Abstract
Purpose: The increasing global risk of nuclear and radiological accidents or attacks has driven renewed research interest in developing medical countermeasures to potentially injurious exposures to acute irradiation. Clinical symptoms and signs of a developing acute radiation injury, i.e. the acute radiation syndrome, are grouped into three sub-syndromes named after the dominant organ system affected, namely the hematopoietic, gastrointestinal, and neurovascular systems. The availability of safe and effective countermeasures against the above threats currently represents a significant unmet medical need. This is the first article within a three-part series covering the nature of the radiation sub-syndromes, various animal models for radiation countermeasure development, and the agents currently approved by the United States Food and Drug Administration for countering the medical consequences of several of these prominent radiation exposure-associated syndromes.
Conclusions: From the U.S. and global perspectives, biomedical research concerning medical countermeasure development is quite robust, largely due to increased government funding following the 9/11 incidence and subsequent rise of terrorist-associated threats. A wide spectrum of radiation countermeasures for specific types of radiation injuries is currently under investigation. However, only a few radiation countermeasures have been fully approved by regulatory agencies for human use during radiological/nuclear contingencies. Additional research effort, with additional funding, clearly will be needed in order to fill this significant, unmet medical health problem.
Introduction
Exposures to ionizing radiation, intended or unintended, are an undisputable reality of life, and such events can at times bring devastating health consequences (Carter et al. Citation2007). Hence, radiological preparedness and radiation countermeasures are grave security issues for the individual and the nation as well (Benjamin et al. Citation2009). Current radiological and nuclear threats can be broadly classified into several groups: (a) detonation of a nuclear bomb or a sophisticated nuclear weapon, (b) explosion of an improvised nuclear device, (c) use of a dirty bomb or a radiological dispersal device, (d) use of a simple radiological device, (e) an attack on a nuclear power plant, and (f) unintended radiological/nuclear accidents (Pellmar & Rockwell Citation2005). The number of people who will need medical attention after a large-scale radiological/nuclear event will undoubtedly be large. Injurious ionizing radiation includes γ- and X-rays, neutrons, α- and β-particles, high-speed electrons, high-speed protons, and other particles capable of producing ions upon impinging with given biologic or non-biologic materials. Radiosensitivity is, by simple definition, the susceptibility of cells, tissues, and organs to respond to molecular ionizations following irradiation with intensely energetic atomic particles. It has been long-recognized that the radiosensitivity of a given tissue within the body can often be related to its mitotic activity and its capacity to ‘self-renew’ (Bergonié & Tribondeau Citation1959; Casarett Citation1980). In general, it has been observed that radiosensitivity of the tissue’s cells is directly related to the rate of cell division and inversely proportional to the extent of cell differentiation (Sevan’kaev & Kozlov Citation1974; Aleksandrova et al. Citation1980).
Radiation exposures from accidents or other sources of radiation can result in different types of radiation injuries, dictating the diagnostic and therapeutic measures. The clinical progression of acute radiation syndrome (ARS) depends on the tissue-absorbed radiation dose and its distribution within the body. Multi-organ involvement and organ system failure need to be taken into consideration (Dorr & Meineke Citation2011). The extent of radiation-induced injury to lymphohematopoietic tissues, gastrointestinal (GI) and neurovascular systems, lung, and skin plays a vital role in the diagnosis, treatment and the prognosis of victims exposed to radiation. Dependable, useful information about the dose and the extent of tissue injury can come from biodosimetry and physical dosimetry. However, these dose estimates are usually not available in the majority of accidents; the estimation of radiation effects can be accomplished on the basis of clinical symptoms as described in the medical treatment protocols for radiation accident victims (Weinstock et al. Citation2008; Gourmelon et al. Citation2010). Since the hematopoietic tissues of all mammals are highly sensitive to ionizing irradiation, diagnostic and therapeutic measures for hematopoietic ARS (H-ARS) are critical to the health and well-being of the exposed individual. The main therapeutic principles behind such strategy are the transplantation of hematopoietic stem cells (HSC), substitution with blood products, and the administration of cytokines or growth factors like granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF). There is a compelling need for internationally accepted strategies for the treatment of potentially lethally-exposed radiation victims (Dainiak et al. Citation2011b).
ARS manifests following whole-body or partial-body irradiation in humans at estimated radiation doses generally above 1 Gray (Gy) and delivered at relatively high dose rates (e.g. ∼0.05 Gy/h or higher). Clinical manifestations of ARS include H-ARS (2–6 Gy), Gastrointestinal ARS (GI-ARS) (>6 Gy) and neurovascular (>10 Gy) sub-syndromes (McCann Citation2006). Though a wide range of radiation doses can trigger both H-ARS and GI-ARS, their impact on mortality is both dose- and time-dependent. Currently, the neurovascular syndrome is generally considered untreatable because at such extremely high levels of radiation exposure, death occurs quickly (e.g. generally within 24–48 h) largely as a result of systemic vascular accidents and multi-organ failure. Symptoms of neurovascular syndrome occur quickly, with nausea and vomiting occurring within minutes of exposure. Again, due to the extremely rapid onset of symptoms and significant, irreversible damage to organs, the syndrome is currently considered fatal, even with full supportive care (Armed Forces Radiobiology Research Institute Citation2013). Individuals exposed to lower doses of radiation resulting in H-ARS and GI-ARS are likely to respond to appropriate countermeasures. Hence, only these two sub-syndromes have been focused on largely for the development of radiation countermeasures. The H-ARS in all models is characterized by a critical loss of HSC and progenitor cell populations, leading to significant, life-threatening blood cytopenias. The GI-ARS is characterized by massive cell death (apoptosis) within the GI epithelium followed by breakdown of the mucosal epithelial barrier and death from fluid and electrolyte loss, sepsis, and intestinal bleeding. The lower lethal doses cause critical bone marrow (BM) injury and modest GI damage. The GI tract demonstrates rapid cell turnover. As a result, it is particularly sensitive to damage by irradiation. Stem cells in the GI tract, essential to epithelial integrity, are damaged by radiation, resulting in impaired cell proliferation and function. Proper intestinal stem cell function is crucial for optimal GI performance (Booth et al. Citation2012). Classification of these ARS-associated sub-syndromes represent an oversimplification of the disease process since multi-organ dysfunction plays a major role (Fliedner et al. Citation2005; Hill Citation2005; Moulder & Cohen Citation2005).
