L, Liu W, et al. MiR-126 correlates with increased disease severity and promotes keratinocytes proliferation and inflammation while suppresses cells’ apoptosis in psoriasis. J Clin Lab Anal. 2018;32(9):e22588. doi: 10.1002/jcla.22588.
- Zheng Y, Cai B, Li X, et al. MiR-125b-5p and miR-181b-5p inhibit keratinocyte proliferation in skin by targeting Akt3. Eur J Pharmacol. 2019;862:172659. doi: 10.1016/j.ejphar.2019.172659.
- Yan JJ, Qiao M, Li RH, et al. Downregulation of miR-145-5p contributes to hyperproliferation of keratinocytes and skin inflammation in psoriasis. Br J Dermatol. 2019;180(2):365–372. doi: 10.1111/bjd.17256.
- Xiao Y, Wang C, Zeng B, et al. miR124-3p/FGFR2 axis inhibits human keratinocyte proliferation and migration and improve the inflammatory microenvironment in psoriasis. Mol Immunol. 2020;122:89–98. doi: 10.1016/j.molimm.2020.04.002.
- Wang Y, Yu X, Wang L, et al. miR-320b is down-regulated in psoriasis and modulates keratinocyte proliferation by targeting AKT3. Inflammation. 2018;41(6):2160–2170. doi: 10.1007/s10753-018-0859-7.
- Tang L, He S, Zhu Y, et al. Downregulated miR-187 contributes to the keratinocytes hyperproliferation in psoriasis. J Cell Physiol. 2019;234(4):3661–3674. doi: 10.1002/jcp.27135.
- Pan M, Huang Y, Zhu X, et al. miR-125b-mediated regulation of cell proliferation through the Jagged-1/Notch signaling pathway by inhibiting BRD4 expression in psoriasis. Mol Med Rep. 2019;19(6):5227–5236. doi: 10.3892/mmr.2019.10187.
- Li R, Qiao M, Zhao X, et al. MiR-20a-3p regulates TGF-β1/Survivin pathway to affect keratinocytes proliferation and apoptosis by targeting SFMBT1 in vitro. Cell Signal. 2018;49:95–104. doi: 10.1016/j.cellsig.2018.06.003.
- A R, Yu P, Hao S, et al. MiR-876-5p suppresses cell proliferation by targeting angiopoietin-1 in the psoriasis. Biomed Pharmacother. 2018;103:1163–1169. doi: 10.1016/j.biopha.2018.04.145.
- Huang D, Zhong X, Jiang Y, et al. Insulin resistance impairs biological agents response in moderate-to-severe plaque psoriasis: insights from a prospective cohort study in China. Br J Dermatol. 2024; doi: 10.1093/bjd/ljae147.
- Cibrian D, Fuente H, Sánchez-Madrid F. Metabolic pathways that control skin homeostasis and inflammation. Trends Mol Med. 2020;26(11):975–986. doi: 10.1016/j.molmed.2020.04.004.
- Lian N, Shi L-Q, Hao Z-M, et al. Research progress and perspective in metabolism and metabolomics of psoriasis. Chin Med J (Engl). 2020;133(24):2976–2986. doi: 10.1097/cm9.0000000000001242.
- Mamizadeh M, Tardeh Z, Azami M. The association between psoriasis and diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Syndr. 2019;13(2):1405–1412. doi: 10.1016/j.dsx.2019.01.009.
- Friis NU, Hoffmann N, Gyldenløve M, et al. Glucose metabolism in patients with psoriasis. Br J Dermatol. 2019;180(2):264–271. doi: 10.1111/bjd.17349.
- Qian H, Kuang Y, Su J, et al. Reductive effect of acitretin on blood glucose levels in Chinese patients with psoriasis. Front Med (Lausanne). 2021;8:764216. doi: 10.3389/fmed.2021.764216.
- Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and the risk of diabetes mellitus: a systematic review and meta-analysis. JAMA Dermatol. 2013;149(1):84–91. doi: 10.1001/2013.jamadermatol.406.
- Ghiasi M, Nouri M, Abbasi A, et al. Psoriasis and increased prevalence of hypertension and diabetes mellitus. Indian J Dermatol. 2011;56(5):533–536. doi: 10.4103/0019-5154.87149.
