Abstract
Introduction
Itch is one of the most burdensome symptoms in epidermolysis bullosa (EB), indicating a hitherto unmet therapeutic need. This review leverages existing data on efficacy of itch treatment in EB to support sound decision making.
Methods
A systematic literature search was performed on 29 March 2022. Studies written later than 1991 and reporting outcomes in patients with EB treated for itch were considered.
Results
Of the 3,099 articles screened, 21 studies met eligibility criteria, comprising 353 patients (65.9%) diagnosed for recessive dystrophic EB. Only two studies (9.5%) evaluated itch as primary endpoint, of which solely one revealed a significant relief of self-reported itch upon topical skin care. In those studies assessing itch as secondary endpoint (19/21, 90.5%), only 36.8% studies (n = 7/19) revealed a statistically significant itch reduction of up to 42%. Methodological limitations (heterogeneity of outcomes, inconsistent data assessment) in addition to limited superiority over control were implicated to account for low treatment efficacy observed in most studies.
Conclusion
Current data quality impairs comparative efficacy analyses of itch treatments in EB. Large scale randomized clinical trials and more personalized approaches applying validated measurement instruments for core outcomes are needed to substantiate evidence-based treatment approaches for EB-associated itch.
Background
Epidermolysis bullosa (EB) comprises a heterogeneous subset of inherited skin fragility disorders with clinical hallmarks including blisters, wounds, and scars, which develop upon minor mechanical trauma (Citation1). Molecular aberrations in at least 16 different genes have hitherto been identified to underlie the classical EB types. These variants cause an impairment of the structural and functional integrity of epithelialised tissues, in particular the epidermis and dermo-epidermal basement membrane zone (BMZ) of the skin, and interfere with key cellular pathways including cell adhesion, differentiation, barrier function and tissue repair (Citation2). Genetic heterogeneity as well as epigenetic, biochemical and environmental factors determine the wide phenotypical variety and severity of EB (Citation3–7). Extracutaneous involvement renders especially the more severe subtypes a systemic disease with multi-organ involvement and high morbidity as well as mortality (Citation8,Citation9).
Itch is one of the most burdensome patient-reported symptoms in EB, causing significant impairment of quality of life (QoL) (Citation10). It affects up to 100% of EB patients (EB simplex, EBS 74%; junctional EB, JEB 100%; dystrophic EB, DEB 93%, i.e., dominant dystrophic EB, DDEB 87.5%, recessive dystrophic EB, RDEB 100%) and usually correlates with disease severity (Citation10–12). In RDEB, mean visual analogue scale (VAS) scores for itch range between seven and eight, thus are comparable to those reported for chronic urticaria, atopic dermatitis and prurigo nodularis (Citation13). Aside from effects of itch on mental health (e.g., sleep disturbances, anxiety, depression), scratching further comprises skin integrity and consequently perpetuates itch through amplification of barrier disruption and release of pro-inflammatory and pruritogenic cytokines and mediators (Citation14–17).
The latter bind to nerve endings in the skin, which themselves promote a neurogenic inflammation (Citation18,Citation19). This distal axon inflammation mediates epidermal fiber damage and loss, and thereby promotes neuropathic itch and pain, stimulating a vicious itch-scratch cycle with negative impact on barrier function and disease severity (Citation19–23).
Against this background, anti-pruritic therapies, through modulation of inflammatory cascades that are induced, dysregulated and chronified in response to the mutation-based disruption of the epidermal barrier, hold potential to improve not only the QoL but also the integrity of skin and mucosa (Citation15,Citation16,Citation24,Citation25).
Indeed, a plethora of pharmacological, behavioral and environmental therapies to treat EB associated itch has been reported in the literature (Citation26,Citation27). Most of these approaches, however, have shown only a moderate or no beneficial effect, underscoring a hitherto unmet therapeutic need (Citation23).
Methodologically, current evidence on treatment modalities for EB largely refers to case reports or case series, which typically reflects inherent challenges in conducting controlled clinical trials in rare disease populations featuring small and heterogeneous sample sizes (Citation23,Citation28). In addition there is a considerable heterogeneity of reported outcomes that poses significant challenges for comparative assessment of available study data (Citation29).
This review systematically evaluates clinical studies of anti-pruritic therapies in EB by summarizing and classifying reported outcomes, interventional impact on QoL and disease severity, as well as safety with the intention to improve the level of scientific and clinical reasoning.
Methods
This research was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (Citation30). The review protocol for reference was registered in PROSPERO (CRD42022348349). Ethical approval was not required for this study.
Eligibility criteria
Comprehensive eligibility criteria were applied following the Population, Intervention, Comparator, Outcome (PICO) framework (Appendix 1) (Citation30). Studies investigating any kind of pharmacological treatment in EB patients that also reported on its impact on symptomatic itch relief, which had been published between 1991, when the first consensus paper on EB terminology and classification had been released, and the search end date of 29 March 2022, were included (Citation31). To account for EB-inherent limitations to high level evidence, retro- and prospective cohort studies, as well as clinical trials were included in analyses. Survey studies were also eligible for inclusion, as they provided standardized data collections of itch as a patient/self-reported outcome in daily care settings. Case reports and case series were not included to focus on reports with standardized procedures and systematic assessments.
Search strategy
We systematically searched the databases MEDLINE (PubMed), EMBASE (embase.com), CINAHL (EBSCO), PsycINFO (EBSCO), Cochrane Central Register of Controlled Trials (CENTRAL) and Web of Science at 29th March 2022. Comprehensive search strategies combining index terms and free text words were developed by two authors (E.W.H.K., S.W.). Searches were limited to articles published between 1991 and 29 March 2022. Animal-only studies, reviews, case reports, case series and meeting abstracts were excluded. The search strategies were reviewed by a second information specialist, using a standardized checklist for Peer Review of Electronic Search Strategies (PRESS) (Citation32). The full search strategies are included in Appendix 2. In addition to the databases searched, cited references of included articles were screened to ensure identification of all potentially eligible studies. The identified citations were uploaded to EndNote 20 (Clarivate Analytics, Philadelphia, USA) and duplicates were removed according to a standardized de-duplication method (Citation30).
