Abstract
Background
Biological therapies are effective for psoriasis, but patient responses vary, often requiring therapy switching or discontinuation.
Objectives
To identify physicians’ prescribing patterns of biological therapies at a referral tertiary center in Saudi Arabia and assess the probability of biologic persistence following treatment initiation.
Methods
We conducted a retrospective study of biologic-naïve adult psoriasis patients who initiated therapy from October 2013 to July 2022 in Dammam. Descriptive statistics and a Kaplan-Meier analysis evaluated treatment persistence at 6, 12, 24, and 36 months.
Results
A total of 151 patients received adalimumab (n = 89), etanercept (n = 17), risankizumab (n = 30), ustekinumab (n = 14), and ixekizumab (n = 1). At 6 months, all therapies demonstrated 100% persistence. At 12 months, persistence was highest for ustekinumab (100%) and lowest for etanercept (88.2%). At 24 months, ustekinumab maintained 100% persistence, followed by risankizumab (96.6%), adalimumab (94.3%), and etanercept (76.4%). At 36 months, risankizumab had the highest persistence (96.6%), followed by adalimumab (83.1%), ustekinumab (78%), and etanercept (70.6%). The most common reasons for discontinuation were lack of effectiveness and intolerability.
Conclusion
This study shows changing psoriasis treatment patterns with new therapies. Risankizumab demonstrated high long-term persistence, while etanercept and ustekinumab showed declining persistence, suggesting evolving treatment considerations.
1. Introduction
Plaque psoriasis (PsO) is a relatively common chronic inflammatory dermatitis. Often stemming from a genetic predisposition, the condition is expressed through plaque, inverse, guttate, pustular, or erythrodermic subtypes (Citation1,Citation2). PsO, which presents as well-circumscribed erythematous patches of skin with silvery-white scales, is the most common subtype (Citation2). While the exact etiology of PsO is still unknown, histological investigation of its lesions suggests an involvement of both innate and adaptive immunity. It is associated with an inflammatory infiltrate of T-lymphocytes, mast cells, neutrophils, and macrophages into the dermis and epidermis, which leads to hyperkeratosis, epidermal acanthosis, parakeratosis, and prominence of the rete ridges (Citation3). According to epidemiological data, PsO affects 2 to 3% of the world’s population, with one-third of cases beginning in childhood (Citation4). Adult PsO has a prevalence of 78.9 per 100,000, significantly higher than the pediatric PsO rate of 40.8 per 100,000 (Citation5).
Current treatments for PsO include topical therapies (vitamin D analogs and corticosteroids), phototherapy (narrowband ultraviolet B radiation, psoralen, and ultraviolet A radiation), conventional disease-modifying agents (methotrexate, ciclosporin, and acitretin), and biologic therapies, such as: TNF inhibitors (etanercept, adalimumab, infliximab, and certolizumab); IL-12/23 inhibitors (ustekinumab); IL-17 inhibitors (secukinumab, ixekizumab, and brodalumab); and IL-23 inhibitors (guselkumab, risakizumab, and tildrakizumab), offering dermatologists several treatment options for their patients (Citation6). Patients with moderate-to-severe PsO may require escalation of therapy with disease-modifying agents or biologic agents, either as monotherapy or combined with other topical or systemic medications (Citation2,Citation7). The choice of biologic treatment for PsO can depend on patient preferences, disease severity, comorbid conditions, or any additional risk factors discovered as new trial data emerge (Citation7,Citation8)
With the increased understanding of the complex pathogenesis of PsO, biologics have revolutionized PsO management. While the efficacy and safety of biologics have been reported in randomized controlled trials (RCTs) (Citation7), these RCTs usually involve highly selected and closely monitored patient populations. Therefore, gathering data from unselected patients in routine clinical practice can provide more generalizable and long-term evidence for various outcomes, such as efficacy or safety outcomes.