- Sommer DM, Jenisch S, Suchan M, et al. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol Res. 2006;298(7):321–328. doi: 10.1007/s00403-006-0703-z.
- Urakaze M, Kobashi C, Satou Y, et al. The beneficial effects of astaxanthin on glucose metabolism and modified low-density lipoprotein in healthy volunteers and subjects with prediabetes. Nutrients. 2021;13(12):4381. doi: 10.3390/nu13124381.
- Alcantara C, Reiche EMV, Simão ANC. Cytokines in psoriasis. Adv Clin Chem. 2021;100:171–204. doi: 10.1016/bs.acc.2020.04.004.
- Davidovici BB, Sattar N, Prinz JC, et al. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol. 2010;130(7):1785–1796. doi: 10.1038/jid.2010.103.
- Cruickshank CN, Trotter MD. The oxygen uptake, glucose utilization and lactic acid production of guinea-pig skin in relation to oxygen tension. Biochem J. 1956;62(1):57–61. doi: 10.1042/bj0620057.
- Tao J, Yang J, Wang L, et al. Expression of GLUT-1 in psoriasis and the relationship between GLUT-1 upregulation induced by hypoxia and proliferation of keratinocyte growth. J Dermatol Sci. 2008;51(3):203–207. doi: 10.1016/j.jdermsci.2008.04.012.
- Elson DA, Ryan HE, Snow JW, et al. Coordinate up-regulation of hypoxia inducible factor (HIF)-1alpha and HIF-1 target genes during multi-stage epidermal carcinogenesis and wound healing. Cancer Res. 2000;60(21):6189–6195. https://www.ncbi.nlm.nih.gov/pubmed/11085544.
- Zhang Z, Zi Z, Lee EE, et al. Differential glucose requirement in skin homeostasis and injury identifies a therapeutic target for psoriasis. Nat Med. 2018;24(5):617–627. doi: 10.1038/s41591-018-0003-0.
- Tang W, Long T, Li F, et al. HIF-1α may promote glycolysis in psoriasis vulgaris via upregulation of CD147 and GLUT1. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2021;46(4):333–344. doi: 10.11817/j.issn.1672-7347.2021.200010.
- Lunt SY, Heiden MGV. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27(1):441–464. doi: 10.1146/annurev-cellbio-092910-154237.
- Weh E, Lutrzykowska Z, Smith A, et al. Hexokinase 2 is dispensable for photoreceptor development but is required for survival during aging and outer retinal stress. Cell Death Dis. 2020;11(6):422. doi: 10.1038/s41419-020-2638-2.
- Zhang R, Shen W, Du J, et al. Selective knockdown of hexokinase 2 in rods leads to age-related photoreceptor degeneration and retinal metabolic remodeling. Cell Death Dis. 2020;11(10):885. doi: 10.1038/s41419-020-03103-7.
- Dayton TL, Jacks T, Heiden MGV. PKM2, cancer metabolism, and the road ahead. EMBO Rep. 2016;17(12):1721–1730. doi: 10.15252/embr.201643300.
- Demeter JB, Elshaarrawi A, Dowker-Key PD, et al. The emerging role of PKM in keratinocyte homeostasis and pathophysiology. Febs J. 2023;290(9):2311–2319. doi: 10.1111/febs.16700.
- Liu Y-Z, Xu M-Y, Dai X-Y, et al. Pyruvate kinase M2 mediates glycolysis contributes to psoriasis by promoting keratinocyte proliferation. Front Pharmacol. 2021;12:765790. doi: 10.3389/fphar.2021.765790.
- Chen C, Yi X, Liu P, et al. CD147 facilitates the pathogenesis of psoriasis through glycolysis and h3k9me3 modification in keratinocytes. Research (Wash D C). 2023;6:0167. doi: 10.34133/research.0167.
- Ghiasi M, Ebrahimi S, Lajevardi V, et al. Efficacy and safety of pioglitazone plus phototherapy versus phototherapy in patients with plaque type psoriasis: a double blinded randomized controlled trial. J Dermatolog Treat. 2019;30(7):664–667. doi: 10.1080/09546634.2018.1544702.