Study selection and data extraction
We screened titles, abstracts, and full texts using Rayyan software for consistency with eligibility criteria and performed data extraction along with risk of bias assessment independently in duplicate (T.W. and C.PU.) (Citation33). Titles or abstracts identified as potentially relevant by both screeners were subjected to full-text review. Inter-rater reliability was assessed at each screening stage and disagreements in title or abstract screening, as well as full-text screening, were resolved by discussion between the two screeners and, when required, with a third reviewer (M.L.). Data from all included studies were extracted by two independent reviewers (T.W., C.PU.) by means of a standardized data collection form, which had been developed by the entire review team following a pilot phase. Information on study characteristics, treatment specifications, and outcomes of interest (itch, disease severity, QoL, adverse events), with consideration of the respective measurement instruments, was retrieved from supplementary materials and clinical trial registries. As inconsistent and incomplete data reporting rendered an integrative/pooled statistical assessment or meta-analysis infeasible, statistical data were only indicated if originally reported. Any disagreements between the two reviewers during the extraction process were resolved by discussion with a third reviewer (M.L.).
Risk of bias and level of evidence assessment
Due to the EB-inherent limitations to study design and trial methodology, risk of bias assessment and level of evidence was determined to improve qualitative assessment of individual study results across the study landscape. Risk of bias was categorized as low, high or unclear by two reviewers (T.W., C.PU.) for each study by applying the Cochrane Collaboration’s tool for selection, performance, detection, attrition, and reporting bias (Citation34). Level of evidence was assessed for each study by two reviewers (T.W., C.PU.) according to the Oxford Center for Evidence-Based Medicine (OCEBM) 2011 Levels of Evidence regarding methodological quality (Citation35). Disagreements among reviewers regarding risk of bias or evaluation of evidence were resolved through discussion. If consensus could not be reached, a third reviewer (M.L.) was consulted to make a final decision.
Data synthesis
Data were synthesized descriptively and listed in tables and charts to determine and illustrate treatment effects on itch, QoL, and disease severity as well as safety (i.e., adverse events). Study features (e.g., study design, EB subtypes, number of subjects), details on treatment modalities (e.g., therapeutic compound, mode of administration, dosage) and outcome measurement instruments were collated to increase interpretability of individual study results in consideration of the heterogeneous study landscape.
Results
Selection of literature
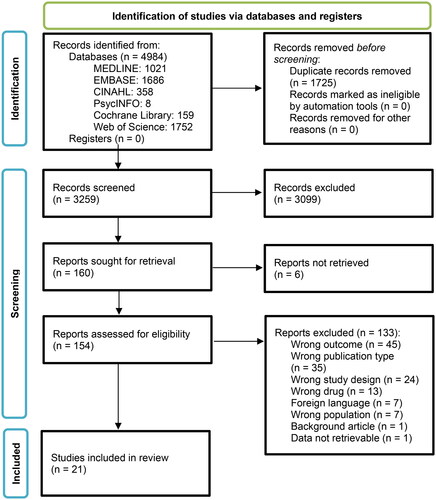
Our search strategy yielded a total of 4,984 records, among which 1,725 duplicates were removed. Based on eligibility criteria, 95.1% (3,099/3,259) records were excluded during title and abstract screening, mainly because they lacked reporting on itch (outcomes) or were ineligible based on study design or intervention. Of the remaining 160 records, six full text reports were not retrievable, resulting in 154 full text manuscripts which were finally screened against the eligibility criteria (). Inter-rater reliability was 99.6% (12 conflicts out of 3,259 records) and 70.6% (47 conflicts out of 160 studies) during title and abstract screening, and full text screening respectively. All conflicts were resolved by discussion.
Study characteristics and risk of bias assessment
A total of 21 studies met the eligibility criteria. Except for two survey studies, all of the identified studies were clinical trials (90.5%, n = 19). All eligible studies reported on interventional effects on itch, ten additionally on QoL, and seven on itch and disease severity, respectively. A large proportion of eligible studies included patients with RDEB (n = 9/21; 42.9%; with in total 102/536 participants, 19%), as well as mixed subtype cohorts (n = 6/21; 28.6%), of whom 61.5% (n = 251/536) were RDEB patients ().
Table 1. Study characteristics.
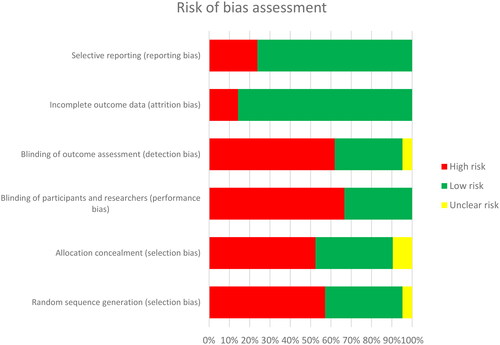
Interventional impact on itch was evaluated as a primary endpoint in 2/21 (9.5%) studies and as a secondary endpoint in 19/21 (90.5%) studies. According to the OCEBM evidence level categorization on treatment benefits, a large proportion (19/21; 90.5%) of the studies showed good or moderate evidence (OCEBM level 2–3) (Citation35). However, the majority of studies were monocentric, open-label, and with only one treatment group (). Therefore, most of the studies (12/21; 57.1%) were classified to harbor a high risk of bias. This is in concordance with a high risk of bias denoted for 46% of the in total six risk items assessed for each individual study. The highest risk was found for items referring to detection, performance, and selection bias (). No study was excluded from this review based on a classification to harbor high or unclear risk of bias.
Figure 2. Summary of risk of bias assessment for included studies using the Cochrane Collaboration tool.
The heterogeneity of reported outcomes on symptomatic itch relief impaired a comparative data assessment across studies in terms of a meta-analysis. For instance, a considerable number of studies lacked information on mean values (38.1%, n = 8/21) and statistical significance (38.1%, n = 8/21). Moreover, treatment effects were often reported only verbatim without further quantification (14.3%; n = 3/21). The use of seven different treatment measurement instruments further impeded efforts of pooled analyses. In two studies, no measurement instrument for itch was reported.