Biologic drug survival, or persistence, has been considered a real-world surrogate for the efficacy and suitability of various biological therapies for PsO. Many studies have investigated persistence with varying results. In Schmitt-Egenolf et al. ustekinumab has demonstrated a greater median persistence (49.3 months, 95% confidence interval (CI): 38.0 to 59.1) compared to etanercept (16.3 months, 95% CI: 14.5 to 19.0). As compared to biologic-exposed patients, biologic-naïve patients had a longer median persistence. Furthermore, Schmitt-Egenolf et al. found that persistence for ustekinumab decreased from a median of 62.3 (95% CI: 45.6 to ∞) months in 2010–2011 to 32.7 months (95% CI: 21.2 to 49.3) in 2014–2016 (Citation9). Similarly, other studies have reported that ustekinumab exhibits the highest drug survival (Citation10,Citation11). Conversely, Leonardi et al. found a statistically (p < 0.001) greater persistence in patients who received ixekizumab compared to secukinumab, adalimumab, ustekinumab, or etanercept after both a 1-year follow-up and up to 3 years of follow-up (Citation12). Moreover, the study by Armstrong et al. found that among 7,997 patients, treatment switching rates were 14.4% and 26% at 12 and 24 months, respectively. In addition, IL-23 inhibitors were associated with the lowest risk of switching compared with TNF, IL-17, and IL-12/23 inhibitors over 24 months (p < 0.001) (Citation13).
To date, there is a lack of data on treatment patterns, including persistence, switching, and discontinuation, in Saudi Arabia. Therefore, this study aims to describe the treatment patterns among patients with PsO who initiated biologic therapies in a Saudi Arabian context.
2. Methods
2.1. Study design, setting, and subjects
We conducted a retrospective observational study at specialized dermatology outpatient clinics in Dammam, Saudi Arabia. Eligible participants included adult patients (≥ 18 years old) with PsO who were biologic-naïve and initiated biological therapies between October 2013 and July 2022. Patients were excluded if they expressed a previous use of biological therapy, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, or Crohn’s disease to ensure that the biological therapies were used solely for PsO. The study was approved by the institutional review board at Dammam Medical Complex (DMC) with IRB# PH15.
2.2. Study aims and definitions
Patient demographics recorded on the index date of starting the biological therapy included age, sex, race, weight, body mass index (BMI), psoriasis type, serum creatinine, disease duration before initiating biological therapy in years, concomitant non-biologic systemic therapies, and comorbidities. Treatment patterns, including persistence, switching, and discontinuation of biologics, were recorded in the follow-up period. Treatment persistence was defined as the time from initiation to discontinuation. Discontinuation of the initial biologic agent was defined as no administration for a duration of 2 prescriptions (depending on the dosing regimen of each medication) plus 1 week to rule out short treatment breaks. The duration was selected in accordance with the half-life of the biological therapy. Reasons for discontinuation include lack of effectiveness, intolerability, and other reasons as recorded in the electronic health records (EHRs). Switching was defined as starting a different biological agent regardless of the time gap between the initial and second biological agent.
2.3. Data analysis
All analyses were performed using R Core Team (2020) software (R Foundation for Statistical Computing, Version 4.0.1, Vienna, Austria). Data were inputted into the Research Electronic Data Capture (REDCap) system in a de-identified manner (Citation14). Descriptive statistics for the baseline data were utilized. Continuous data were presented as medians with interquartile ranges (IQR), and categorical variables were presented as frequencies and percentages. For the treatment persistence analysis, we fitted a time-to-event model (i.e. Kaplan-Meier Model) for each biological therapy. Time 0 was the day of initiation of the biological therapy, with the event being the discontinuation or switching of biological therapy for any reason (whichever occurred first). Events were censored when there was a loss of follow-up. Then, the absolute probability of treatment persistence was calculated from the Kaplan-Meier model at 6, 12, 24, and 36 months. The Kaplan-Meier model included biological therapy as the only covariate. As Kaplan-Meier models are non-parametric, it has fewer assumptions, such as non-informative censoring without testing for parallelism. We visualized the data for discontinuation or switching for each biological agent using the ggplot2 package (Citation15). Switching patterns were visually analyzed using the ggalluvial package (Citation16). The formal sample size was not calculated given the descriptive nature of the study.
