Abstract
Purpose: To evaluate the enhancement effect of two combined radiation-sensitizing agents in mammalian cells at small doses as compared to large doses using the linear-quadratic (LQ) mathematical model.
Methods and materials: Data on clonogenic assays concerning the radio-enhancement effects of combined halogenated pyrimidines and hyperthermia or combined cisplatin and hyperthermia, as published in earlier reports, were analyzed according to the LQ-formula: S(D)/S(0) = exp−(αD + βD2). Effects of sensitizing agents on the linear parameter α and the quadratic parameter β are compared in order to evaluate differences depending on the applied dose, the possible relations to mechanisms of radiation sensitization and to derive suggestions for applications.
Results: The values of the linear parameter α, which determines the effectiveness at low doses, are for all cell lines and all conditions more increased than the values of the parameter β which has a higher contribution at larger radiation doses. The combination of hyperthermia with halogenated pyrimidines to radiation as well as the combination of hyperthermia and cisplatin to radiation significantly increases the value of the linear parameter α, as compared to radiation alone or radiation combined with a single agent.
Conclusions: The radiation enhancement factors of the values of linear and quadratic parameters demonstrate that the sensitizing agents have a larger effect on the linear parameter which is dominant at low radiation doses as is used in fractionated-radiation treatment in the clinic. Moreover, the effect is even further increased when two radiation sensitizers are used.
Introduction
Radiotherapy of cancer is often combined with hyperthermia or chemotherapy to increase effectiveness of the treatment. A useful mathematical model to analyze radiation sensitization is the linear quadratic (LQ) model (Barendsen et al. Citation2001). The LQ-parameters α (Gy−1) and β (Gy−2) represent the radiation sensitivity. The parameter α particularly reflects the effectiveness at low doses. A previous publication applied this model to analyze the enhancement of radiation effectiveness by hyperthermia or incorporation of halogenated pyrimidines at low and high radiation doses for reproductive death of cultured cells (Franken and Barendsen Citation2014). The incentive for this analysis was that data on surviving fractions as a function of dose for two conditions provide more useful information about radiation sensitization than is presented by a single enhancement factor. The results showed that the values of the linear parameter α are more increased than the values of the quadratic parameter β, which provides a higher contribution at higher doses.
Hyperthermia (HT) refers to heat treatment of cancer cells or tumours by increasing the temperature to a level between 39 °C and 43 °C (Horsman and Overgaard Citation2007). Hyperthermia is a very effective radiation-and chemo-sensitizer as shown both experimentally and clinically to enhance the anti-cancer effects (Bergs et al. Citation2007). In vitro studies on the combination of hyperthermia and radiation have shown a synergistic interaction between the two modalities (Bergs et al. Citation2007). Hyperthermia has an effect on potentially lethal damage (PLD) and sublethal damage (SLD) and therefore influences both the linear and the quadratic parameter (Kok et al. Citation2014).
Halogenated pyrimidines (HP) are well known radiation sensitizers and have been suggested to be particularly effective in rapidly growing tumours (Franken et al. Citation1997, Citation1999). Radiation sensitization is probably caused by an enhancement of SLD and/or PLD due to incorporation of HP in the DNA, yielding an increase in the amount of radiation-induced DNA damage. Labelling depends on the growth fraction, cell loss, cell cycle time and potential doubling time. Of special importance for sensitization is the rate at which non-cycling cells are recruited into the proliferative compartment during exposure to HPs and a course of radiotherapy. However, even in rapidly growing tumours, cells may, after proliferative cycles, move into a non-proliferative stage. This might compromise the degree of radiation sensitization if resting cells are less affected by HP, or are better able to cope with additional damage by repair of PLD or SLD.