A good medicinal for large-scale, radiological exposure contingencies should ideally be easy to handle and to administer, stable under extreme conditions, thermoresistant, inexpensive, deployable, and must meet medical needs and therapeutic support. Radiation countermeasures have been categorized into three classes based on the time of administration in relation to radiation exposure (Stone et al. Citation2004): radioprotectors, radiomitigators, and radiation therapeutics. Radioprotectors (prophylaxis agents) are administered prior to radiation exposure to prevent radiation injury to tissue during the early phase of radiochemical events. Such agents include the free radical scavengers (e.g. amifostine and vitamin E isomers or other tocols) and must be present at the time of radiation exposure (Weiss & Landauer Citation2009; Singh et al. Citation2013). Radiomitigators are administered soon after radiation exposure in order to stimulate recovery of injured tissues, but prior to the appearance of overt symptoms. Radiomitigators include immunomodulating agents such as 5-androstenediol, interleukin-12, G-CSF, PEGylated G-CSF, GM-CSF, and CBLB502 (truncated flagellin), as well as and angiotensin-converting enzyme inhibitors (ACE; captopril) (Seed Citation2005; Dumont et al. Citation2010; Singh et al. Citation2012, Citation2014a; Moulder Citation2014). Radiation therapeutics are agents administered once symptoms manifest in order to accelerate regeneration of tissue and organs (MacVittie et al. Citation1994; Wagemaker et al. Citation1998; Farese et al. Citation2001, Citation2013; Drouet et al. Citation2004; Herodin et al. Citation2007; Dumont et al. Citation2010). G-CSF, PEGylated G-CSF, GM-CSF, and cellular therapy falls under this class (Singh et al. Citation2015a). The outcome of any given countermeasure for the purpose of limiting the risk of potential radiation lethality may depend on the time between radiation exposure and countermeasure administration.
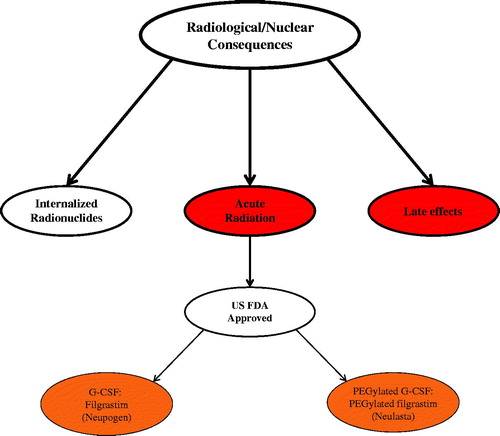
Despite significant advances over the past six decades to develop safe, non-toxic, and effective radiation countermeasures for radiation injuries, only a few agents have been approved by the United States Food and Drug Administration (US FDA) for human use (Singh et al. Citation2016b). This is the first article within a three-part series presenting a comprehensive review of radiation countermeasures. Here we have discussed radiation sub-syndromes, animal models for developing radiation countermeasures, and agents approved by US FDA for ARS ().
Figure 1. Current status of radiation countermeasures for radiological and nuclear threats. In 2002, the FDA issued the Animal Rule to expedite the development of medical countermeasures (MCM) against chemical, biological, radiological, and nuclear threats. Since then, two countermeasures (Neupogen and Neulasta) have been approved by the FDA for ARS following the Animal Rule. Color coding: red, acute, early arising injuries and chronic, late-arising injuries; burnt orange, FDA fully approved medicals.
Radiation-induced damage
The nucleus of the cell is often considered the primary target of ionizing radiation as it is a large organelle that houses vital reproductive machinery (the genome). However, recent experimental evidence demonstrates that this concept is often inadequate for explaining additional, non-nuclear effects of radiation, specifically the response of the mitochondria as an important source of coding deoxyribonucleic acid (DNA) (Kam & Banati Citation2013).
The biological effects of ionizing radiation have been categorized into two types: direct and indirect. Direct effects are when highly energetic (with ionizing potential) subatomic elements (photons, neutrons, alpha and β-particles) impinge and are absorbed by tissue cells while interacting with biological molecules causing ‘direct’ molecular damage to vital ‘targets’. The atoms of the target molecules can get ionized or excited, resulting in a chain of reactions ending in direct structural and functional alterations of those targets. Alternatively, radiation may interact with free or bound intracellular water to generate reactive oxygen species or free radicals, which in turn damage critical biomolecular targets. This is known as an indirect action of radiation.
Direct damage
Ionizing radiation is a DNA-damaging physical agent capable of causing lesions in cellular DNA, including double-strand breaks, single-strand breaks, DNA cross links, and more than 20 types of base damage. Exposure of cells to sublethal doses of ionizing radiation usually produces within each exposed cell an estimated average of: 40 double-strand breaks, 1000 single-strand breaks, and 3000 damaged bases per Gy (Prise et al. Citation2005). DNA double-strand breaks are generally considered critical mediators of radiation-induced cell death. Although cells have developed complex DNA repair mechanisms, a large number of breaks, often in close association, are unrepairable or misrepaired, leading to cell death as well. The latter is largely the result of the radiation-damaged cell’s inability to fully navigate the cell cycle and pass through cell division.
Indirect damage
In addition to its direct action, ionizing radiation is also capable of causing cellular damage indirectly via free radial generation. The indirect mechanism is responsible for approximately two thirds of the biological damage produced by highly energetic photons from X-rays or γ-rays. These energetic photons interact with available cellular water producing highly reactive and toxic free radicals. These processes occur largely at the time of radiation exposure, or within microseconds of the irradiation, interacting chemically with DNA, producing molecule-modifying radicals that serve to ‘fix’ (makes permanent) the damage by the ionizing irradiation. Oxidation of lipids and proteins induced by the free radicals generated by radiation exposure can serve as a major trigger for healthy tissue damage (Daly Citation2012).
There are additional indirect mechanisms by which ionizing radiation exerts its toxic effects; one prominent indirect mechanism is through an inflammatory process that itself reduces free radicals. After radiation exposure, there is an initiation of a pro-inflammatory reaction in both exposed and adjoining tissues alike, leading to the production of several pro-inflammatory chemokines and cytokines soon after irradiation. These agents comprise Interleukin-1, Interleukin-6, tumor necrosis factor and transforming growth factor-β. Transforming growth factor-β is of particular importance in pathophysiology of radiation injury specifically with regard to facilitating radiation-induced lung and skin fibrosis. Ultimately, these mediators start a long-term inflammatory response leading to chronic inflammation and tissue injury (Kim et al. Citation2014). The pro-inflammatory reaction is largely responsible for many of the long-term problems associated with radiation injury, whereas free radical generation is associated with acute as well as delayed toxicities.
In addition to local effects, systemic effects also occur after irradiation. These include the immunosuppressive actions of ionizing radiation on the lymphohematopoietic systems. Similar to local effects of radiation exposure, systemic effects appeared to be mediated indirectly, in part, through the production of pro-inflammatory cytokines, oxidative stress, and damage to vital biomolecules, specifically DNA. Other factors include the production of adenosine triphosphate, heat shock proteins, and uric acid. The combination of some or all of these factors leads to compromised cellular and tissue integrity (Mavragani et al. Citation2016).
Radiation sub-syndromes for organ system/tissue injury
Since most of the radiation countermeasures under development are for specific sub-syndromes, it is reasonable to briefly discuss these sub-syndromes.