Outcome measurement instruments
In total 18 different measurement tools were used across all studies to assess outcomes on itch (n = 7), QoL (n = 5) and disease severity (n = 6) (). In four studies, disease severity was evaluated using a second measurement instrument to corroborate and substantiate an observed therapeutic impact of the investigated drug clinically () (Citation36–39).
Figure 3. Number of outcome measurement instruments applied to assess itch, QoL or disease severity.
BEBSS: Birmingham Epidermolysis Bullosa Severity Score; DLQI: Dermatology Life Quality Index; EBDASI: Epidermolysis Bullosa Disease Activity and Scarring Index; GSS: global severity score; iscorEB: instrument for scoring clinical outcomes of research for epidermolysis bullosa; NRS: numerical rating scale; PedsQL: Pediatric Quality of Life Inventory; QOLEB: quality of life evaluation in epidermolysis bullosa; TBSA: total body surface area; VAS: visual analogue scale.
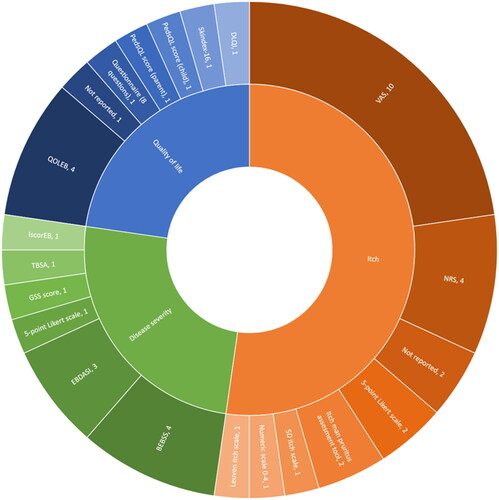
Table 2. Measurement instruments used to measure itch, QoL and disease severity.
16/18 (88.8%) of the measurement instruments used were validated for the respective outcome. Notably, information on the person who had completed the self-reported questionnaire (i.e., affected individuals themselves, their caregivers or medical staff) was rarely provided. Likewise, contextually relevant aspects of assessment (i.e., day time; procedural versus interval itch; maximum/peak itch intensity versus mean itch scores; symptomatic quality, e.g., neuropathic) were inconsistently specified. Furthermore, information on whether the used instruments were applied in patients’ native language and age-appropriate was missing. A more comprehensive analysis and assessment of validity (i.e., content-, construct-, criterion-related validity) and reliability of study data was infeasible based on published study information. Retrieving additional data on outcome assessment procedures through e.g., contacting corresponding authors was beyond the scope of this research.
Treatment approaches
In total, 17 different treatment approaches were extracted from 19 clinical trials (). To facilitate a structured assessment, this study categorized treatments by route of application, i.e., either systemic (oral, intravenous or subcutaneous) or local (topical, intralesional injection or transplantation) as well as by modality (i.e., small molecule, cell or protein therapies).
Table 3. Overview of local and systemic treatment modalities reporting on itch outcomes for EB patients.
In addition, two cross-sectional survey studies evaluated a total of nine different treatment approaches (e.g., bathing products, topical products), with at least two to a maximum of 16 different therapeutic products (e.g., bleach, pimecrolimus, hydrocortisone). Notably, the variability and incomplete reporting of substances, their content and composition in addition to inconsistent data on modes of application impaired an unambiguous allocation of the two survey studies to any category.
Treatment outcomes
A comprehensive overview on treatment outcomes is provided in .
Cell therapy
Local therapies
A statistically non-significant reduction in local itching within the treatment area was reported in five out of nine children with EB (aged 4 months to 14 years) after transplantation of an allogeneic viable bilayer skin construct derived from human neonatal foreskin keratinocytes and dermal fibroblasts. As a limitation, results were reported only verbatim and measurement instruments were not specified by the authors. Consequently, age-appropriateness of the assessment approach remains unclear. Considering the underage study population, the outcomes thus should be interpreted with caution (Citation41).
Similarly, intralesional injection of allogeneic fibroblasts in five adult RDEB patients failed to show a significant reduction in local itch at 12 months, while demonstrating the highest level of evidence (i.e., randomized, placebo controlled testing) in this category (Citation42).
Systemic therapies
Infusion of allogeneic bone marrow derived mesenchymal stromal cells (BM-MSCs) significantly improved mean severity (–15.44 points) as well as frequency (–13.89 points), and consequences of itch (–12.64 points, e.g., lesions from scratching, sleep disturbances) on the Leuven itch scale (range for each item 0–100) in ten adult RDEB patients aged 26–55 years (Citation37). Treatment was associated with a non-significant trend toward improvement of QoL (quality of life evaluation in epidermolysis bullosa, QOLEB) and disease severity (Birmingham Epidermolysis Bullosa Severity Score, BEBSS; Epidermolysis Bullosa Disease Activity and Scarring Index, EBDASI) at day 60 (Citation37).
Intravenous allogeneic human umbilical cord blood derived mesenchymal stem cells (hUCB-MSCs) demonstrated a significant reduction in mean itch by two points on VAS 0–10 in six RDEB patients aged eight to 60 years. In addition, significant mean improvements in QoL and disease severity by 6.2 points in QOLEB and 5.6 points in BEBSS were observed, respectively (Citation39).
Infusion of allogeneic ATP-binding cassette subfamily B5 positive dermal mesenchymal stem cells (ABCB5+ MSCs) resulted in a significant median reduction in itch by 14.3% on day 35 (numerical rating scale, NRS), in a cohort of 16 RDEB patients. In this study, a significant improvement in disease severity was only observed in analyses of distinct subscores (instrument for scoring clinical outcomes of research for epidermolysis bullosa IscorEB clinician subscore −18.2%, EBDASI activity score −13.0%), while no relevant change in the QOLEB was determined (Citation38).
A single infusion of allogenic multilineage-differentiating stress-enduring (Muse) cells in two RDEB and three DDEB patients (aged 17–49 years) revealed no improvement in itch after four weeks. In contrast, a temporary aggravation was noted between weeks 12 and 20 (Citation43).