3. Results
3.1. Demographics and baseline characteristics
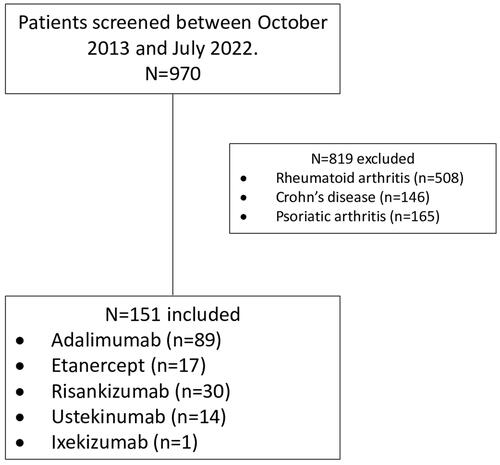
Of the 970 patient charts evaluated, 151 patients were included, and 819 patients were excluded. The included patients were categorized based on the biological agent received; 89 received adalimumab, 17 received etanercept, 30 received risankizumab, 14 received ustekinumab, and 1 received ixekizumab ().
Baseline characteristics were included in . Of the total number of patients, the median (IQR) age was 34 (26–44), and 51% were male patients. The mean duration of the disease was 1 year among the whole sample, whereas the risankizumab group exhibited the highest mean disease duration of 4.5 years. Of the included total sample, 77.4% were not on non-biologic systemic therapy and 16.6% and 6% received methotrexate and ciclosporin, respectively. All patients received topical therapy. Most of the included patients had no morbid conditions (90.7%), whereas 2.6%, 2%, 7.3%, 1.3%, and 0.7% had hypertension, hyperlipidemia, diabetes mellitus, cardiovascular disease, or depression, respectively.
Table 1. Baseline characteristics.
3.2. Persistence, switching, and discontinuation of biologics patterns
At 6 months, 100% of the patients persisted on all biological therapies. However, at 12 months, ustekinumab demonstrated the highest probability of treatment persistence with 100%, whereas etanercept presented the lowest with 88.2% probability. At 24 months, ustekinumab and risankizumab groups displayed the highest treatment persistence with 100% and 96.6% probability, respectively. Etanercept patients revealed the lowest treatment persistence with 88.2% probability. At 36 months, risankizumab expressed the highest treatment persistence with 96.6% probability, whereas etanercept displayed the lowest treatment persistence with 70.6% (). The persistence probability of patients is shown in . However, there was no significant difference between patients in different biological therapy categories regarding persistence probability (p = 0.38).
Figure 2. Kaplan–Meier curve of persistence probabilities. Strata was the biological treatment. The event was discontinuation or switching the biological agent. Patients at risk in each group were presented for each biological therapy events were reported between brackets. Tick marks on the curves are indicative of censoring data (loss of follow-up).
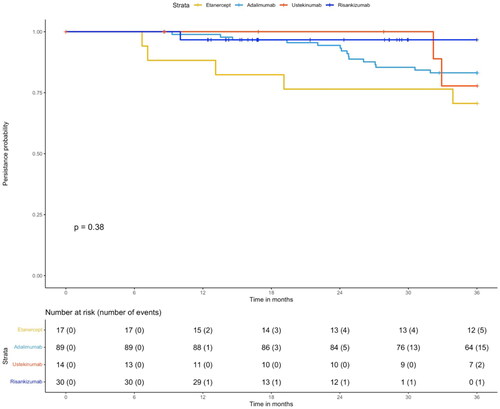
Table 2. Probability of persistence.
The initiation, discontinuation, and switching patterns by year are shown in (Panel A and Panel B). In 15 instances, physicians switched patients’ treatments from adalimumab to other biological therapies: 2 patients (13.3%) were switched to etanercept, 7 patients (46.7%) to ustekinumab, and 6 patients (40%) to risankizumab. Additionally, there were 5 cases where patients were switched from etanercept to adalimumab (100%), and 2 cases where patients were switched from ustekinumab to risankizumab (100%) (see ). Several reasons for discontinuation or switching are shown in . Lack of effectiveness was the main reason for the discontinuation or switching for etanercept (11.7%) and adalimumab (5.6%) at 12–24 months, as well as for adalimumab at 24–36 months (10.1%).