Hematopoietic syndrome (H-ARS)
H-ARS (development of neutropenia, thrombocytopenia, and anemia), emerges at the lowest end of the scale of radiation doses that leads to acute injury. It is a result of the relative high radiosensitivities of committed progenitors in specific lineages of neutrophils and thrombocytes (Inoue et al. Citation1995; Goans et al. Citation1997; Dainiak Citation2002; Waselenko et al. Citation2004). Radiation doses of 2 Gy and above leads to significantly decreased blood leukocyte counts and immunosuppression, rendering exposed victims susceptible to secondary infections. Exposure may lead to lethal hemorrhage, BM failure, and/or microbial infections. Without any treatment, death may occur within 2–4 weeks post-irradiation. Therefore, reconstitution or protection of the hematopoietic systems is a foremost concern (Gianni et al. Citation1989; Laterveer et al. Citation1996).
Rates of lineage-commitment of stem cells and subsequent proliferation and differentiation of associated progenitorial cells within lymphohematopoietic tissues determine the time required for each symptom to manifest. The time until symptoms manifest, the level of the nadir based on cell count, and the recovery period (time it takes to return to baseline) for each cell lineage have been characterized as secondary endpoints to investigate the degree of hematopoietic damage. Pluripotent stem cells localized in the BM are able to survive mid-range doses of radiation that result in H-ARS; however, when the dose increases, the fraction of surviving stem cells decreases and the recovery of various progenitorial blood cell lineages is delayed. Radiation countermeasures for H-ARS are largely based on the stimulation of the recovery of various hematopoietic cell lineages.
The harmful effects of radiation exposure on hematopoiesis have been well-established and studies conducted in various animal models have been valuable (Dainiak Citation2002; Waselenko et al. Citation2004; Stone et al. Citation2004; Augustine et al. Citation2005; Williams et al. Citation2010). Various animal models have been used to develop radiation countermeasures for H-ARS and the three models that have been used extensively in the majority of studies are mice, canines, and non-human primates (NHP) (Stone et al. Citation2004; Augustine et al. Citation2005; Dumont et al. Citation2010). The most well-defined and widely used endpoint for H-ARS evaluation after total-body irradiation (TBI) is the radiation dose that results in the death of approximately 50% of the population, i.e. the LD50 value. Development of H-ARS occurs more quickly in mice, with 50% the population dead within 30 days (d) after irradiation (LD50/30); for larger animal models such as the NHP, a 60-d period is considered a more appropriate measured time frame (LD50/60) (Farese et al. Citation2012a).
Gastrointestinal syndrome (GI-ARS)
The GI tract is especially sensitive to irradiation and lethality as a result of GI failure is the primary endpoint for GI-ARS. GI radiation injury, leading to enterocyte depletion, breakdown of mucosal barrier, and mucositis with diarrhea, occurs as a result of several sequential and concurrent pathophysiological events. Other components of the GI tract also contribute to system dysfunction; immune system, the enteric muscularis, microvasculature and nervous systems, as well as the resident microbes (bacteria and fungi), though, GI injury is typically accredited to the death of clonogenic crypt epithelial stem cells (Withers & Elkind Citation1968). The pathological features of GI injury is characterized by protein alterations, differences in redox status, secondary consequences due to inflammation and release of cytokines, and the functional consequences of cell death (Denham et al. Citation2001). Cellular compartments may add to and regulate organ dysfunction, but the vital event in the pathophysiology of GI damage is enterocyte loss, with vascular injury contributing more heavily at higher radiation doses (Ch’ang et al. Citation2005). The physiological component involves early vomiting and diarrhea, which are normal GI-related symptoms of radiation exposure. Vomiting is the reflexive act of forcefully ejecting the stomach contents through the mouth by coordinated muscle contraction. Clinical studies have demonstrated that patients receiving TBI or upper-abdominal irradiation frequently display nausea, retching, and vomiting as side-effects. These symptoms can exacerbate electrolyte and fluid loss and possibly lead to death (Hall & Giaccia Citation2012). At radiation doses below the threshold for GI-ARS, but at the mid to upper range of radiation doses for H-ARS, breakdown of the mucosal barrier allows bacteria to translocate into peripheral circulation, that can lead to sepsis and death, in the setting of concomitant immunosuppression (Geraci et al. Citation1985). With extended times following exposure, substantial tissue remodeling occurs as a consequence of the initial radiation-induced injury and serves to alter the structure, absorption potential, and motility of the gut. Fibrosis makes it more stiff and susceptible to adhesions, stenosis, and perforation (Carr Citation2001).
A current, well-used endpoint for studying GI-ARS is animal lethality within 10 d after high dose, high dose rate irradiation (LD50/10). At low rates of radiation exposure, this ‘time’ parameter, along with other correlates of gut injury change, often substantially (Brennan et al. Citation1998). The conventional histological endpoint in mice is the regenerating crypt numbers counted in the cross section of jejunum (3.5 d for mouse jejunum); jejunal crypt cell survival may not always correlate with animal survival due to the impact of probable simultaneous damage to the immunohematopoietic system (Mason et al. Citation1989). Functional analysis of GI damage includes the bacterial translocation into the peripheral blood stream, GI motility and permeability, and citrulline levels in blood plasma (Kobayashi et al. Citation1991; Krimsky et al. Citation2000; Lutgens et al. Citation2005). Radiation-stimulated vomiting as an endpoint has been extensively studied in canines; however, the mechanisms underlying this symptom are not entirely clear, as induced responses do not precisely correlate between humans, NHP, or canines (Dubois et al. Citation1984; Lang et al. Citation1986; Danquechin Dorval et al. Citation1985; Makrauer et al. Citation1999). Abdominal radiation exposure has been demonstrated to triple the occurrence of colonic giant migrating contractions, with more than half initiating in the small intestine which leads to diarrhea (Otterson et al. Citation1988, Citation1992).
Neurovascular syndrome
Clinical presentation of the neurovascular syndrome generally occurs following TBI or local cranial doses well in excess of 10 Gy in humans. Although this syndrome is recognized as a distinct clinical entity, it clearly has a role in the presentation of cardiovascular sub-syndrome as well. This syndrome arises from localized changes in the nervous system and includes injury to the blood-brain barrier, impaired capillary circulation, inflammation of the meninges, interstitial edema, petechial hemorrhages, acute inflammation, and hypertrophy of perivascular astrocytes (Jurkovic & Vokrouhlicky Citation1963; Schultheiss et al. Citation1995; O’Connor & Mayberg Citation2000; Warrington et al. Citation2013). Signs and symptoms comprise of continued and severe vomiting, nausea, accompanied by headache, disorientation, neurologic deficits, loss-of-balance, confusion, and seizures. Physical examination may demonstrate ataxia, papilledema, and reduced or absence of deep tendon and corneal reflexes (Lopez & Martin Citation2011). Damage resulting from particularly high radiation exposure has been considered untreatable. Therefore, the research community has concentrated its focus finding preventative and mitigating countermeasures for the H-ARS, GI-ARS, pulmonary and cutaneous sub-syndromes exclusively.