Protein therapy
Topical applications of protein therapies have been investigated for a potential effect on local itching only. For this purpose, an acellular skin substitute matrix with purified type I bovine collagen was applied weekly to ten RDEB patients with chronic wounds with six-months to six-years of persistence. Seven participants (aged 8–24 years) completed the study, and reported a significant reduction of average wound itch on week eight (–0.89, VAS 0–4) compared to baseline (Citation44).
Small molecules
Local therapies
All five studies identified demonstrated a good level of evidence (OCEBM level 2). Two publications reported on the same registered clinical trial (ESSENCE study), referring to the largest study cohort analyzed in this review, with a total of 169 patients (SD-101 cream, NCT02384460) (Citation45,Citation46). One of these clinical investigations reported only on the vehicle arm (allantoin 0%, SD-101-0.0) (Citation46), while the other publication reported on both, the vehicle and verum (allantoin 6%, SD-101-6.0) arms (Citation45). In total, the trial included 82 patients in the verum cohort, while 87 participants had received placebo. Both, children and adults, with either EBS, DEB, or JEB were enrolled. Trial participants applied the topical treatment to their entire integument on a daily basis. Compared to baseline, antipruritic effects were detected in both cohorts already at the first visit on day seven as well as after three months, with a mean improvement of −0.5 and −0.3 (Itch Man Pruritus Assessment Tool 0–5) for verum and placebo on day seven, respectively. After three months, mean improvement was −0.7 for placebo, while for this timepoint no verum data was reported. Overall, treatment effects on itch did not reach statistical significance when comparing verum to placebo (Citation45,Citation46).
In a study on daily application of low-dose calcipotriol in ultraphil ointment in six patients with RDEB, a significant reduction in wound itching down to 1.83 (VAS, 0–10) was demonstrated on day 28 compared to placebo (5.52, p < 0.0001) (Citation47).
Two additional studies, oleogel-S10 in ten DEB patients and diacereine 1% in 15 EBS children, revealed no reduction in localized itching within the treatment area (Citation48,Citation49).
Systemic therapies
In a single-arm, open-label study, the humanized monoclonal anti-IgE antibody omalizumab was subcutaneously administered in eleven children older than seven years and adults with RDEB and high serum IgE levels. The treatment regime was 150 mg every four weeks. A significant itch reduction of 42% compared to baseline (VAS 0–10, p < 0.0001) was observed in ten patients that completed the trial and received omalizumab for at least three treatment cycles. Itch relief was accompanied by a significant improvement in QoL (Dermatology Life Quality Index DLQI, p < 0.0001) and disease severity (BEBSS reduction by 15%, p < 0.0005) after the last cycle (Citation50). As a drawback, the QoL measurement instrument (DLQI) was not validated for underage subjects (n = 4/10; 40% of the cohort that completed the trial) (Citation51).
Of the six publications on systemically administered small molecules, one clinical trial focused on itch relief in patients with RDEB (n = 13) and JEB (n = 1) upon treatment with the neurokinin 1 receptor (NK1) receptor antagonist serlopitant. It is the only clinical trial in this systematic review that evaluated itch as a primary endpoint. However, the randomized, double-blind, placebo-controlled trial revealed only a non-significant trend toward reduction of generalized itch after eight weeks on the NRS scale, and localized itching at sites of dressing changes did not improve (Citation52).
Orally administered epigallocatechin-3-gallate (green tea extract) showed a non-significant itch reduction in 17 RDEB patients (Citation53). In a study on cyproheptadine, using ‘standard dosing’ (which, however, was not further specified in the publication), three out of ten patients reported ‘relief of itching’ (not further quantified) after six weeks (Citation54).
Daily intravenous infusion of gentamicin for up to 28 days in five children with JEB (two with intermediate JEB and three with severe JEB) did not show consistent improvement of itch, QoL or disease severity (Citation55). Similarly, six children with EBS who were given erythromycin orally for five months did not experience any relief from itching (Citation56).
Survey studies
The survey studies retrospectively analyzed patient-reported data on various topical and systemic treatment modalities. Both studies were classified as having a low level of evidence (OCEBM level 4), which reflects inherent limitations in terms of objectivity (lack of systematic application and evaluation, absence of reproducible and comparable conditions). Danial et al. included 146 individuals with EB to examine the impact of various products, applications, and techniques on itch alleviation. Patients were asked to name the treatments they had used and to rate their effectiveness in relieving itch (Citation57). Commonly used treatments included ‘creams,’ ‘topical corticosteroids,’ ‘oils,’ oral hydroxyzine, topical diphenhydramine and vaporizing rubs. Significantly lower itch intensity correlated with higher frequency of ‘creams’ (p = 0.05) or ‘lotions’ (p = 0.04) use but was independent of the product used (Citation40).
Another survey study was conducted to investigate the effect of cannabinoid-based medicines (CBM) on EB-specific symptoms in 71 patients with EBS, JEB, and DEB. Patients’ reported data revealed a median improvement on NRS by three points (IQR: −1.25 to −5, p < 0.001, 95% CI 2.59–3.10) upon CBM intake (which was predominantly inhaled, followed by topical and oral, and sublingual application). 40/44 (90.9%) and 46/48 (95.8%) patients indicated an improvement of generalized itch and overall EB symptoms on a 5-D Likert scale, respectively (Citation58). Notably, the authors were unable to objectively verify the Δ9-tetrahydrocannabinol (THC) content and composition of CBM products, which, however, impacts the mechanism of action and scope of potential side effects (Citation59).
Adverse events
Of the 21 trials assessed, only 13 reported on treatment-related adverse events. Overall, there was no statistically significant increase of adverse events reported for verum cohorts. (). In one study on ABCB5+ MSCs, two serious adverse events (classified as hypersensitivity reactions) were reported, which resolved shortly after withdrawal of the treatment (Citation38).
Table 4. Adverse events reported in clinical studies.