Figure 3. (A) Initiation, discontinuation, or switching patterns are shown for each year. Each initiation, discontinuation, or switching case was drawn in the graph for a specific year. The size of the dot represents the counts. Red is for discontinuation/switching, and blue is for initiation. (B) A tornado graph for each year for initiation (right side) and discontinuation or switching (left side) counts.
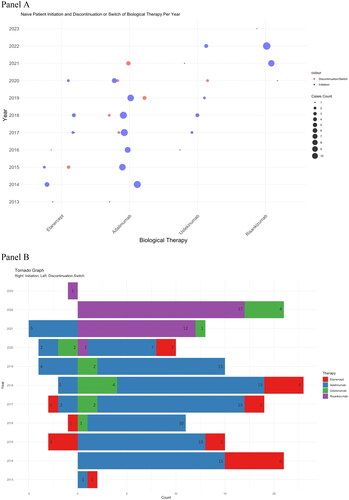
Figure 4. Switching between biological therapies patterns. Left: from, Right: to. Number of switches was shown in the graph. The width of the square is proportional to the number of patients using the agent. Note: 1 patient in the adalimumab group discontinued without switching to another agent.
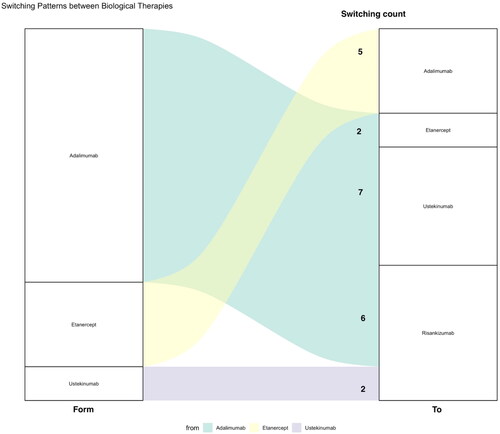
Table 3. Reasons for discontinuation or switching.
3.3. Dose intensification
We observed no dose intensifications in the cohort except for 2 ustekinumab cases in which patients had their dose intensified within 12–24 months.
4. Discussion
This study provides insights into the prescribing patterns and persistence of biological therapies among patients with PsO, as no previous studies have been conducted on this topic in Saudi Arabia. Patients in this cohort mainly received the following biological therapies: etanercept, adalimumab, ustekinumab, and risankinumab. There was 1 patient who received ixekizumab that was excluded from this discussion, as this patient continued the therapy without issues. In Saudi Arabia, although non-binding to practitioners, a national guideline was developed in 2014 and updated in 2020 by an expert board of dermatologists and pharmacists in PsO management (Citation17,Citation18). The 2015 guideline included only a few agents that had been registered by the Saudi Food and Drug Administration (SFDA) at the time: etanercept (2004 approval), adalimumab (2008 approval), infliximab (2006 approval), ustekinumab (2009 approval) (Citation19–22). The choice of the agent is dependent on the type of psoriasis, but for patients with chronic plaque psoriasis, anti-TNF agents etanercept and adalimumab were considered to be the first choice at the time. In our single-center study, we noted that from 2013 to 2015, the main agents for initiation were indeed etanercept and adalimumab as the initial biological therapy. These agents continued to be the main two agents until 2020. Adalimumab had higher rates of initiation by clinicians, perhaps due to its long-term efficacy data with psoriasis area and severity index (PASI) 90 (i.e. 90% or greater reduction in PASI score) in 50% of patients compared to 27% of the patients who received etanercept. In addition, adalimumab has a more convenient dosing regimen of every other week compared to the weekly dosing of etanercept. There were no initiations of infliximab in our cohort, although it was recommended in PsO patients who require a more rapid control of their symptoms (Citation17). Clinicians’ unfavorable views of infliximab could be related to patient factors, its required intravenous infusion method of administration, infusion reactions (in up to 22% of patients), or the development of antibodies to infliximab with related lower efficacy, thus impeding its prescription in outpatient settings despite its long-term PASI 90 efficacy of up to 45% (Citation23,Citation24).