- Hafez VG, Bosseila M, Halim MREA, et al. Clinical effects of "pioglitazone", an insulin sensitizing drug, on psoriasis vulgaris and its co-morbidities, a double blinded randomized controlled trialx1. J Dermatolog Treat. 2015;26(3):208–214. doi: 10.3109/09546634.2014.932324.
- Chi C-C, Lee C-Y, Liu C-Y, et al. Effects of antidiabetic drugs on psoriasis: a meta-analysis. Eur J Clin Invest. 2021;51(2):e13377. doi: 10.1111/eci.13377.
- Chang G, Wang J, Song J, et al. Efficacy and safety of pioglitazone for treatment of plaque psoriasis: a systematic review and meta-analysis of randomized controlled trials. J Dermatolog Treat. 2020;31(7):680–686. doi: 10.1080/09546634.2019.1610552.
- Chen P, Chen X, Lei L, et al. The efficacy and safety of pioglitazone in psoriasis vulgaris: a meta-analysis of randomized controlled trials. Medicine (Baltimore). 2020;99(32):e21549. doi: 10.1097/md.0000000000021549.
- Demerjian M, Man M-Q, Choi E-H, et al. Topical treatment with thiazolidinediones, activators of peroxisome proliferator-activated receptor-gamma, normalizes epidermal homeostasis in a murine hyperproliferative disease model. Exp Dermatol. 2006;15(3):154–160. doi: 10.1111/j.1600-0625.2006.00402.x.
- Mao-Qiang M, Fowler AJ, Schmuth M, et al. Peroxisome-proliferator-activated receptor (PPAR)-gamma activation stimulates keratinocyte differentiation. J Invest Dermatol. 2004;123(2):305–312. doi: 10.1111/j.0022-202X.2004.23235.x.
- Ramot Y, Mastrofrancesco A, Camera E, et al. The role of PPARγ-mediated signalling in skin biology and pathology: new targets and opportunities for clinical dermatology. Exp Dermatol. 2015;24(4):245–251. doi: 10.1111/exd.12647.
- Mirabelli M, Chiefari E, Puccio L, et al. Potential benefits and harms of novel antidiabetic drugs during COVID-19 crisis. Int J Environ Res Public Health. 2020;17(10):3664. doi: 10.3390/ijerph17103664.
- Buysschaert M, Baeck M, Preumont V, et al. Improvement of psoriasis during glucagon-like peptide-1 analogue therapy in type 2 diabetes is associated with decreasing dermal γδ T-cell number: a prospective case-series study. Br J Dermatol. 2014;171(1):155–161. doi: 10.1111/bjd.12886.
- Nauck MA, Quast DR, Wefers J, et al. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol Metab. 2021;46:101102. doi: 10.1016/j.molmet.2020.101102.
- Drucker DJ, Rosen CF. Glucagon-like peptide-1 (GLP-1) receptor agonists, obesity and psoriasis: diabetes meets dermatology. Diabetologia. 2011;54(11):2741–2744. doi: 10.1007/s00125-011-2297-z.
- Lin L, Xu X, Yu Y, et al. Glucagon-like peptide-1 receptor agonist liraglutide therapy for psoriasis patients with type 2 diabetes: a randomized-controlled trial. J Dermatolog Treat. 2022;33(3):1428–1434. doi: 10.1080/09546634.2020.1826392.
- Faurschou A, Knop FK, Thyssen JP, et al. Improvement in psoriasis after treatment with the glucagon-like peptide-1 receptor agonist liraglutide. Acta Diabetol. 2014;51(1):147–150. doi: 10.1007/s00592-011-0359-9.
- Brauchli YB, Jick SS, Curtin F, et al. Association between use of thiazolidinediones or other oral antidiabetics and psoriasis: a population based case-control study. J Am Acad Dermatol. 2008;58(3):421–429. doi: 10.1016/j.jaad.2007.11.023.
- Su Y-J, Chen T-H, Hsu C-Y, et al. Safety of metformin in psoriasis patients with diabetes mellitus: a 17-year population-based real-world cohort study. J Clin Endocrinol Metab. 2019;104(8):3279–3286. doi: 10.1210/jc.2018-02526.