Discussion
In this systematic review we gathered data on the efficacy and safety of itch treatments from EB studies with the aim to improve the scientific and clinical decision making process for targeting one of the most burdensome and therapeutically challenging symptoms in this population. Twenty-one studies were classified eligible for analyses. However, methodological shortcomings inherent to rare disease clinical research, the heterogeneity and incomplete reporting of outcomes, as well as lacking information on applied measurement instruments hampering validity assessment, significantly impaired efforts of data pooling and comparative assessment.
We identified a significant heterogeneity of outcomes and outcome measurement instruments reported in the literature for the assessment of the impact of study interventions on itch. Variations in trial design (e.g., endpoints, inclusion and exclusion criteria, frequency and time points of measurements) as well as incomplete information on contextually relevant aspects of assessment procedures (e.g., day time, symptom quality, questionnaire respondent, age and language validation) impaired subtype- and age- specific stratification of treatment efficacy as well as feasibility of data pooling and cross trial comparison/comparative analyses. Occasionally, measurement instruments were used that lacked validation for distinct subcohorts of the study population (Citation50), which may have had a confounding impact on effect sizes (Citation60,Citation61).
The diversity in key trial features is in concordance with data from a recent scoping review revealing great heterogeneity among 1,280 reported outcomes pertaining to 80 different outcome domains (the ‘what to measure’) for EB. Moreover, these outcome domains had been assessed by a diverse range of in total 200 measurement instruments (the ‘how to measure’) in 207 studies (Citation29). In the context of EB, which features a high intra- and inter-individual disease heterogeneity per se, such heterogeneity constitutes a major shortcoming to clinical research efficacy. Considering the growing trial landscape competing for a limited number of eligible patients, initiatives to foster a more homogenous reporting and assessment of core outcomes are thus highly warranted (Citation62).
A small number of eligible patients is a prototypic feature of EB trials. It requires an optimal balance between maximum bias reduction (through e.g., restrictive inclusion criteria, timing and frequency of measurements, blinding, randomization) and feasibility (acceptable trial burden e.g., invasiveness of investigations, access to verum arm, travel burden, individual risk to benefit assessment) for the EB patient cohort that is typically severely affected, vulnerable, geographically dispersed and often underage () (Citation28). As the inter- and intra-individual heterogeneity of patient cohorts and a variable disease course additionally favor random imbalance in covariates and bias, trials were often designed to be open-label (n = 12/21, 57.1%), and allocated to a single group (n = 9/21, 42.9%) while baseline was serving as comparator (n = 14/21, 66.7%). Consequently, the overall formal quality of data evidence of the assessed studies was mediocre. Moreover, randomization was often infeasible or actively omitted (n = 12/21, 57.1%) to ensure distribution of verum to all participants, thereby raising recruitment, compliance, adherence and trial efficacy. Being less affected by these factors, reporting and attrition bias, however, were often low for the reported outcomes ().
Despite a wide variety of topical and systemic treatment modalities (n = 83) that have been evaluated in this work, statistically significant anti-itch effects were observed in only eight studies (38.1%). These trials mainly involved (R)DEB patients, the EB cohort who is reported to suffer most from pruritus () (Citation12). Notably, these studies were actually powered for primary endpoints other than symptomatic itch relief, assessing e.g., wound healing, disease severity, safety, and blister formation. Conversely, the only one trial reporting on an interventional impact on itch as primary endpoint, failed to reach statistical significance for this outcome (Citation52). These data further underscore that the formal quality of data evidence for efficacy of antipuritic treatment modalities is currently sparse.
With regard to topical therapeutics, significant improvement of itch within the treatment area was only observed upon weekly application of collagen type I wound dressings as well as daily low-dose calcipotriol ointment (Citation44,Citation47). Notably, narrow therapeutic impact or non-superiority of topical applications compared to control has also been linked to the observation that regular (i.e., intensified) per protocol application of topical (skin care) products commonly serving as control or placebo, has the potential to effectively and independently reduce itch. Occasionally, control thereby may even outperform verum (Citation45,Citation46,Citation57).
Remarkably, oral agents did not yield statistically significant itch relief in this analysis, despite mediating a presumably generalized, systemic effect.
Considering the interpretation of reported effect sizes, we want to emphasize that statistical significance per se does neither imply clinical meaningfulness (i.e., a change that the patient can discern) nor a minimally important difference. The latter refers to the point at which a change in symptoms or experienced disease burden becomes large enough that most patients would want to change their therapy to get this benefit (Citation63). This outcome also covers aspects of patient-centeredness, particularly with regard to treatment burden (such as feasibility and invasiveness of route of administration considering e.g., tolerability of oral intake in the background of mucosal EB lesions or tolerability of topical applications to large, vulnerable skin areas). Data supporting such an impact of any therapeutic modality was not extractable.
To corroborate observed effect sizes, value assessment may include the correlation of symptomatic itch relief, QoL and disease severity outcomes, as these scores have shown a strong interdependence (Citation22,Citation23). However, these items were simultaneously reported in only a limited number of studies to underpin patient-reported data. Improvement of parameters for itch (VAS), QoL (DLQI, QOLEB) and disease severity (BEBSS; total body surface area, TBSA) was concurrent and significant in two trials (omalizumab, hUCB-MSCs), and for itch and disease severity in one trial (ABCB5+ MSCs), respectively (Citation38,Citation39,Citation50).
Overall, these analyses revealed no statistically significant increase of adverse events reported for verum cohorts (). However, adverse events were only inconsistently and incompletely reported, which impeded a thorough risk-benefit assessment. Such an analysis, however, would be of particular relevance considering the vulnerable and often underage study population and need for long-term therapy. Those adverse events reported were rarely rated serious, with the exemption of hypersensitivity reactions associated with infusions of ABCB5+ MSCs. The latter, however, were observed only in isolated cases and without any reported sequelae.