Pulmonary syndrome
The lung is a highly radiosensitive organ in terms of its susceptibility to acute and chronic inflammation and the subsequent induction of fibrosis that can be life-threatening (Travis Citation2001). Exposure to high doses of internally deposited radionuclides or to high external thoracic doses, commonly manifest over time in life-threatening lung pathologies. These pathologies can be both cancers (stochastic) or non-cancer-types (non-stochastic) pathologies and all carry relatively high mortality risks. Pulmonary pneumonitis and fibrosis, two ‘non-stochastic-types’ of induced pathologies, tend to develop more quickly, especially following higher thoracic exposures than do lung cancers that are more prevalent following lower levels of radionuclides and longer post-exposure survival periods (Dagle & Sanders Citation1984). The majority of animal models demonstrate a higher tolerance to pulmonary radiation compared with humans. The time-frames for the earlier arising lung injuries to fully manifest are variable, ranging from a few months to a half of a year or more following irradiation; inflammatory pneumonitis within 2–4 months after irradiation and fibrosis 4–6 months post-irradiation (McLaughlin et al. Citation1966; Sharplin & Franko Citation1989a, Citation1989b; Marks et al. Citation2003). Histologically, pneumonitis is indicated by interstitial and airspace edema, predominantly macrophages as inflammatory infiltrate, and loss of epithelium (Travis Citation2001). Though clinical indications may not progress for weeks to months, irradiation immediately elicits a cascade of molecular and cellular events which advance during a clinical latent period; this course involves epithelial cells, endothelial cells, fibroblasts, macrophages, profibrotic and proinflammatory cytokines, and induced expression of various genes, as well as stimulation of transcription factors (Vujaskovic et al. Citation2000; Haston et al. Citation2002).
There are several animal models that have been previously developed and refined for analyzing pulmonary effects of irradiation (Chen et al. Citation2004; Epperly et al. Citation2004; Carpenter et al. Citation2005). The murine model is the most well-characterized animal model in terms of pulmonary damage; however, within this species, there is a remarkable deal of inconsistency in histopathological sequelae and severity of lung damage between different strains of animals in response to thoracic irradiation (Travis & Tucker Citation1986; Jackson et al. Citation2010, Citation2011, Citation2012). Murine lung pathology diverges from that of human pathology in respect to blood supply, relative thickness of the septa and pleura, and lobularity (McLaughlin et al. Citation1966). The three well-defined large animal models, NHP, canine, and swine, have their strengths and weaknesses relative to evaluating pulmonary damage. In the case of swine, limited data is available for either partial body or TBI though their lungs have a similar physiology to humans (Hopewell et al. Citation2000; Mandel et al. Citation1980). For canines, there is a plethora of information on the radiation-induced pulmonary response (Slauson et al. Citation1976), though the pathophysiology differs from that of the human. Despite the significant similarities in the physiology of the human to NHP, limited data is available in respect of radiation-induced pulmonary damage (Williams et al. Citation2010). However, there is a recent study that has clearly demonstrated delayed, potentially fatal lung injury in the NHP. In this study, thoraxes of the animals were exposed to radiation and supportive care was provided according to a standardized treatment protocol. The primary endpoint in this study was mortality at 180 d post-irradiation. A relative multi-parameter analysis was used, concentrating on the lethal dose response relationship (Garofalo et al. Citation2014a). This study defines the dose response and time course of the delayed pulmonary sequelae and may provide an effective platform for the MCM efficacy testing against the delayed pulmonary sub-syndrome.
Since the pulmonary sub-syndrome may be associated with elevated mortality and morbidity, appropriate treatments following high-doses of radiation should effectively serve to delay onset of the syndrome. All aforementioned animal models need to be validated for specific injury to the lungs to better understand the mechanisms leading to radiation injury (DiCarlo et al. Citation2012).
Cutaneous syndrome
Accidental radiation exposure of human skin with significantly elevated doses (e.g. ∼15–20 Gy or greater) leads to a discrete clinical manifestation, distinguished by a temporary and faint erythema after a few hours (h), followed by acute erythema, blistering, and necrosis. The necrosis generally occurs 10–30 d after exposure depending on severity of the injury, but in the most severe cases, such necrosis may appear within 48 h (Peter et al. Citation1994, Citation1997; Peter & Gottlober Citation2002). Early treatment strategies should comprise the use of systemic and topical anti-inflammatory agents as early as possible following exposure. These treatments should be continued throughout the acute and subacute phases, as this decreases the demand for surgical intervention, once necrosis has appeared (Peter & Gottlober Citation2002). The latest treatment strategy relies on the excision of the affected tissue based on the reconstructed skin dose in order to prevent recurrent necrosis and is followed by therapy with autologous keratinocytes and combined with allogeneic stem cell administration (Lataillade et al. Citation2007; Bey et al. Citation2010). There has been an increased interest in using stromal cells/mesenchyml stem cells, progenitor cells, and adipose stromal cells for cell and gene therapy for treatment of cutaneous syndrome and H-ARS (Semont et al. Citation2006; Lataillade et al. Citation2007; Abdel-Mageed et al. Citation2009; Agay et al. Citation2010; Bey et al. Citation2010; Saha et al. Citation2011; Forcheron et al. Citation2012; Singh et al. Citation2014b, Citation2014c). Swine have been used extensively to study the cutaneous effects of radiation exposure. The data from NHP for cutaneous effects are limited (Williams et al. Citation2010). There are several countermeasures, such as PrC-210 (Peebles et al. Citation2012), Fibroblast growth factor-P (Zhang et al. Citation2011), Pravastatin (Holler et al. Citation2009), Plerixafor (Kim et al. Citation2012), and curcumin (Okunieff et al. Citation2006; Ryan et al. Citation2013), that have demonstrated efficacy for cutaneous radiation syndrome in different animal models. A summary of the main sub-syndromes and their symptoms is provided in .
Table 1. Major sub-syndromes of ARS and their symptoms.
Delayed radiation-induced syndrome following acute irradiation
Surviving acute, relatively high dose ionizing radiation exposure of the whole-body carries additional health risks that often have dire outcomes. These pathological syndromes are delayed in expression, more chronic in nature, and associated with evolving pathologies within multiple organ systems of the body, including the BM, GI tract, lung, heart, kidney and the central nervous system. Often, especially in the older literature, these ‘delayed effects’ are referred to as ‘late effects’ of radiation exposures. Nevertheless, when fully expressed, these ‘delayed’ or ‘late effects’ of acute radiation exposure (often labeled currently with the acronym as ‘DEARE’) syndrome ultimately results in multiple organ system failure and death (Fliedner et al. Citation2005; Satyamitra et al. Citation2016).
Effective countermeasures for the fully expressed DEARE syndrome have yet to be fully developed. However, the early use of protective/mitigative medicinals at the time of acute exposure, or shortly thereafter, would likely minimize the severity of the initial radiation induced damage that underlies the expression of such delayed onset pathologies. Several research groups are focusing on developing medicinals that specifically target radiation-injured vasculature of various organ systems. Further comments on this area of research are provided under the countermeasure section.