- Singh S, Bhansali A. Randomized placebo control study of metformin in psoriasis patients with metabolic syndrome (systemic treatment cohort). Indian J Endocrinol Metab. 2017;21(4):581–587. doi: 10.4103/ijem.IJEM_46_17.
- Singh S, Bhansali A. Randomized placebo control study of insulin sensitizers (metformin and pioglitazone) in psoriasis patients with metabolic syndrome (Topical Treatment Cohort). BMC Dermatol. 2016;16(1):12. doi: 10.1186/s12895-016-0049-y.
- Heerspink HJL, Perkins BA, Fitchett DH, et al. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134(10):752–772. doi: 10.1161/circulationaha.116.021887.
- Ma S-H, Wu C-Y, Lyu Y-S, et al. Association between sodium-glucose co-transporter 2 inhibitors and risk of psoriasis in patients with diabetes mellitus: a nationwide population-based cohort study. Clin Exp Dermatol. 2022;47(12):2242–2250. doi: 10.1111/ced.15385.
- Tezuka Y, Sekine O, Hirano A, et al. A prospective, open-label short-term pilot study on modification of the skin hydration status during treatment with a sodium-glucose cotransporter-2 inhibitor. Diabetes Ther. 2021;12(1):431–440. doi: 10.1007/s13300-020-00950-7.
- Ali K, Mohammed SR, Deonarine R, et al. Sodium-glucose co-transporter-2 inhibitor-induced pruritus: itching for answers. Cureus. 2021;13(8):e17573. doi: 10.7759/cureus.17573.
- Yan D, Afifi L, Jeon C, et al. The metabolomics of psoriatic disease. Psoriasis (Auckl). 2017;7:1–15. doi: 10.2147/ptt.S118348.
- Ni C, Chiu MW. Psoriasis and comorbidities: links and risks. Clin Cosmet Investig Dermatol. 2014;7:119–132. doi: 10.2147/ccid.S44843.
- Zhang F, Han L, Wang B, et al. Annexin A6 polymorphism is associated with pro-atherogenic lipid profiles and with the downregulation of methotrexate on anti-atherogenic lipid profiles in psoriasis. J Clin Med. 2022;11(23):7059. doi: 10.3390/jcm11237059.
- Kanda N, Hoashi T, Saeki H. Nutrition and psoriasis. Int J Mol Sci. 2020;21(15):5405. doi: 10.3390/ijms21155405.
- Pietrzak A, Chabros P, Grywalska E, et al. Serum lipid metabolism in psoriasis and psoriatic arthritis - an update. Arch Med Sci. 2019;15(2):369–375. doi: 10.5114/aoms.2018.74021.
- Nowowiejska J, Baran A, Flisiak I. Aberrations in lipid expression and metabolism in psoriasis. Int J Mol Sci. 2021;22(12):6561. doi: 10.3390/ijms22126561.
- Salihbegovic EM, Hadzigrahic N, Suljagic E, et al. Psoriasis and dyslipidemia. Mater Sociomed. 2015;27(1):15–17. doi: 10.5455/msm.2014.27.15-17.
- Zeng C, Wen B, Hou G, et al. Lipidomics profiling reveals the role of glycerophospholipid metabolism in psoriasis. GigaScience. 2017;6(10):1–11. doi: 10.1093/gigascience/gix087.
- Ruipérez V, Casas J, Balboa MA, et al. Group V phospholipase A2-derived lysophosphatidylcholine mediates cyclooxygenase-2 induction in lipopolysaccharide-stimulated macrophages. J Immunol. 2007;179(1):631–638. doi: 10.4049/jimmunol.179.1.631.
- Elferink RPJO, Bolier R, Beuers UH. Lysophosphatidic acid and signaling in sensory neurons. Biochim Biophys Acta. 2015;1851(1):61–65. doi: 10.1016/j.bbalip.2014.09.004.
- Liu P, Zhou Y, Chen C, et al. Lysophosphatidylcholine facilitates the pathogenesis of psoriasis through activating keratinocytes and T cells differentiation via glycolysis. J Eur Acad Dermatol Venereol. 2023;37(7):1344–1360. doi: 10.1111/jdv.19088.