Cisplatin is a widely used anti-cancer drug which is often combined with radiotherapy (Bergs et al. Citation2007). Cisplatin based chemo-radiotherapy is standard treatment for several different types of cancers, e.g. cervical carcinoma and locally advanced non-small cell lung cancer (NSCLC). Many studies have been carried out on the radiation-sensitizing effect of cisplatin. Results vary from a clear cisplatin-induced radiation sensitization to only an additive effect on cell survival. For both cisplatin and radiation DNA is the main target (Rabik and Dolan Citation2007).
In the present study we evaluated whether radiotherapy combined with two sensitizing agents, HPs + hyperthermia or cisplatin + hyperthermia, further increased the radiation effectiveness. Possible differences in comparison with enhancement by single agents might be of interest for clinical considerations concerning the application of combined treatments and might provide insights in mechanisms of sensitization.
Methods and materials
In vitro experiments for radiation combined with either halogenated pyrimidines and hyperthermia or with cisplatin and hyperthermia have been published in several earlier communications (Van Bree et al. Citation1997; Franken et al. Citation2001, Citation2013). In this study we performed linear quadratic analyses on these published data and evaluated enhancement factors of the LQ-parameters, which reflect the increase in radiation sensitivity as a result of the combined treatment. The following sections describe the combination treatments and analysis of the data.
Cell cultures
Cultures of cells from rodents R1, V79, MOS and RUC-II and of human origin SIHA, SW-1573 and RKO were used. The rodent cell lines grow in minimal essential medium. All media were supplied from Gibco-BRL life technologies, Breda, The Netherlands. SIHA is a cervical carcinoma cell line growing in EMEM; SW1573 is a non-small cell squamous lung carcinoma cell line growing in Leibowitz-15; RKO is a colon cancer cell line which is growing in Mc Coys 5A medium with 25 mM Hepes. The media are supplemented with 10% fetal bovine serum (FBS) and 2 mM glutamine. All cell lines were maintained at 37 °C in an incubator with humidified air supplemented with 5% CO2 except for the SW1573 which medium did not need additional supply of CO2. Cell cycle times ranged from 12–16 h for the rodent cells and 20–24 h for the cells of human origin.
Clonogenic assay
Clonogenic assays were conducted as described elsewhere (Franken et al. Citation2006).
Irradiation
Radiation treatments were performed with single doses (0, 2, 4, 6 and 8 Gy) of γ-rays from a 137Cs source at a dose rate of about 0.5 Gy/min.
Hyperthermia
Hyperthermia treatment of cells at 41.0 °C (HT41), 42.0 °C (HT42) or 43.0 °C (HT43) was performed for 1 h immediately prior to irradiation. Hyperthermia was carried out by submerging the culture dishes in a thermostatically controlled waterbath. Temperature was checked in parallel dishes and the desired temperature (±0.1 °C) was reached in approximately 5 min. The atmosphere of the waterbath was adjustable by a connection with air and CO2 supplies. The rodent, SIHA and RKO cells were heated in a 5%CO2/95% air atmosphere with an air inflow of 2 l/min. The SW1573 cells did not need a supply of CO2.
Halogenated pyrimidines
Cells were incubated with 4 μM bromodeoxyuridine, (BrdUrd), chlorodeoxyuridine (CldUrd) or iododeoxyuridine (IdUrd) (all from Sigma-Aldrich, Zwijndrecht, The Netherlands), for 72 h before irradiation as described earlier (Van Bree et al. Citation1997; Franken et al. Citation2001).
Cisplatin
Treatment with cisplatin (Platosin®, Pharmacie, Haarlem, The Netherlands) was started at 1 h before radiation at a concentration of 1 or 5 μM.
Linear quadratic (LQ) analyses
Cell survival curves obtained from combined treatments were analyzed using the linear-quadratic formula: S(D)/S(0) = exp - (αD + βD2) in which D is the radiation dose, S(D) is the surviving fraction after radiation dose D, S(0) the surviving fraction after dose 0 and α and β are constants which determine the radiation sensitivity of which the values depend on culture conditions including radiation sensitizing agents (Barendsen Citation1994; Barendsen et al. Citation2001). From the values of the LQ parameters the enhancement factors for lethal and sublethal damage were calculated:
α-EF = enhancement factor α.