Furthermore, ustekinumab (IL-12/IL-23 inhibitor, 2009 approval) was reserved as a second line after an anti-TNF therapy failure or contraindication to anti-TNF therapy in the Saudi 2015 guideline and a third line in the 2020 consensus (Citation17,Citation18). Our study showed that ustekinumab was initiated in 9 (13.2%) patients between 2016 to 2019. The 2020 Saudi consensus (Citation18) presented a shift toward the favorability of IL-23 antagonists, namely guselkumab (2017 approval) and risankizumab (2019 approval) after the failure of systemic therapies (Citation25,Citation26). Those with a suboptimal response or failure may try IL-17 antagonists, such as secukinumab and ixekizumab, and if no response is noted, ustekinumab (IL-12/IL-23 inhibitor) can be used as the third line (Citation18). While both guselkumab and risankizumab are registered by the SFDA, our institution’s formulary had risankizumab as the choice of anti-IL-23 agent. The longer half-life (28 days) allows the drug to be dosed every 12 weeks as maintenance therapy, which is advantageous over guselkumab (dosing regimen of every 8 weeks) (Citation18). The results from UltIMMA-1 and 2 trials showed a PASI 90 of 81.9% and 80.6%, respectively, at 1 year of follow-up for risankizumab, whereas a lower PASI 90 of 76.3% was reported at 48 weeks of follow-up in the VOYAGE 1 trial for guselkumab (Citation27,Citation28). Reflecting on these benefits, physicians at our institution started to prescribe risankizumab, first for 1 patient in 2020 (10% of initiations during this year) and then the vast majority of the biological therapies initiated in 2021 (92.3% of biological therapy initiations) and 2022 (80.6% of biological therapy initiations).
Persistence patterns as illustrated in and show risankizumab to exhibit the highest probability of persistence at 36 months, which is consistent with clinical trials, being the most effective therapy available for PsO (Citation27). Our results are similar to a real-world retrospective data analysis from Australia, in which guselkumab had the highest probability of drug survival at 1 and 5 years with 94.2%, followed by ixekizumab, which had a 1-year survival rate of 87.2% and a 5-year survival rate of 59.4% . Etanercept had the lowest survival rate at 1 year (57.7% ) and at 5 years (30.3% ) (Citation29). Additionally, real-world data from the United States (US) confirm the drug survival favorability of the IL-23 antagonist class, followed by IL-12/23, IL-17, and TNF inhibitors (Citation13). Our data did not include ixekizumab, however, the study by Lockshin et al. showed that patients receiving ixekizumab displayed a 64% lower risk of discontinuation compared to TNFi (Hazard Ratio [HR] = 0.36; 95% CI: 0.27 to 0.47) and a 31% lower risk compared to other IL-17i (HR = 0.69; 95% CI 0.55 to 0.87) after adjusting for biologic experience and other covariates (Citation30). In addition, in the study by Mastorino et al. which included a total of 189 patients who received ixekizumab, drug survival was achieved in 86.2% of patients at 54 weeks, 75.7% at 104 weeks, 69.3% at 156 weeks, 67.4% at 208 weeks, and 65.5% at 260 weeks. Similar to our data, the main reason for biologic therapy discontinuation was primary failure (38.1%). This was followed by secondary failure (31.8%), adverse events (AEs) (4.8%), and 16 patients (25.4%) interrupted the treatment for other reasons (Citation31). Another Italian study found that drug survival was 88% at 24 months for IL-23 inhibitors (guselkumab, risakizumab, and tildrakizumab) compared to 75% for IL-17 inhibitors (secukinumab, ixekizumab, and brodalumab). Furthermore, there was a lower probability of drug interruption for risankizumab (HR= 0.42; 95% CI: 0.26 to 0.69, p = 0.001) and guselkumab (HR= 0.49; 95% CI: 0.24 to 0.99, p = 0.046) when compared with secukinumab (Citation32).