Chronic radiation/low dose radiation-induced syndromes
Chronic irradiation of the body at relatively high dose-rates and with accumulation of comparatively high total-body doses will elicit, non-stochastically, largely the same spectrum of pathologies seen under acute, high dose exposures. By contrast, very low dose-rates and low cumulative exposures, stochastically driven syndromes (e.g. cancers) dominate the spectrum of radiation-induced diseases. The principal drivers of these two major classes of induced responses involve not only the physical nature of the irradiation (dose, dose-rate, radiation quality) but also the genetic and physiological makeup of the species being irradiated (e.g. mouse versus man). Studies of chronic radiation syndromes in both mouse and canines provide good examples of the latter. Depending on the daily rate of irradiation and duration of exposure, the noted pathological syndromes change from ‘acute-type’ effects (as observed as H-ARS type responses) to classic late effect-type pathologies, characterized by the predominance of neoplastic diseases. In chronically irradiated canines under prolonged course (e.g. duration or near-duration of life) exposures, the time-frame for induction of different types of cancers appear quite specific, occurring in time-dependent waves: first the hematopoietic neoplasias and subsequently the non-neoplastic solid tumors (Seed et al. Citation2002c). Studies of chronically irradiated mice show similar responses, but the noted spectrum of induced cancers are restricted by the strain of mice being irradiated (Hoel et al. Citation2005).
FDA animal rule
The Animal Rule states that the FDA may grant marketing approval of a drug under development to treat or prevent a serious or life-threatening condition caused by a permanently disabling or lethal toxic substance, if animal efficacy studies adequately establish that the drug will produce a clinical benefit in humans (Aebersold Citation2012; US Food and Drug Administration Citation2015b). Drugs being developed in this manner should follow the existing requirements for establishing the safety of new drugs. The FDA will rely on the animal efficacy studies if the following four criteria are met:
The pathophysiological mechanism of injury/toxicity by the stimulus/agent is understood, as well as the role of the product in the prevention or significant reduction of said injury or toxicity.
The efficacy of the product is demonstrated in more than one animal species with a response predictive of human response, or a single animal species that has been adequately characterized for predicting the human response.
The animal study endpoints clearly related to the desired result in humans, normally reduced mortality or morbidity.
The effective dose can be calculated from kinetic and pharmacodynamics of the product (or relevant data) in both humans and animals.
Only when all of these criteria are met, it is reasonable to expect a drug tested in animals to be indicative of human efficacy.
The Animal Rule is intended to be used in the development of drugs to counter debilitating or lethal conditions resulting from either accidental or deliberate exposure to chemical, biological, radiological, or nuclear substances when human clinical studies for efficacy are not feasible or ethical.
The FDA may also take into account additional data when determining if the animal data is sufficient; such data can come from different types of studies, including, but not limited to: in vitro studies, other animal studies, and human studies. The data from human studies can be from studies for the same indication or from a relevant indication not utilizing the Animal Rule for approval. This additional data may be supportive, but well-controlled animal efficacy studies remain a strict requirement to receive approval.
Although human data from other studies evaluating a particular drug for a different indication can be used in support of drug approval for an indication that seemingly requires the use of the Animal Rule, the FDA encourages the drug manufacturer, i.e. the applicant, to consider first evaluating the prospective new drug for its relevant indication using the traditional regulatory pathway. Such a relevant indication may include one where the drug targets the same pathway in the pathophysiological cascade as that indication being considered for evaluation using the Animal Rule. The Animal Rule specifies that the species utilized in the efficacy studies must be appropriate in regards to both the condition or injury and the drug being evaluated. Though no particular species are specified in the Animal Rule, only animals that can be scientifically justified in terms of key characteristics comparable to the human disease or condition should be utilized; likewise, the mechanism by which the drug produces its effects should also be comparable to that of humans, to determine appropriate doses and schedule for human efficacy. The number of animals utilized in the animal efficacy studies will depend upon the differences between that particular animal model and humans in terms of nature and clinical significance.
If a study utilizes a sufficiently well-characterized animal model to yield adequate support for approval, a confirmatory study also utilizing that model may still be required; ideally this conformational study would be conducted in another laboratory, but with reasonable justification and reasoning, the same laboratory may be acceptable.
Additional requirements for drug approval through the Animal Rule are as follows:
A plan outlining the approach to field studies that would evaluate the safety and clinical benefit of the drug, in the event that such a study would be ethical or feasible (i.e. Emergency event where the drug is administered).
Restrictions regarding distribution, administration and use, to ensure safety (i.e. special training of institute or healthcare practitioners, certain follow-up procedures or record keeping, etc.).
The label should include information that the drug was approved based solely on animal efficacy studies for ethical and feasibility reasons along with all other information the FDA requires on drug labels at the time of approval. These details should be communicated before administering or dispensing, if possible.
Any and all products approved via the Animal Rule must follow post-marketing, record keeping, and safety reporting regulations. Additional information regarding the procedures for withdrawing, or submitting promotional materials, etc., can also be found in the regulations outlined in the Animal Rule. To date, only two radiomitigators have received FDA approval under Animal Rule as illustrated in .
Table 2. Radiation countermeasures approved by U.S. FDA under animal rule.
Animal models for development of radiation countermeasures (for both acute- and chronic-induced injuries)
There are a large number of radiation countermeasures at various stages of development to treat or prevent radiation injuries. Under this section, we have focused on the animal models used for the development of promising radiation countermeasures (). Rodent (mice), canine, and NHP models are used most frequently for the development of radiation countermeasures and studying the injuries caused by radiation exposure. Some studies have used additional animal models such as rats, guinea pigs, rabbits, ferrets, and minipigs to study the effects of radiation exposure for the development of selected radiation countermeasures (Martin et al. Citation1998; King et al. Citation1999; Augustine et al. Citation2005; Williams et al. Citation2010, Citation2012; Sanzari et al. Citation2013; Krigsfeld et al. Citation2014a; Shim et al. Citation2014).
Table 3. Animal models for ARS and types of ARS sub-syndromes.
There are, by contrast to the above, relatively few MCM specifically investigated and developed for the purpose of countering late-arising pathologies associated with chronic or protracted exposures. However, the few countermeasures that have been designed and studied, include the ‘ACE inhibitor-like’ agents to counter late-arising kidney disease, captopril to counter tissue fibrosis (e.g. pulmonary fibrosis), and several anti-cancer related agents such as the aminothiols, and inhibitors of topoisomerase (Grdina et al. Citation1991; Cohen et al. Citation2012; Medhora et al. Citation2014; Gokare et al. Citation2016). Though emphasis is given to animal models for ARS, all models discussed below have also been used in studies for late effects (Williams et al. Citation2012).