Individualized approaches employing (potentially) predictive biomarkers (e.g., pruritogenic lead cytokines used as surrogates for the response to certain immunomodulatory therapies) constitute an innovative approach to effectively target itch mediators. This option seems particularly attractive in view of the rapidly expanding immunomodulatory therapy options. Profiling of inflammatory signatures and identification of lead cytokines in patients with EB pruriginosa prompted the administration of omalizumab (Citation50). Furthermore, repurposing of IL-4/IL-13 antibody dupilumab, janus kinase inhibitors (i.e., baricitinib, upadacitinib), as well as the phosphodiesterase-4-inhibitor apremilast, induced a self-reported itch reduction, however, hitherto only in case reports and case series (Citation14,Citation40,Citation58,Citation64). Methodological issues (e.g., detection, performance, and reporting bias; optimal dosing), as well as dose finding and determination of long term outcomes remain to be addressed by large scaled studies.
Conclusions
As translational perspectives and therapeutic potential for EB broadens, a steadily growing number of innovative therapeutic components from bench as well as repurposed drugs have entered clinical testing to correct, modify or palliate the symptoms of this devastating genodermatosis, including burdensome itch (Citation65). Our data underscore the considerable lack of high level evidence based on large-scale studies sufficiently powered to demonstrate a clinically meaningful effect. In addition, the use of a variety of different outcome measurement instruments impeded comparative assessment of heterogeneous and small-sized study populations. Therefore, current evidence is insufficient to unequivocally support recommendations on treatment prioritization for EB associated itch based on efficacy and safety data from clinical trials. Against this background, common efforts to boost international collaboration, coordination and consensus, including consistent use of core outcomes with adequate and validated measurement instruments, are crucial for efficient data generation out of size-limited EB patient cohorts. A collaborative approach will likewise foster the identification and validation of pilot data on predictive biomarker signatures and therapeutic targets, which hold the potential to more effectively meet the challenges of disease heterogeneity by addressing patient-specific pathogenic traits of EB.
Author contributions
T.W., C.PU, E.W.H.K., S.W., C.P., M.C.B. and M.L. developed the concept and designed the systematic review. T.W., C.PU., E.W.H.K., and M.L. contributed to acquisition, analysis, or interpretation of data. T.W., C.PU., and M.L. wrote the initial manuscript. All authors contributed to critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript. M.L. and M.C.B. provided supervision.
Supplemental Material
Download Zip (29.6 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this review are available from the corresponding authors upon reasonable request.
Additional information
Funding
References
- Fine JD. Inherited epidermolysis bullosa. Orphanet J Rare Dis. 2010;5(1):12. doi:10.1186/1750-1172-5-12.
- Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183(4):614–627. doi:10.1111/bjd.18921.
- Turczynski S, Titeux M, Pironon N, et al. Marked intrafamilial phenotypic heterogeneity in dystrophic epidermolysis bullosa caused by inheritance of a mild dominant glycine substitution and a novel deep intronic recessive COL7A1 mutation. Br J Dermatol. 2016;174(5):1122–1125. doi:10.1111/bjd.14312.
- Odorisio T, Di Salvio M, Orecchia A, et al. Monozygotic twins discordant for recessive dystrophic epidermolysis bullosa phenotype highlight the role of TGF-beta signalling in modifying disease severity. Hum Mol Genet. 2014;23(15):3907–3922. doi:10.1093/hmg/ddu102.
- Schumann H, Kiritsi D, Pigors M, et al. Phenotypic spectrum of epidermolysis bullosa associated with alpha6beta4 integrin mutations. Br J Dermatol. 2013;169(1):115–124. doi:10.1111/bjd.12317.
- Kiritsi D, Kern JS, Schumann H, et al. Molecular mechanisms of phenotypic variability in junctional epidermolysis bullosa. J Med Genet. 2011;48(7):450–457. doi:10.1136/jmg.2010.086751.
- McGrath JA, Ashton GH, Mellerio JE, et al. Moderation of phenotypic severity in dystrophic and junctional forms of epidermolysis bullosa through in-frame skipping of exons containing non-sense or frameshift mutations. J Invest Dermatol. 1999;113(3):314–321. doi:10.1046/j.1523-1747.1999.00709.x.
- Fine JD, Mellerio JE. Extracutaneous manifestations and complications of inherited epidermolysis bullosa: part I. Epithelial associated tissues. J Am Acad Dermatol 2009;61(3):367–384; quiz 85-6. doi:10.1016/j.jaad.2009.03.052.
- Fine JD, Mellerio JE. Extracutaneous manifestations and complications of inherited epidermolysis bullosa: part II. Other organs. J Am Acad Dermatol. 2009;61(3-4):387–402. doi:10.1016/j.jaad.2009.03.053.
- Schräder NHB, Korte EWH, Duipmans JC, et al. Identifying Epidermolysis Bullosa patient needs and perceived treatment benefits: an explorative study using the patient benefit index. J Clin Med. 2021;10(24):5836. doi:10.3390/jcm10245836.
- Danial C, Adeduntan R, Gorell ES, et al. Prevalence and characterization of pruritus in epidermolysis bullosa. Pediatr Dermatol. 2015;32(1):53–59. doi:10.1111/pde.12391.
- Snauwaert JJ, Yuen WY, Jonkman MF, et al. Burden of itch in epidermolysis bullosa. Br J Dermatol. 2014;171(1):73–78. doi:10.1111/bjd.12885.
- Jeon IK, On HR, Kim SC. Quality of life and economic burden in recessive dystrophic epidermolysis Bullosa. Ann Dermatol. 2016;28(1):6–14. doi:10.5021/ad.2016.28.1.6.
- Castela E, Tulic MK, Rozières A, et al. Epidermolysis bullosa simplex generalized severe induces a T helper 17 response and is improved by apremilast treatment. Br J Dermatol. 2019;180(2):357–364. doi:10.1111/bjd.16897.
- Esposito S, Guez S, Orenti A, et al. Autoimmunity and cytokine imbalance in inherited epidermolysis Bullosa. Int J Mol Sci. 2016;17(10):1625. doi:10.3390/ijms17101625.
- Huitema L, Phillips T, Alexeev V, et al. Immunological mechanisms underlying progression of chronic wounds in recessive dystrophic epidermolysis bullosa. Exp Dermatol. 2021;30(12):1724–1733. doi:10.1111/exd.14411.