This was defined as the ratio of the value of α after combination treatment and the value of α for radiation alone.
β-EF = enhancement factor β.
This was defined the ratio of the value of β after combination treatment and the value of β for radiation alone.
Statistical analysis
Means and standard deviations (SD) were calculated for all data points from at least three different experiments. The means were compared by the Student’s t-test analysis using SPSS software (SPSS Inc., Chicago, IL); p < 0.05 was considered as significantly different.
Results
Radiation enhancement with halogenated pyrimidines and hyperthermia
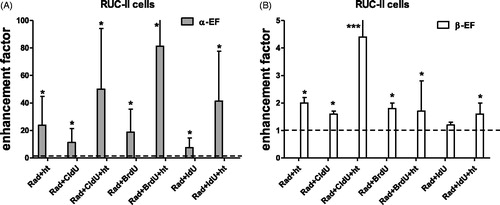
In ) the enhancement factor of the linear (α) and quadratic (β) parameter for R1 and RKO cells after treatment with a halogenated pyrimidine (CldU, BrdU and IdU), hyperthermia and combined treatment is presented. The value of α was significantly enhanced. This is why for the MOS, V79 and SW1573 only the enhancement factors of α is presented (). In ) the enhancement factors for the RUC-II cells are presented. It can be observed that for this cell line besides the value of the parameter α and also for some conditions the β value was significantly increased. This can be due to the very low radiation sensitivity of this cell line. It is clear that for all cell lines the value of α was already increased after a duo-modality treatment of ionizing radiation with HP or ionizing radiation with hyperthermia. Treatment with the tri-modality further increased the value of α. For R1, MOS, V79 and SW1573 cells the α-enhancement factor of the tri-modality treatment was significantly increased compared to the α-enhancement factor of the duo-modality treatment except for the BrdU. For the RUC-II cells only the β-enhancement factor of the tri-modality with HT + CldU was significantly higher than the β-enhancement factor duo-modality treatments.
Figure 1. Enhancement factor of the linear (α) and quadratic (β) parameters after combining radiation with a halogenated pyrimidine (BrdU, IdU or CldU) or hyperthermia and tri-modality treatment. R1 cells (A), RKO cells (B), α-enhancement for MOS, V79 and SW1573 cells (C). The value of 1 represents treatment with radiation alone. The values were derived from radiation dose survival curves with and without sensitizing agents. Each experiment was performed at least three times. Error bars are SD. *Significant difference from radiation only, **significant difference from duo-modality radiation and hyperthermia, ***significant difference from both duo-modalities. All p at least <0.05.
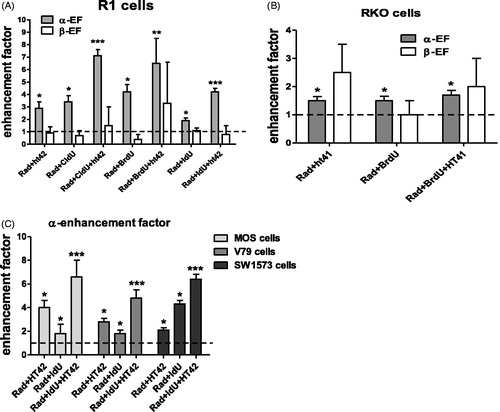
Figure 2. α-Enhancement factors for RUC-II cells (A) and β-enhancement factors for RUC-II cells (B) after combined radiation with a halogenated pyrimidine (BrdU, IdU or CldU) or hyperthermia and tri-modality treatment. The value of 1 represents treatment with radiation alone. The values were derived from radiation dose survival curves with and without sensitizing agents. Each experiment was performed at least three times. Error bars are SD. *Significant difference from radiation only, ***significant difference from both duo-modalities. All p at least <0.05.