Mice
Mice are used frequently as a basic animal model for pharmaceutical research. Among the many advantages to using the mouse as an animal model, the most important is their relatively small size and ease of handling, as well as physiological, and genetic similarities to humans. The mouse’s short generation time, body size, and an accelerated lifespan (one mouse year roughly approximates 30 human years) provide advantages in terms of the space, cost, and time required for studies. Another advantage to using the mouse model is the full complement of research reagents available; this model has the greatest number and range of reagents currently available compared to those of any other animal model. The main disadvantage of the rodent model is small body thickness, which does not account for the intrinsic heterogeneity of radiation dose distribution inherent to human exposure. The majority of investigators have used inbred mouse strains, although several groups have used hybrid strains (Stone et al. Citation2004; Augustine et al. Citation2005; Williams et al. Citation2010). The most common mouse strains used are C3H/HeN, B6D2F1/J, BALB/c, CD2F1, and C57BL/6. These strains show significant variation in their response to acute irradiation, as demonstrated by their range of LD50/30 values (6.5–9.0 Gy), with the BALB/c being most sensitive and the C57BL/6 most resistant (‘drift’ in the dose-response relationship and LD50/30 may occur in any laboratory). Further, various strains exhibit significant variation in the type and frequency of late-arising pathologies that develop following either acute or late expressed health effects; e.g. AKR strain exhibits a propensity to develop thymic lymphoma, whereas the CBA or RFM strains exhibit propensities to the myeloproliferative disorders, particularly to myeloid leukemia (Shin et al. Citation2010; Rivina et al. Citation2014). The BALB/c strain has been reported to have a double-strand DNA repair defect that may account for its enhanced radiation sensitivity (Okayasu et al. Citation2000). In general, mouse models appear quite appropriate for the initial efficacy testing of new radiation countermeasures, as screening protocols are well established and general response patterns documented within radiobiological literature. A large number of radiation countermeasures for ARS have been investigated in murine models (Singh et al. Citation2013, Citation2014a, Citation2014b; Rosen et al. Citation2015). The strain differences outlined above suggest that radiation countermeasures should be tested in more than one mouse strain; in terms of countermeasures for ARS, one resistant (C57BL/6 or CD2F1) and another sensitive strain (C3H/HeN or BALB/c) should be tested for other ‘delayed-type’ response patterns, specific, pathology-susceptible strains need to be considered and utilized in testing.
The mouse model has been used to study combined injury (radiation plus wound, burn, or hemorrhage) as well as for the evaluation of countermeasures effective against combined injuries (Kiang et al. Citation2012, Citation2014a, Citation2015; Elliott et al. Citation2015). Efficacy of several radiation countermeasures such as captopril, PEGylated G-CSF, ghrelin, ciprofloxacin etc., has been established using the combined injury model in mice (Jiao et al. Citation2009; Kiang et al. Citation2010, Citation2014b; Swift et al. Citation2015).
Though several animal models have been studied for pulmonary response to radiation exposure, mice are the most widely used model for lung injury. The fibrosis-prone C57BL/6J is relatively poor model of lung injury due to the pleural effusions (Williams et al. Citation2012). C3H and CBA appear to closely resemble closely the human lung response.
Mice have been used extensively to characterize the health effects (both stochastic and non-stochastic types) and associated potential mitigative treatments of protracted ionizing radiation exposures: e.g. Carnes and Grdina (Citation1992) reported on the stochastic anti-cancer actions of amifostine and its long-term survival sparing effects in mice subjected to fractionated regimens of relatively low dose exposures of fission neutrons (Carnes & Grdina Citation1992). Similarly, Yasushi Kataoka from the Grdina laboratory at the University of Chicago reported on the anti-mutagenic actions of amifostine of mice chronically exposed to whole-body cobalt-60 (60Co) γ-rays or to fission neutrons (Kataoka et al. Citation1992).
Rat
Rats have been used in a relatively limited number of studies for radiation countermeasure development compared to mice. There are numerous reports establishing the rat model for studying radiation injury, both of an acute, early arising nature as well as the more chronic, delayed-type injuries, along with complementary studies of various types of radiation countermeasures (Geraci et al. Citation1995; Moulder et al. Citation1998; Williams et al. Citation2010; Jenrow et al. Citation2010; Kma et al. Citation2012; Gao et al. Citation2013). Rats have been used to study radiation-induced pneumonitis and lung fibrosis (Ghosh et al. Citation2009; Gao et al. Citation2012). A study using a single high dose of TBI capable of inducing pneumonitis, a dose relevant to radiological terrorism or nuclear accidents, was used to demonstrate that a short course of EUK-207, a synthetic superoxide dismutase (SOD)/catalase mimetic, starting 7 d post-irradiation and stopping before appearance of pneumonitis, mitigates radiation-induced pneumonitis and pulmonary fibrosis, as well as increased survival in rats (Gao et al. Citation2012; Doctrow et al. Citation2013). EUK-207 mitigation of lung fibrosis was also demonstrated using a model of whole-thoracic irradiation. Another agent, Human ghrelin, attenuated intestinal injury and mortality after TBI in male Sprague Dawley rats (Wang et al. Citation2015). Captopril has been shown to mitigate radiation-induced lung injury in rats (Medhora et al. Citation2012). Wistar rats have been used to demonstrate the efficacy of rifaximin against TBI (Jahraus et al. Citation2010). SOD, as well as SOD mimetics, have been reported to be effective in mitigating both early- and late-arising, radiation-induced lung injury in various rodent models (Vujaskovic et al. Citation2002). In addition to late-arising lung injury, rats have been used in studies of late-arising, non-stochastic type of injuries to several major organ systems of the body; e.g. chronic kidney injuries with mitigative responses of (a) captopril and dexamethasone (Geraci et al. Citation1995), (b) ACE inhibitors (Ward et al. Citation1989; Moulder et al. Citation1998), and (c) the central nervous system and associated mitigative countermeasures (Geraci et al. Citation1995; Robbins et al. Citation2010). Countermeasures assessed to date certainly include, but not limited to captopril and dexamethasone.
Rats have also been used to study combined injury (radiation plus wound) (DiCarlo et al. Citation2010; Zawaski et al. Citation2014). Laminin 332 deposition was found to be diminished in the irradiated skin of WAG/RijCmcr rats when subjected to combined radiation and wound skin injury (Jourdan et al. Citation2011). Rat research reagents are not as common as those for mice, making analysis of some parameters difficult.
Guinea pig
The guinea pig is a small, easily handled, and readily accessible animal model that has been used for experimental purposes for a century or more, with biological similarities to humans making them useful in various fields of research. There are large numbers of reports studying effects of radiation exposure and countermeasures using both X-rays and γ-rays (Bianchi Citation1963; Osmond et al. Citation1966; Jacquet et al. Citation1994). Several countermeasures such as metformin, N-acetyl cysteine, corticosteroids, piracetam, and barium chloride have been evaluated using the guinea pig as an animal model (Rosenthal et al. Citation1960; Hilf et al. Citation1961; Wisniewski et al. Citation1972; Altas et al. Citation2006; Gulbahar et al. Citation2009; Zhu & Liu Citation2009; Mujica-Mota et al. Citation2014). This model has also been used to specifically study radiation-induced nasal and ear injuries and mitigation by various agents.