Moreover, our data showed that the persistence rates of ustekinumab were 100% and 78% at 2 and 3 years, respectively. In Spain, a retrospective study of 148 patients who received ustekinumab found that the survival rates were 82% at 2 years, 66% at 5 years, and 58% at 8 years. The median survival rate was 80 months (95% CI 36.9 to 123.01 months). The reasons for discontinuation were primary failure (lack of initial efficacy prior to 16 weeks of treatment), followed by secondary failure and other reasons (Citation33).
A retrospective, multicentric, multi-country study by Torres et al. confirmed our results. The authors found that IL-23 inhibitors (guselkumab, risankizumab) had the highest drug survival rates at 6, 12, and 24 months. The IL-17 inhibitors secukinumab and ixekizumab were associated with a lower cumulative probability of drug survival registered at month 36 as compared with guselkumab. Furthermore, risankizumab, guselkumab, and ixekizumab were less likely to be discontinued than secukinumab (Citation34). The recently published systematic review and meta-analysis with a total of 69 studies aggregating the drug survival outcomes of 48,704 patients found that guselkumab and risankizumab displayed the highest drug survival rate compared with other biologics (Citation35). Moreover, when compared to adalimumab, the meta-analysis by Mourad et al. showed that etanercept had a lower drug survival in 5 years with a pooled estimate of 41.1%, compared to 50.1% for adalimumab (Citation36). Given the ample of evidence in favor of risankizumab, clinicians at our institution mostly switched patients to this agent ().
The current study offers insights into the current management practices for PsO patients receiving biological therapies in Saudi Arabia. Although it was a single-center study, this could be a first sample representing many centers affiliated with the Saudi Ministry of Health. Nonetheless, this study is subject to several limitations. Given the retrospective nature of the study, which relied on electronic health records, assessing patient adherence levels was not possible; such a factor could contribute to variations in treatment effectiveness and drug survival rates. As the sample size was notably small, increasing the sample size may have produced better estimates. We also noted a lack of PASI scores and body surface area (BSA) involvement reporting by clinicians in patient records. Reporting such metrics is recommended to enhance communication among providers, support management decisions, and enable further research into the effectiveness of these therapies in real-world settings. Additionally, dosing optimization can be done prior to transitioning patients to alternative therapies. For example, patients receiving adalimumab therapy can be optimized by an increase in the dosage frequency from 40 mg every other week to 40 mg every week. We noted that such a dose optimization was not implemented for all cases of anti-TNF inhibitors, as it was only realized in two cases of ustekinumab who required switching afterwards.
Lastly, as these therapies can be very costly, there is a need for more robust real-world data that evaluate their cost effectiveness in Saudi Arabia. The study by Alruthia et al. the first of its kind in Saudi Arabia, presented real-world comparative data for risankizumab and adalimumab in PsO management (Citation37). In 70 patients who were analyzed for effectiveness, there was no statistically significant reduction in PASI scores between the two groups. From the Saudi Arabian public health sector, the mean annual cost of these therapies at their recommended dosing was $9,433.20 USD for adalimumab and $16,638.33 USD for risankizumab. The economic evaluation examined four scenarios with varying dosage patterns and costs: first, actual acquisition costs of both agents, with adalimumab administered every other week; second, actual acquisition costs but with adalimumab administered weekly; third, the cheapest biosimilar alternative for adalimumab administered weekly versus the actual acquisition cost of risankizumab; and fourth, the cheapest biosimilar alternative for adalimumab administered weekly versus a 40% risankizumab price discount. The fourth scenario provided a lower cost and a greater reduction in PASI scores in 71.25% of the bootstrap distributions (cost saving), which favors risankizumab therapy when the discounted price is considered. Notably, Alruthia et al. underscored the importance of economic evaluations in guiding therapeutic decisions, especially in contexts where the financial burden of treatment is a major concern for both the healthcare system and the patient.