Radiation enhancement with cisplatin and hyperthermia
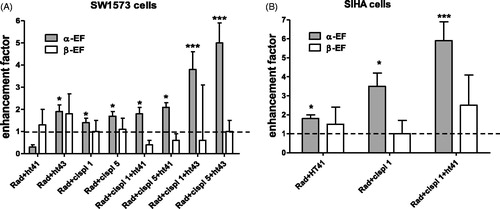
In the enhancement factor of the linear and quadratic LQ-parameters of SW1573 and SIHA cells after radiation treatment with cisplatin, hyperthermia or tri-modality treatment is presented. It can be observed that only the value of the linear parameter α was increased significantly. Like the HP treatment also cisplatin treatment enhanced the radiation effect, as reflected in an increased value of α. Ionizing radiation with hyperthermia also increased the value of the linear parameter. It can be observed that the α-enhancement increased with temperature for the SW1573 cells. Treatment with the tri-modality of cisplatin, hyperthermia and ionizing radiation significantly increased the value of α further, compared to the duo-modality treatments with either cisplatin and hyperthermia or radiation and hyperthermia.
Figure 3. Enhancement factor of the linear and quadratic parameters of SW1573 (A) and SIHA (B) cells after treatment with cisplatin, hyperthermia or combined treatment. The values were derived from radiation dose survival curves with and without sensitizing agents. The value of 1 represents treatment with radiation alone. Each experiment was performed at least three times. Error bars are SD. *Significant difference from radiation only, ***significant difference from both duo-modalities. All p at least <0.05.
In the values of the α-enhancement factors for all treatments are summarized.
Table 1. Values of the enhancement factor of the linear parameter alpha for the different sensitizing agents.
Discussion
In this paper the combined treatment of radiation and hyperthermia with an additional third radiation sensitizing agent, halogenated pyrimidines or cisplatin, was evaluated in terms of enhanced radiation sensitivity. Results showed that combining radiation with two types of sensitizing agents yielded a substantially higher enhancement factor of the linear component α compared to the quadratic component β. This implies that the enhancement at low radiation doses is considerably higher than at doses of 5 Gy and more. Since in clinical applications fraction doses up to 3 Gy are commonly applied, this implies that these tri-modality treatments are likely to be clinically effective to improve tumour control. The increased radiation sensitivity of combined treatments can be explained by different mechanisms, as discussed below.
The interaction of hyperthermia with ionizing radiation probably results from inhibition of repair of radiation-induced DNA damage by hyperthermia which was recently described (Krawczyk et al. Citation2011; Bergs et al. Citation2013; Oei et al. Citation2015). It was demonstrated that hyperthermia treatment combined with ionizing radiation led to inhibition of the cellular capacity to repair PLD (Oei et al. Citation2015).
The effects of combining hyperthermia with radiation sensitization by halogenated pyrimidines on PLD were studied by Van Bree et al. (Citation1997). It has been suggested that radiation sensitization by HP might be attributed to the conversion of low LET lesions into high LET lesions through an increase in the clustering or severity of radiation-induced DNA damage. Our data show that additional cell kill in HP-radiation sensitized cells can be achieved by adding hyperthermia, as supported by an increase in the value of linear parameter α.