Rabbit
The rabbit is a readily available, non-aggressive animal model used in experiments to understand the physiological and pathological processes affecting human beings. This animal model has been used to study the radiation-induced injuries and investigate radiation countermeasures using different radiation sources. There are several reports for studying amifostine using this animal model (Bohuslavizki et al. Citation1998, Citation1999; Hakim et al. Citation2005). Fms (mcdonough feline sarcoma viral oncogene homolog)-related tyrosine kinase-3 ligand (FLT3L) has also been tested for efficacy and compared with G-CSF’s therapeutic efficacy either alone or in combination using 60Co γ-irradiated New Zealand White rabbits. Results demonstrated that FLT3L was more efficacious than G-CSF in terms of survival and protection of hematopoietic tissue when either recombinant was used singly, but showed still further therapeutic benefit when the two were used in combination (Gratwohl et al. Citation1998).
Ferret
Ferrets have been used for studies in pharmacology, toxicology, and virology, and they are considered a very appropriate animal model to mimic human radiation-induced vomiting and retching (King Citation1988). Another advantage of using ferrets as an animal model for radiation-induced vomiting is that the prodromal response appears at lower doses and with an earlier onset time as compared to other species. The effective dose at which 50% of ferrets exhibited an emetic response to radiation is similar to that of humans (Sanzari et al. Citation2013). Data from the ferret studies have been used to develop a mathematical model for the human emetic response to radiation as well as to identify the dopaminergic mechanisms that play a minor role in radiation-induced emesis in the ferret (Benson et al. Citation2008).
The LD50/30 for ferrets following radiation exposure was estimated as 1.5 Gy and the survival curves for γ-irradiated ferrets were comparable to those for the proton-irradiated animals (Krigsfeld et al. Citation2014a). The ferret’s high degree of radiosensitivity highlights differences between various mammalian species relative to their sensitivity to acute injury and subsequent survival patterns. Many large animal species exhibit comparably high radiosensitivities that have been attributed previously to the relatively low efficiency of progenitorial compartments within lymphohematopoietic tissues to maintain and to reconstitute seriously compromised tissues following acute irradiation (Vriesendorp & van Bekkum Citation1984). Despite such differences in progenitorial function between species, it is well-recognized that progenitorial BM cells of the various mammalian species have similar sensitivities to ionizing radiation. The latter observation appears incongruous with the fact that different species, such as the ferret, can exhibit markedly different survival patterns following ionizing irradiation. Again however, a number of researchers (experimental hematologists) suggest that these differences in acute radiation sensitivities expressed among species likely relate to species-specific differences in the required number of HSCs to reconstitute/regenerate the hematopoietic system (and related renewing systems) following acute, potentially fatal irradiation-related injury (Vriesendorp & van Bekkum Citation1984). More recent studies have suggested that other factors may be involved in these varying survival patterns among species: e.g. disseminated intravascular coagulation (DIC) may be a significant, contributing factor leading to ferret death at the LD50 dose of radiation (Krigsfeld et al. Citation2014a). DIC is a serious and life-threatening condition in which the coagulation mechanism is over activated, causing bleeding and clotting to occur at the same time (Krigsfeld et al. Citation2014b). This condition may be the major mechanism by which relatively low doses of radiation causes death in large mammals.
Minipig
Swine (specifically the Göttingen minipig) have been used as large animal models in medical research procedures and are now regarded as an appropriate animal model for many types of drug validation studies (Agay et al. Citation2010; Forcheron et al. Citation2012; Shim et al. Citation2014; Singh et al. Citation2016c). This is consistent with previous basic radiobiological investigations utilizing young, first-generation crosses of swine; e.g. Large White crossed with Land Race strains, in basic radiobiological studies of radiation dose, dose-rate, and radiation quality (Lemaire & Maas Citation1984). The body thickness of a minipig is comparable to that of a young human, resulting in radiation absorption patterns that are quite similar (Zoetelief et al. Citation1984). The pathophysiology of H-ARS in the minipig is similar to that observed in humans, NHP, and canines; however, this model has proven to be very sensitive to TBI with an LD50/30 less than that of the canine, NHP, and that suspected of the human as well (Moroni et al. Citation2011a, Citation2011b). The low LD50/30 value of the minipig, 1.73 Gy (Moroni et al. Citation2011b), has been suggested to be due to the abnormally high radiosensitivity of its vascular/endothelial cells. Recent studies suggest that radiation-induced DIC may be a contributing factor to the radiosensitivity of minipigs (Krigsfeld et al. Citation2014a, Citation2014b). It is important to note that the study being referred to was conducted using X-rays; however, it is believed that the findings would be comparable for γ-rays as well. This degree of high radiosensitivity may ultimately limit the minipig’s utility as a model for human ARS.
A report which used a small number of minipigs to study high-dose irradiation-induced GI-ARS (abdominal irradiation with 10 and 15 Gy 60Co γ-radiation, 1.4317 Gy/min) suggests that the minipig mimics human GI-ARS (Shim et al. Citation2014). This study also evaluated plasma citrulline levels as a biomarker for radiation-induced GI damage. Another report used TBI (5–12 Gy 60Co γ-radiation, 0.6 Gy/min) in Göttingen minipigs to study GI-ARS (Elliott et al. Citation2014) and demonstrated a dose-dependent occurrence of parameters associated with GI-ARS (plasma citrulline, diarrhea, vomiting, bacterial translocation, and intestinal crypt loss). This study was limited however not only by the small number of animals used per radiation dose, but also by the early occurring deaths (≤ 10 d post-irradiation), suggesting the need of supportive care in order to extend survival time to allow sufficient time to assess crypt epithelial cell transit time and evaluate countermeasure efficacy. To date, there is still no comprehensive study defining the GI-ARS dose-response relationship and survival/probit curve for acute TBI in the minipig.
Göttingen minipigs have been used to establish a single-high-radiation-dose-induced lung injury model and to characterize a thoracic computed tomography-based method to measure the progression of radiation-induced lung damage (Lee et al. Citation2016). Peribronchial opacification, lung volume loss, and interlobular septal thickening are three quantifiable CT parameters for monitoring the advancement of radiation-induced lung injury and appear to be valuable tools for preclinical studies.
The attributes described above suggest that the minipig might well serve as an additional large animal model alongside NHP and canines for studying radiation-induced H- and GI-ARS and for the investigation of potential radiation countermeasure efficacy. In this regard, the minipig model has been used recently to validate the efficacy of G-CSF and PEGylated G-CSF as radiomitigators for the radiation-induced H-ARS. It was determined that G-CSF enhanced survival, stimulated recovery from neutropenia, and induced mobilization of hematopoietic progenitor cells (Moroni et al. Citation2013; Sanzari et al. Citation2015).