- Pitroda SP, Wakim BT, Sood RF, et al. STAT1-dependent expression of energy metabolic pathways links tumour growth and radioresistance to the Warburg effect. BMC Med. 2009;7(1):68. doi: 10.1186/1741-7015-7-68.
- Semba RD, Zhang P, Adelnia F, et al. Low plasma lysophosphatidylcholines are associated with impaired mitochondrial oxidative capacity in adults in the Baltimore longitudinal study of aging. Aging Cell. 2019;18(2):e12915. doi: 10.1111/acel.12915.
- Kim D, Khin PP, Lim OK, et al. LPA/LPAR1 signaling induces PGAM1 expression via AKT/mTOR/HIF-1α pathway and increases aerobic glycolysis, contributing to keratinocyte proliferation. Life Sci. 2022;311(Pt B):121201. doi: 10.1016/j.lfs.2022.121201.
- Trombino S, Servidio C, Laganà AS, et al. Viscosified solid lipidic nanoparticles based on naringenin and linolenic acid for the release of cyclosporine a on the skin. Molecules. 2020;25(15):3535. doi: 10.3390/molecules25153535.
- Ren J, Chung SH. Anti-inflammatory effect of alpha-linolenic acid and its mode of action through the inhibition of nitric oxide production and inducible nitric oxide synthase gene expression via NF-kappaB and mitogen-activated protein kinase pathways. J Agric Food Chem. 2007;55(13):5073–5080. doi: 10.1021/jf0702693.
- Miller CC, Ziboh VA. Induction of epidermal hyperproliferation by topical n-3 polyunsaturated fatty acids on guinea pig skin linked to decreased levels of 13-hydroxyoctadecadienoic acid (13-hode). J Invest Dermatol. 1990;94(3):353–358. doi: 10.1111/1523-1747.ep12874482.
- Camp RD, Mallet AI, Woollard PM, et al. The identification of hydroxy fatty acids in psoriatic skin. Prostaglandins. 1983;26(3):431–447. doi: 10.1016/0090-6980(83)90178-8.
- Ogawa E, Owada Y, Ikawa S, et al. Epidermal FABP (FABP5) regulates keratinocyte differentiation by 13(S)-HODE-mediated activation of the NF-κB signaling pathway. J Invest Dermatol. 2011;131(3):604–612. doi: 10.1038/jid.2010.342.
- Sitter B, Johnsson MK, Halgunset J, et al. Metabolic changes in psoriatic skin under topical corticosteroid treatment. BMC Dermatol. 2013;13(1):8. doi: 10.1186/1471-5945-13-8.
- Trong HN, Tat TN, Anh TTN, et al. Efficacy of adding oral simvastatin to topical therapy for treatment of psoriasis: the Vietnamese experience. Open Access Maced J Med Sci. 2019;7(2):237–242. doi: 10.3889/oamjms.2019.060.
- Kim T-G, Byamba D, Wu WH, et al. Statins inhibit chemotactic interaction between CCL20 and CCR6 in vitro: possible relevance to psoriasis treatment. Exp Dermatol. 2011;20(10):855–857. doi: 10.1111/j.1600-0625.2011.01343.x.
- Kulkarni NM, Muley MM, Jaji MS, et al. Topical atorvastatin ameliorates 12-O-tetradecanoylphorbol-13-acetate induced skin inflammation by reducing cutaneous cytokine levels and NF-κB activation. Arch Pharm Res. 2015;38(6):1238–1247. doi: 10.1007/s12272-014-0496-0.
- Okopień B, Buldak L, Bołdys A. Fibrates in the management of atherogenic dyslipidemia. Expert Rev Cardiovasc Ther. 2017;15(12):913–921. doi: 10.1080/14779072.2017.1408410.
- Imamura T, Takata I, Ogasawara M, et al. Clofibrate treatment of psoriasis with hypertriglycemia--clinical, histological and laboratory analysis. Nihon Hifuka Gakkai Zasshi. 1991;101(6):623–628. https://www.ncbi.nlm.nih.gov/pubmed/1920893.