- Wally V, Lettner T, Peking P, et al. The pathogenetic role of IL-1beta in severe epidermolysis bullosa simplex. J Invest Dermatol. 2013;133(7):1901–1903. doi:10.1038/jid.2013.31.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171(1):217–228 e13. doi:10.1016/j.cell.2017.08.006.
- Schmidt D, Díaz P, Muñoz D, et al. Characterisation of the pathophysiology of neuropathy and sensory dysfunction in a mouse model of recessive dystrophic epidermolysis bullosa. Pain. 2022;163(10):2052–2060. doi:10.1097/j.pain.0000000000002599.
- von Bischhoffshausen S, Ivulic D, Alvarez P, et al. Recessive dystrophic epidermolysis bullosa results in painful small fibre neuropathy. Brain. 2017;140(5):1238–1251. doi:10.1093/brain/awx069.
- Mack MR, Wendelschafer-Crabb G, McAdams BD, et al. Peripheral neuro-immune pathology in recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 2015;135(4):1193–1197. doi:10.1038/jid.2014.500.
- de Vere Hunt I, Halley M, Sum K, et al. A qualitative exploration of the experiences of itch for adults living with epidermolysis bullosa. Br J Dermatol. 2022;187(2):261–263. doi:10.1111/bjd.21031.
- Papanikolaou M, Onoufriadis A, Mellerio JE, et al. Prevalence, pathophysiology and management of itch in epidermolysis bullosa. Br J Dermatol. 2021;184(5):816–825. doi:10.1111/bjd.19496.
- Cianfarani F, Zambruno G, Castiglia D, et al. Pathomechanisms of altered wound healing in recessive dystrophic epidermolysis Bullosa. Am J Pathol. 2017;187(7):1445–1453. doi:10.1016/j.ajpath.2017.03.003.
- Callewaert C, Nakatsuji T, Knight R, et al. IL-4Ralpha blockade by dupilumab decreases staphylococcus aureus colonization and increases microbial diversity in atopic dermatitis. J Invest Dermatol. 2020;140(1):191–202 e7. doi:10.1016/j.jid.2019.05.024.
- Goldschneider KR, Good J, Harrop E, et al. Pain care for patients with epidermolysis bullosa: best care practice guidelines. BMC Med. 2014;12(1):178. doi:10.1186/s12916-014-0178-2.
- Pope E, Lara-Corrales I, Mellerio J, et al. A consensus approach to wound care in epidermolysis bullosa. J Am Acad Dermatol. 2012;67(5):904–917. doi:10.1016/j.jaad.2012.01.016.
- Prodinger C, Diem A, Ude-Schoder K, et al. Profiling trial burden and patients’ attitudes to improve clinical research in epidermolysis bullosa. Orphanet J Rare Dis. 2020;15(1):182. doi:10.1186/s13023-020-01443-3.
- Korte EWH, Welponer T, Kottner J, et al. Heterogeneity of reported outcomes in epidermolysis bullosa clinical research: a scoping review as a first step towards outcome harmonization. Br J Dermatol. 2023;189(1):80–90. doi:10.1093/bjd/ljad077.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71.
- Fine JD, Bauer EA, Briggaman RA, et al. Revised clinical and laboratory criteria for subtypes of inherited epidermolysis bullosa. A consensus report by the Subcommittee on Diagnosis and Classification of the National Epidermolysis Bullosa Registry. J Am Acad Dermatol. 1991;24(1):119–135. doi:10.1016/0190-9622(91)70021-s.
- McGowan J, Sampson M, Salzwedel DM, et al. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–46. doi:10.1016/j.jclinepi.2016.01.021.
- Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi:10.1186/s13643-016-0384-4.
- Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(oct18 2):d5928–d5928. doi:10.1136/bmj.d5928.
- Group OLoEW. “The Oxford Levels of Evidence 2”. Oxford Centre for Evidence-Based Medicine. Available from: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence.
- Petrof G, Lwin SM, Martinez-Queipo M, et al. Potential of systemic allogeneic mesenchymal stromal cell therapy for children with recessive dystrophic epidermolysis Bullosa. J Invest Dermatol. 2015;135(9):2319–2321. doi:10.1038/jid.2015.158.
- Rashidghamat E, Kadiyirire T, Ayis S, et al. Phase I/II open-label trial of intravenous allogeneic mesenchymal stromal cell therapy in adults with recessive dystrophic epidermolysis bullosa. J Am Acad Dermatol. 2020;83(2):447–454. doi:10.1016/j.jaad.2019.11.038.
- Kiritsi D, Dieter K, Niebergall-Roth E, et al. Clinical trial of ABCB5+ mesenchymal stem cells for recessive dystrophic epidermolysis bullosa. JCI Insight. 2021;6(19):22. doi:10.1172/jci.insight.151922.
- Lee SE, Lee S-J, Kim S-E, et al. Intravenous allogeneic umbilical cord blood-derived mesenchymal stem cell therapy in recessive dystrophic epidermolysis bullosa patients. JCI Insight. 2021;6(2):606. doi:10.1172/jci.insight.143606.
- Shehadeh W, Sarig O, Bar J, et al. Treatment of epidermolysis bullosa pruriginosa-associated pruritus with dupilumab. Br J Dermatol. 2020;182(6):1495–1497. doi:10.1111/bjd.18855.
- Fivenson DP, Scherschun L, Choucair M, et al. Graftskin therapy in epidermolysis bullosa. J Am Acad Dermatol. 2003;48(6):886–892. doi:10.1067/mjd.2003.502.
- Venugopal SS, Yan W, Frew JW, et al. A phase II randomized vehicle-controlled trial of intradermal allogeneic fibroblasts for recessive dystrophic epidermolysis bullosa. J Am Acad Dermatol. 2013;69(6):898–908 e7. doi:10.1016/j.jaad.2013.08.014.
- Fujita Y, Komatsu M, Lee SE, et al. Intravenous injection of muse cells as a potential therapeutic approach for epidermolysis Bullosa. J Invest Dermatol. 2021;141(1):198–202 e6. doi:10.1016/j.jid.2020.05.092.