5. Conclusion
The pattern of biological therapy varies between biological agents. While adalimumab was the most prescribed biological agent, the risankizumab group had the highest persistence rate over a 36-month follow-up, followed by the adalimumab and ustekinumab groups, respectively. The etanercept group had the lowest probability of persistence at 36 months, which suggests evolving considerations in treatment selection or patient responses.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386(9997):983–994. doi: 10.1016/S0140-6736(14)61909-7.
- Raharja A, Mahil SK, Barker JN. Psoriasis: a brief overview. Clin Med (Lond). 2021;21(3):170–173. doi: 10.7861/CLINMED.2021-0257.
- Silverberg N, Lebwohl M, Kellen R. Efficacy and safety of ustekinumab in adolescents. Pediatric Health Med Ther. 2016;7:109–120. doi: 10.2147/PHMT.S75836.
- The National Psoriasis Foundation [Internet]. Get the facts about psoriasis and psoriatic arthritis. [cited 2024 May 22]. Available from: https://www.psoriasis.org/psoriasis-statistics/.
- Tollefson MM, Crowson CS, McEvoy MT, et al. Incidence of psoriasis in children: a population-based study. J Am Acad Dermatol. 2010;62(6):979–987. doi: 10.1016/J.JAAD.2009.07.029.
- Menter A, Griffiths CE. Current and future management of psoriasis. Lancet. 2007;370(9583):272–284. doi: 10.1016/S0140-6736(07)61129-5.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84(2):432–470. doi: 10.1016/J.JAAD.2020.07.087.
- Thatiparthi A, Martin A, · Jeffrey L, et al. Biologic treatment algorithms for moderate-to-severe psoriasis with comorbid conditions and special populations: a review. Am J Clin Dermatol. 2021;22(4):425–442. doi: 10.1007/s40257-021-00603-w.
- Schmitt-Egenolf M, Freilich J, Stelmaszuk-Zadykowicz NM, et al. Drug persistence of biologic treatments in psoriasis: a Swedish National Population Study. Dermatol Ther (Heidelb). 2021;11(6):2107–2121. doi: 10.1007/S13555-021-00616-7.
- Graier T, Salmhofer W, Jonak C, et al. Biologic drug survival rates in the era of anti-interleukin-17 antibodies: a time-period-adjusted registry analysis. Br J Dermatol. 2021;184(6):1094–1105. doi: 10.1111/bjd.19701.
- Egeberg A, Ottosen MB, Gniadecki R, et al. Safety, efficacy and drug survival of biologics and biosimilars for moderate-to-severe plaque psoriasis. Br J Dermatol. 2018;178(2):509–519. doi: 10.1111/BJD.16102.
- Leonardi C, Zhu B, Malatestinic WN, et al. Real-world biologic adherence, persistence, and monotherapy comparisons in US patients with psoriasis: results from IBM MarketScan® Databases. Adv Ther. 2022;39(7):3214–3224. doi: 10.1007/S12325-022-02155-9.
- Armstrong AW, Patel M, Li C, et al. Real-world switching patterns and associated characteristics in patients with psoriasis treated with biologics in the United States. J Dermatolog Treat. 2023;34(1):2200870. doi: 10.1080/09546634.2023.2200870.
- Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/J.JBI.2019.103208.
- Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer-Verlag; 2016. doi: 10.1007/978-3-319-24277-4.
- Brunson J. Ggalluvial: layered grammar for alluvial plots. J Open Source Software. 2020;5(49):2017. doi: 10.21105/JOSS.02017.
- Hamadah IR, Al Raddadi AA, Bahamdan KA, et al. Saudi practical guidelines on biologic treatment of psoriasis. J Dermatolog Treat. 2015;26(3):223–229. doi: 10.3109/09546634.2014.946882.
- Fatani MIA, Hamadah IRA, Alajlan MA, et al. Saudi consensus statement on biologic treatment of chronic plaque psoriasis (2020). J Dermatolog Treat. 2022;33(4):1916–1930. doi: 10.1080/09546634.2021.1950601.