- Kanemaru K, Matsuyuki A, Nakamura Y, et al. Obesity exacerbates imiquimod-induced psoriasis-like epidermal hyperplasia and interleukin-17 and interleukin-22 production in mice. Exp Dermatol. 2015;24(6):436–442. doi: 10.1111/exd.12691.
- Barrea L, Megna M, Cacciapuoti S, et al. Very low-calorie ketogenic diet (VLCKD) in patients with psoriasis and obesity: an update for dermatologists and nutritionists. Crit Rev Food Sci Nutr. 2022;62(2):398–414. doi: 10.1080/10408398.2020.1818053.
- Villarreal-Calderón JR, Cuéllar RX, Ramos-González MR, et al. Interplay between the adaptive immune system and insulin resistance in weight loss induced by bariatric surgery. Oxid Med Cell Longev. 2019;2019:3940739–3940714. doi: 10.1155/2019/3940739.
- Egeberg A, Sørensen JA, Gislason GH, et al. Incidence and prognosis of psoriasis and psoriatic arthritis in patients undergoing bariatric surgery. JAMA Surg. 2017;152(4):344–349. doi: 10.1001/jamasurg.2016.4610.
- Bonvini A, Coqueiro AY, Tirapegui J, et al. Immunomodulatory role of branched-chain amino acids. Nutr Rev. 2018;76(11):840–856. doi: 10.1093/nutrit/nuy037.
- Armstrong AW, Wu J, Johnson MA, et al. Metabolomics in psoriatic disease: pilot study reveals metabolite differences in psoriasis and psoriatic arthritis. F1000Res. 2014;3:248. doi: 10.12688/f1000research.4709.1.
- Wu N, Yang M, Gaur U, et al. Alpha-ketoglutarate: physiological functions and applications. Biomol Ther (Seoul). 2016;24(1):1–8. doi: 10.4062/biomolther.2015.078.
- Xia X, Cao G, Sun G, et al. GLS1-mediated glutaminolysis unbridled by MALT1 protease promotes psoriasis pathogenesis. J Clin Invest. 2020;130(10):5180–5196. doi: 10.1172/jci129269.
- Lou F, Sun Y, Xu Z, et al. Excessive polyamine generation in keratinocytes promotes self-RNA sensing by dendritic cells in psoriasis. Immunity. 2020;53(1):204–216.e210. doi: 10.1016/j.immuni.2020.06.004.
- Conciatori F, Ciuffreda L, Bazzichetto C, et al. mTOR Cross-talk in cancer and potential for combination therapy. Cancers (Basel). 2018;10(1):23. doi: 10.3390/cancers10010023.
- Suto T, Karonitsch T. The immunobiology of mTOR in autoimmunity. J Autoimmun. 2020;110:102373. doi: 10.1016/j.jaut.2019.102373.
- Aboudehen K. Regulation of mTOR signaling by long non-coding RNA. Biochim Biophys Acta Gene Regul Mech. 2020;1863(4):194449. doi: 10.1016/j.bbagrm.2019.194449.
- Popova NV, Jücker M. The role of mTOR signaling as a therapeutic target in cancer. Int J Mol Sci. 2021;22(4):1743. doi: 10.3390/ijms22041743.
- Buerger C. Epidermal mTORC1 signaling contributes to the pathogenesis of psoriasis and could serve as a therapeutic target. Front Immunol. 2018;9:2786. doi: 10.3389/fimmu.2018.02786.
- Chamcheu JC, Chaves-Rodriquez M-I, Adhami VM, et al. Upregulation of PI3K/AKT/mTOR, FABP5 and PPARβ/δ in human psoriasis and imiquimod-induced murine psoriasiform dermatitis model. Acta Derm Venereol. 2016;96(6):854–856. doi: 10.2340/00015555-2359.
- Lembo S, Caprio RD, Balato A, et al. The increase of mTOR expression is consistent with FoxO1 decrease at gene level in acne but not in psoriasis. Arch Dermatol Res. 2020;312(1):77–80. doi: 10.1007/s00403-019-01959-0.