- Gorell ES, Leung TH, Khuu P, et al. Purified type I collagen wound matrix improves chronic wound healing in patients with recessive dystrophic epidermolysis bullosa. Pediatr Dermatol. 2015;32(2):220–225. doi:10.1111/pde.12492.
- Paller AS, Browning J, Nikolic M, et al. Efficacy and tolerability of the investigational topical cream SD-101 (6% allantoin) in patients with epidermolysis bullosa: a phase 3, randomized, double-blind, vehicle-controlled trial (ESSENCE study). Orphanet J Rare Dis. 2020;15(1):158. doi:10.1186/s13023-020-01419-3.
- Murrell DF, Paller AS, Bodemer C, et al. Wound closure in epidermolysis bullosa: data from the vehicle arm of the phase 3 ESSENCE Study. Orphanet J Rare Dis. 2020;15(1):190. doi:10.1186/s13023-020-01435-3.
- Guttmann-Gruber C, Piñón Hofbauer J, Tockner B, et al. Impact of low-dose calcipotriol ointment on wound healing, pruritus and pain in patients with dystrophic epidermolysis bullosa: a randomized, double-blind, placebo-controlled trial. Orphanet J Rare Dis. 2021;16(1):473. doi:10.1186/s13023-021-02062-2.
- Schwieger-Briel A, Kiritsi D, Schempp C, et al. Betulin-based oleogel to improve wound healing in dystrophic epidermolysis bullosa: a prospective controlled proof-of-concept study. Dermatol Res Pract. 2017;2017:5068969–5068910. doi:10.1155/2017/5068969.
- Wally V, Hovnanian A, Ly J, et al. Diacerein orphan drug development for epidermolysis bullosa simplex: a phase 2/3 randomized, placebo-controlled, double-blind clinical trial. J Am Acad Dermatol. 2018;78(5):892–901 e7. doi:10.1016/j.jaad.2018.01.019.
- Chen F, Guo Y, Zhou K, et al. The clinical efficacy and safety of anti-IgE therapy in recessive dystrophic epidermolysis bullosa. Clin Genet. 2022;101(1):110–115. doi:10.1111/cge.14062.
- Basra MK, Fenech R, Gatt RM, et al. The dermatology life quality index 1994–2007: a comprehensive review of validation data and clinical results. Br J Dermatol. 2008;159(5):997–1035. doi:10.1111/j.1365-2133.2008.08832.x.
- Chiou AS, Choi S, Barriga M, et al. Phase 2 trial of a neurokinin-1 receptor antagonist for the treatment of chronic itch in patients with epidermolysis bullosa: a randomized clinical trial. J Am Acad Dermatol. 2020;82(6):1415–1421. doi:10.1016/j.jaad.2019.09.014.
- Chiaverini C, Roger C, Fontas E, et al. Oral epigallocatechin-3-gallate for treatment of dystrophic epidermolysis bullosa: a multicentre, randomized, crossover, double-blind, placebo-controlled clinical trial. Orphanet J Rare Dis. 2016;11(1):31. doi:10.1186/s13023-016-0411-5.
- Neufeld-Kaiser W, Sybert VP. Is cyproheptadine effective in the treatment of subjects with epidermolysis bullosa simplex-Dowling-Meara? Arch Dermatol. 1997;133(2):251–252. doi:10.1001/archderm.1997.03890380125030.
- Mosallaei D, Hao M, Antaya RJ, et al. Molecular and clinical outcomes after intravenous gentamicin treatment for patients with junctional epidermolysis bullosa caused by nonsense variants. JAMA Dermatol. 2022;158(4):366–374. doi:10.1001/jamadermatol.2021.5992.
- Chiaverini C, Fontas E, Vabres P, et al. Oral erythromycin therapy in epidermolysis bullosa simplex generalized severe. Br J Dermatol. 2015;173(2):563–564. doi:10.1111/bjd.13672.
- Danial C, Adeduntan R, Gorell ES, et al. Evaluation of Treatments for Pruritus in Epidermolysis Bullosa. Pediatr Dermatol. 2015;32(5):628–634. doi:10.1111/pde.12486.
- Jiang X, Wang H, Lee M, et al. Epidermolysis Bullosa pruriginosa treated with baricitinib. JAMA Dermatol. 2021;157(10):1243–1244. doi:10.1001/jamadermatol.2021.3174.
- Schräder NHB, Gorell ES, Stewart RE, et al. Cannabinoid use and effects in patients with epidermolysis bullosa: an international cross-sectional survey study. Orphanet J Rare Dis. 2021;16(1):377. doi:10.1186/s13023-021-02010-0.
- Kimberlin CL, Winterstein AG. Validity and reliability of measurement instruments used in research. Am J Health Syst Pharm. 2008;65(23):2276–2284. doi:10.2146/ajhp070364.
- Kwon J, Freijser L, Huynh E, et al. Systematic review of conceptual, age, measurement and valuation considerations for generic multidimensional childhood patient-reported outcome measures. Pharmacoeconomics. 2022;40(4):379–431. doi:10.1007/s40273-021-01128-0.
- Drucker AM, Morra DE, Prieto-Merino D, et al. Systemic immunomodulatory treatments for atopic dermatitis: update of a living systematic review and network meta-analysis. JAMA Dermatol. 2022;158(5):523–532. doi:10.1001/jamadermatol.2022.0455.
- Speeckaert R, Belpaire A, Herbelet S, et al. Credibility and generalization of the minimally important difference concept in dermatology: a scoping review. JAMA Dermatol. 2022;158(11):1304–1314. doi:10.1001/jamadermatol.2022.3511.
- Kwon IJ, Kim SE, Kim SC, et al. Efficacy of oral JAK1 or JAK1/2 inhibitor for treating refractory pruritus in dystrophic epidermolysis bullosa: a retrospective case series. J Dermatol. 2024;51(3):441–447. doi:10.1111/1346-8138.17079.
- Hou PC, Del Agua N, Lwin SM, et al. Innovations in the treatment of Dystrophic Epidermolysis Bullosa (DEB): current landscape and prospects. Ther Clin Risk Manag. 2023;19:455–473. doi:10.2147/TCRM.S386923.