- Food and Drug Administration [Internet]. Enbrel® (etanercept). [cited 2024 May 24]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/103795s5503lbl.pdf.
- Food and Drug Administration [Internet]. HUMIRA® (adalimumab) injection. [cited 2024 May 22]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125057s410lbl.pdf.
- Food and Drug Administration [Internet]. REMICADE. [cited 2024 May 24]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/103772s5359lbl.pdf.
- Food and Drug Administration [Internet]. STELARA® (ustekinumab) injection. [cited 2024 May 22]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761044lbl.pdf.
- Subedi S, Gong Y, Chen Y, et al. Infliximab and biosimilar infliximab in psoriasis: efficacy, loss of efficacy, and adverse events. Drug Des Devel Ther. 2019;13:2491–2502. doi: 10.2147/DDDT.S200147.
- Reich K, Nestle FO, Papp K, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005;366(9494):1367–1374. doi: 10.1016/S0140-6736(05)67566-6.
- Food and Drug Administration [Internet]. SKYRIZI® (risankizumab-rzaa) injection. [cited 2024 May 22]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761105s014lbl.pdf.
- Food and Drug Administration [Internet]. TREMFYA® (guselkumab) injection. [cited 2024 May 22]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761061s001lbl.pdf.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392(10148):650–661. doi: 10.1016/S0140-6736(18)31713-6.
- Blauvelt A, Papp KA, Griffiths CEM, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–417. doi: 10.1016/J.JAAD.2016.11.041.
- Ting S, Lowe P, Smith A, et al. Drug survival of biologics in psoriasis: an Australian multicentre retrospective study. Australas J Dermatol. 2024;65(4):350–357. doi: 10.1111/AJD.14254.
- Lockshin B, Cronin A, Harrison RW, et al. Drug survival of ixekizumab, TNF inhibitors, and other IL-17 inhibitors in real-world patients with psoriasis: the Corrona Psoriasis Registry. Dermatol Ther. 2021;34(2):e14808. doi: 10.1111/DTH.14808.
- Mastorino L, Dapavo P, Burzi L, et al. Drug survival, effectiveness and safety of ixekizumab for moderate-to-severe psoriasis up to 5 years. J Eur Acad Dermatol Venereol. 2024;38(3):568–575. doi: 10.1111/JDV.19682.
- Mastorino L, Dapavo P, Susca S, et al. Drug survival and clinical effectiveness of secukinumab, ixekizumab, brodalumab, guselkumab, risankizumab, tildrakizumab for psoriasis treatment. J Dtsch Dermatol Ges. 2024;22(1):34–42. doi: 10.1111/DDG.15251.
- Galache Osuna C, Gómez-Vila B, Aubán Pariente J, et al. Ustekinumab drug survival in patients with psoriasis: a retrospective study of real clinical practice. Medicina (Kaunas). 2020;56(11):584. doi: 10.3390/MEDICINA56110584.
- Torres T, Puig L, Vender R, et al. Drug survival of interleukin (IL)-17 and IL-23 inhibitors for the treatment of psoriasis: a retrospective multi-country, multicentric cohort study. Am J Clin Dermatol. 2022;23(6):891–904. doi: 10.1007/S40257-022-00722-Y.
- Thomas SE, Barenbrug L, Hannink G, et al. Drug survival of IL-17 and IL-23 inhibitors for psoriasis: a systematic review and meta-analysis. Drugs. 2024;84(5):565–578. doi: 10.1007/S40265-024-02028-1/FIGURES/3.
- Mourad A, Straube S, Armijo-Olivo S, et al. Factors predicting persistence of biologic drugs in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2019;181(3):450–458. doi: 10.1111/BJD.17738.
- AlRuthia Y, Alfakhri AH, Alharbi I, et al. Comparative effectiveness and cost evaluation of risankizumab and adalimumab in the management of psoriasis: a real-world study in Saudi Arabia. Cost Eff Resour Alloc. 2023;21(1):95. doi: 10.1186/S12962-023-00504-1.