- Freedman SJ, Sun Z-YJ, Poy F, et al. Structural basis for recruitment of CBP/p300 by hypoxia-inducible factor-1 alpha. Proc Natl Acad Sci U S A. 2002;99(8):5367–5372. doi: 10.1073/pnas.082117899.
- Kim YJ, Lee S, Jin J, et al. Cassiaside C inhibits M1 polarization of macrophages by downregulating glycolysis. Int J Mol Sci. 2022;23(3) doi: 10.3390/ijms23031696.
- Tovar-Castillo LE, Cancino-Díaz JC, García-Vázquez F, et al. Under-expression of VHL and over-expression of HDAC-1, HIF-1alpha, LL-37, and IAP-2 in affected skin biopsies of patients with psoriasis. Int J Dermatol. 2007;46(3):239–246. doi: 10.1111/j.1365-4632.2006.02962.x.
- Rosenberger C, Solovan C, Rosenberger AD, et al. Upregulation of hypoxia-inducible factors in normal and psoriatic skin. J Invest Dermatol. 2007;127(10):2445–2452. doi: 10.1038/sj.jid.5700874.
- Reynoso-Roldán A, Roldán ML, Cancino-Diaz JC, et al. Vascular endothelial growth factor production is induced by histone deacetylase 1 and suppressed by von Hippel-Lindau protein in HaCaT cells. Clin Invest Med. 2012;35(6):E340–350. doi: 10.25011/cim.v35i6.19205.
- Kim JH, Bae HC, Kim J, et al. HIF-1α-mediated BMP6 down-regulation leads to hyperproliferation and abnormal differentiation of keratinocytes in vitro. Exp Dermatol. 2018;27(11):1287–1293. doi: 10.1111/exd.13785.
- Li Y, Su J, Li F, et al. MiR-150 regulates human keratinocyte proliferation in hypoxic conditions through targeting HIF-1α and VEGFA: implications for psoriasis treatment. PloS One. 2017;12(4):e0175459. doi: 10.1371/journal.pone.0175459.
- Varshney P, Saini N. PI3K/AKT/mTOR activation and autophagy inhibition plays a key role in increased cholesterol during IL-17A mediated inflammatory response in psoriasis. Biochim Biophys Acta Mol Basis Dis. 2018;1864(5 Pt A):1795–1803. doi: 10.1016/j.bbadis.2018.02.003.
- Lann D, LeRoith D. Insulin resistance as the underlying cause for the metabolic syndrome. Med Clin North Am. 2007;91(6):1063–1077, viii. doi: 10.1016/j.mcna.2007.06.012.
- Tu C, Wang S, Hu X, et al. Lipopolysaccharide induces TREM-1-dependent HIF-1α expression in human keratinocyte cell line. Cell Biol Int. 2016;40(12):1357–1365. doi: 10.1002/cbin.10693.
- Dehpouri T, Rokni GR, Narenjbon NA, et al. Evaluation of the glycemic effect of methotrexate in psoriatic arthritis patients with metabolic syndrome: a pilot study. Dermatol Reports. 2019;11(1):7965. doi: 10.4081/dr.2019.7965.
- Subramanian S, Trence DL. Immunosuppressive agents: effects on glucose and lipid metabolism. Endocrinol Metab Clin North Am. 2007;36(4):891–905; vii. doi: 10.1016/j.ecl.2007.07.003.
- Pearce DJ, Klinger S, Ziel KK, et al. Low-dose acitretin is associated with fewer adverse events than high-dose acitretin in the treatment of psoriasis. Arch Dermatol. 2006;142(8):1000–1004. doi: 10.1001/archderm.142.8.1000.
- Cai J, Cui L, Wang Y, et al. Cardiometabolic comorbidities in patients with psoriasis: focusing on risk, biological therapy, and pathogenesis. Front Pharmacol. 2021;12:774808. doi: 10.3389/fphar.2021.774808.
- Chiu H-Y, Chang W-L, Shiu M-N, et al. Psoriasis is associated with a greater risk for cardiovascular procedure and surgery in patients with hypertension: a nationwide cohort study. J Dermatol. 2018;45(12):1381–1388. doi: 10.1111/1346-8138.14654.