It is also shown that adding hyperthermia to combined cisplatin and radiation further enhanced radiation sensitivity, also reflected in an increased value of α. The increased value of α by adding cisplatin to radiation was demonstrated earlier by Bergs et al. (Citation2006). Wilkins et al. (Citation1993), who investigated the effect of cisplatin and radiation on repair of PLD in confluent cultures of two different brain tumour cell lines, also reported a cisplatin-induced radiation sensitization due to inhibition of PLD repair. Their results indicate that the radiation sensitizing effect of cisplatin is caused by the inhibition of post-irradiation recovery. The mechanism of cisplatin-induced radiation sensitization might be due to the inhibition of the DNA-repair, NHEJ and HR, pathways (Myint et al. Citation2002). The Ku protein complex, which plays an important role in NHEJ, was demonstrated to reduce the ability to translocate on DNA containing cisplatin-DNA adducts compared to undamaged DNA. This resulted in a decreased interaction between Ku and DNA-PKcs (Turchi et al. Citation2000). As reviewed by Bergs et al. (Citation2007), it has been demonstrated by several studies that all three agents applied together yield a clear enhancement of effects compared to single- or bi-modality treatments. Hyperthermia increases radiation sensitivity by inhibiting homologous recombination repair and in addition cisplatin increases the radiation sensitivity by disrupting endjoining repair and base excision repair (Bergs et al. Citation2006, Citation2013; Oei et al. Citation2015). This explains the increased effect for the tri-modality strategy.
Our results can be further explained by a mechanism of interaction of two DNA double-strand breaks induced in close proximity by individual particles which yields chromosome aberrations (Franken et al. Citation2011; Krawczyk et al. Citation2012, Franken and Barendsen Citation2014). This mechanism of cell inactivation is suggested to dominate the responses at low doses up to 3 Gy and is most relevant in clinical radiotherapy with fraction doses in this range. The mechanism yielding cell inactivation described by the quadratic parameter β is suggested to involve interaction of two DNA double-strand breaks produced by independent ionizing particles. Additional studies of the mechanisms involved in movement of damaged sites and the induction of different types of damage by hyperthermia and different agents interacting with damage from radiation are subject of ongoing research.
Conclusion
It can be stated that that the sensitizing agents have a larger effect on the value of linear parameter, α, which is dominant at low radiation doses as is used in fractionated-radiation treatment in the clinic than on the value of the quadratic parameter, β. Moreover, the value of α is even further increased when two radiation sensitizers are used.
Franken_et_al._Supplemental_Figures.zip
Download Zip (124.3 KB)Acknowledgements
The Dutch Cancer Foundation (nos. UVA 2008-4019 and 2012-5540) is acknowledged for financing personnel support.
Disclosure statement
The authors report no conflicts of interest. The authors alone are response for the content and writing of the paper.
References
- Barendsen GW, Van Bree C, Franken NA. 2001. Importance of cell proliferative state and potentially lethal damage repair on radiation effectiveness: implications for combined tumor treatments (review). Int J Oncol. 19:247–256.
- Barendsen GW. 1994. RBE-LET relationships for different types of lethal radiation damage in mammalian cells: comparison with DNA dsb and an interpretation of differences in radiosensitivity. Int J Radiat Biol. 66:433–436.
- Bergs JW, Franken NA, Haveman J, Geijsen ED, Crezee J, van Bree C. 2007. Hyperthermia, cisplatin and radiation trimodality treatment: a promising cancer treatment? A review from preclinical studies to clinical application. Int J Hyperthermia. 23:329–341.
- Bergs JW, Krawczyk PM, Borovski T, ten Cate R, Rodermond HM, Stap J, Medema JP, Haveman J, Essers J, van Bree C, et al. 2013. Inhibition of homologous recombination by hyperthermia shunts early double strand break repair to non-homologous end-joining. DNA Repair (Amst). 12:38–45.
- Bergs JW, Franken NAP, ten Cate R, Van Bree C, Haveman J. 2006. Effects of cisplatin and gamma-irradiation on cell survival, the induction of chromosomal aberrations and apoptosis in SW-1573 cells. Mutat Res. 594:148–154.
- Franken NA, Barendsen GW. 2014. Enhancement of radiation effectiveness by hyperthermia and incorporation of halogenated pyrimidines at low radiation doses as compared with high doses: implications for mechanisms. Int J Radiat Biol. 90:313–317.
- Franken NA, Oei AL, Kok HP, Rodermond HM, Sminia P, Crezee J, Stalpers LJ, Barendsen GW. 2013. Cell survival and radiosensitisation: modulation of the linear and quadratic parameters of the LQ model (Review). Int J Oncol. 42:1501–1515.
- Franken NA, Ruurs P, Ludwików G, van Bree C, Kipp JB, Darroudi F, Barendsen GW. 1999. Correlation between cell reproductive death and chromosome aberrations assessed by FISH for low and high doses of radiation and sensitization by iodo-deoxyuridine in human SW-1573 cells. Int J Radiat Biol. 75:293–299.
- Franken NA, Van Bree C, Veltmaat MA, Rodermond HM, Haveman J, Barendsen GW. 2001. Radiosensitization by bromodeoxyuridine and hyperthermia: analysis of linear and quadratic parameters of radiation survival curves of two human tumor cell lines. J Radiat Res. 42:179–190.
- Franken NA, Van Bree CV, Kipp JB, Barendsen GW. 1997. Modification of potentially lethal damage in irradiated Chinese hamster V79 cells after incorporation of halogenated pyrimidines. Int J Radiat Biol. 72:101–109.
- Franken NAP, Rodermond HM, Stap J, Haveman J, van Bree C. 2006. Clonogenic assay of cells in vitro. Nat Protoc. 1:2315–2319.
- Franken NAP, ten Cate R, Krawczyk PM, Stap J, Haveman J, Aten J, Barendsen GW. 2011. Comparison of RBE values of high-LET α-particles for the induction of DNA-DSBs, chromosome aberrations and cell reproductive death. Radiat Oncol. 6:64.
- Horsman MR, Overgaard J. 2007. Hyperthermia: a potent enhancer of radiotherapy. Clin Oncol. 19:418–426.
- Kok HP, Crezee J, Franken NA, Stalpers LJ, Barendsen GW, Bel A. 2014. Quantifying the combined effect of radiation therapy and hyperthermia in terms of equivalent dose distributions. Int J Radiat Oncol Biol Phys. 88:739–745.
- Krawczyk PM, Borovski T, Stap J, Cijsouw T, ten Cate R, Medema JP, Kanaar R, Franken NA, Aten JA. 2012. Chromatin mobility is increased at sites of DNA double-strand breaks. J Cell Sci. 125:2127–3213.
- Krawczyk PM, Eppink B, Essers J, Stap J, Rodermond H, Odijk H, Zelensky A, van Bree C, Stalpers LJ, Buist MR, et al. 2011. Temperature-controlled induction of BRCA2 degradation and homologous recombination deficiency sensitizes cancer cells to PARP-1 inhibition. Proc Natl Acad Sci USA. 108: 9851–9856.
- Myint WK, Ng C, Raaphorst P. 2002. Examining the non-homologous repair process following cisplatin and radiation treatments. Int J Radiat Biol. 78:417–424.
- Oei AL, Vriend LE, Crezee J, Franken NA, Krawczyk PM. 2015. Effects of hyperthermia on DNA repair pathways: one treatment to inhibit them all. Radiat Oncol. 10:165.
- Rabik CA, Dolan ME. 2007. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treatment Rev. 33:9–23.
- Turchi JJ, Henkels KM, Zhou Y. 2000. Cisplatin-DNA adducts inhibit translocation of the Ku subunits of DNA-PK. Nucl Acids Res. 28:4634–4641.
- Van Bree C, Franken NA, Bakker PJ, Klomp-Tukker LJ, Barendsen GW, Kipp JB. 1997. Hyperthermia and incorporation of halogenated pyrimidines: radiosensitization in cultured rodent and human tumor cells. Int J Radiat Oncol Biol Phys. 39:489–496.
- Wilkins DE, Heller DP, Raaphorst GP. 1993. Inhibition of potentially lethal damage recovery by cisplatin in a brain tumor cell line. Anticancer Res. 13:2137–2142.