Canine
The canine hematopoietic and immune systems are similar to those of humans. There is a large data base and well-characterized model of H-ARS using different radiation qualities (60Co γ-ray, X-ray, and mixed field neutron:γ), dose rates and partial body irradiations, with and without medical management in the beagle (Stone et al. Citation2004; Augustine et al. Citation2005; Williams et al. Citation2010). Further, canines have been used extensively (primarily under U.S. Department of Energy funding) in developing basic radiobiological relationships between various parameters of radiation exposure, namely dose, dose-rate, radiation quality, and the nature of exposure (acute versus chronic exposures, fractionated versus continuous exposures, cancer risk projections, etc.) (Seed et al. Citation1984, Citation2002a, Citation2002b; Benjamin et al. Citation1986; Mays et al. Citation1986; Thompson Citation1989).
There is a wealth of information on the pulmonary response to irradiation in the canine model, of which a significant proportion entailed using inhaled radionuclides (Hahn et al. Citation1999; Muggenburg et al. Citation1999; Williams et al. Citation2010, Citation2012; Wilson et al. Citation2011). However, as with rodents, the physiology of the canine differs from that of a human; nevertheless, some investigators group the canine and rodent models together in respect to pulmonary anatomy and function. Despite the extensive database on canine response to irradiation and with the exception of work on countermeasures for the internally deposited radionuclides (Thompson Citation1989), MCM research directed toward late-health effects is fairly sparse.
OrbeShield (beclomethasone dipropionate), under development by Soligenix, Inc. (Princeton, NJ) for the treatment of GI-ARS, has been tested in the canine model, and it demonstrated a survival benefit. Canines received TBI (12 Gy, 0.7 Gy/min, Clinac600 linear accelerator), followed by autologous BM infusion and supportive care. This study demonstrated that OrbeShield, when administered 2 or 24 h after exposure to lethal dose of TBI, provided significant survival benefits (Georges et al. Citation2012). This study also suggests that OrbeShield has the potential to rescue inflamed tissues in the radiation-damaged GI mucosa and improve survival when therapy is initiated as late as 24 h after high-dose irradiation. This is a promising study demonstrating significant efficacy in the canine model (Soligenix Inc Citation2016). Amifostine, G-CSF and GM-CSF have also been evaluated in the canine model, demonstrating beneficial effects against radiation injury (MacVittie et al. Citation1990; Nothdurft et al. Citation1992, Citation1997; Herrera et al. Citation1995; Kouvaris et al. Citation2007; Li et al. Citation2011; Yu et al. Citation2011).
NHP
The NHP model is considered by many investigators (and to some extent, the FDA itself) to be the gold standard of animal models for acute radiation injury and countermeasure analyses, as it shares 95+% DNA sequence identity with humans and a high degree of similarity in terms of receptors and pathways of physiological responses. This model most closely reproduces the clinical, histopathological, and pathophysiological aspects of radiation injury in humans. However, NHPs tend to exhibit greater innate radioresistance (relative to potentially lethal BM marrow failure and associated early deaths) to acute TBI than either swine, canines, and most likely humans as well (Carsten Citation1984).
Due to the longer life span and similar supportive care requirements, it is possible to link the dose-effect relationships between NHP and humans. In addition to the rhesus macaque, several studies have been conducted using baboons to evaluate radiation injuries as a result of X-irradiation (Mahmud et al. Citation2004), 60Co γ-rays (Norol et al. Citation2000), and mixed field exposure (Herodin et al. Citation1992). Promising radiation countermeasures under development for H-ARS have been tested in NHP for efficacy and pharmacokinetics/pharmacodynamics. Some important radiation countermeasures tested for efficacy in the NHP model include G-CSF (filgrastim, Neupogen) (Farese et al. Citation2013; Farese & MacVittie Citation2015), PEGylated G-CSF (PEGfilgrastim/Neulasta) (Hankey et al. Citation2015), CBLB502 (Entolimod) (Krivokrysenko et al. Citation2012, Citation2015), interleukin-12 (HemaMax) (Basile et al. Citation2012; Gluzman-Poltorak et al. Citation2014a, Citation2014b), AEOL 10150 (meso-porphyrin mimetic) (Garofalo et al. Citation2014b), 5-Androstenediol (Stickney et al. Citation2006, Citation2007), amifostine (WR2721, Ethyol) (Geary et al. Citation1989), B-190 (Vasin et al. Citation2014) and γ-tocotrienol (Singh et al. Citation2016a).
U.S. FDA-approved countermeasures
There are currently seven agents approved by the FDA for radiation protection. G-CSF and PEGylated G-CSF received FDA approval in 2015 as radiomitigators for H-ARS (U.S. Food and Drug Administration Citation2015a; National Institute of Allergic and Infectious Diseases Citation2015). Amifostine is the only agent classified as a cytoprotectant and is approved for narrow clinical indications associated with radiotherapy/chemotherapy (Seed Citation2005; Brizel Citation2007). Other agents include Potassium iodide (ThyroShield), trisodium calcium diethylenetriaminepentaacetate (Ca-DTPA), Trisodium zinc diethylene triamine pentaacetate (Zn-DTPA), and Prussian Blue (ferric hexacyanoferrate) as chelators used in blocking or reducing body burdens of internalized radioisotopes following isotope exposures as well as the anti-emetic agents, granisetron and ondansetron (Singh et al. Citation2015b). General information concerning the above-listed radiation injury-countering medicinals, excepting amifostine, can be reviewed from the U.S. government website for the Centers for Disease Control and Prevention (Centers for Disease Control and Prevention Citation2016).
Countermeasures approved for ARS
As stated above, only two radiation countermeasures for ARS have been fully approved by US FDA; both agents are radiomitigators for H-ARS and both have been found to be effective in the NHP when used with a highly, trigger-based supportive care (Farese & MacVittie Citation2015; National Institute of Allergic and Infectious Diseases Citation2015). In addition to these agents, myelopoiesis-stimulating growth factor, Leukine (sargramostim) and other cytokines, along with amifostine have US FDA approval for use, but again for limited, oncologic indications as discussed below.
G-CSF/neupogen/filgrastim
The radiomitigative potential of G-CSF was evaluated in different strains of mice (Tanikawa et al. Citation1990; Hosoi et al. Citation1992; Patchen & MacVittie Citation1994; Sureda et al. Citation1998), canines (beagle) (Schuening et al. Citation1989; MacVittie et al. Citation1990, Citation1991; Schuening et al. Citation1993; Nash et al. Citation1994), minipigs (Moroni et al. Citation2013), and NHP (Farese et al. Citation2013). Since G-CSF is not species-specific, almost all of these studies have been conducted with human recombinant G-CSF. These studies findings demonstrated that G-CSF always enhance survival and blood neutrophil recovery across various species regardless of the radiation source (γ-ray, X-ray). Additional reports suggest G-CSF is an effective mitigator of injury against mixed field (neutron and γ-photon) irradiation in mice (Cary et al. Citation2012). The radiomitigative efficacy of G-CSF is dependent on the extent of radiation exposure, dose of G-CSF, treatment schedule in relation to irradiation, and the period of the treatment. G-CSF has been used in several radiation accident victims with proven positive outcome (Singh et al. Citation2015a). In March 2015, G-CSF was approved by the FDA for adult and pediatric human use to mitigate H-ARS (Farese & MacVittie Citation2015; U.S. Food and Drug Administration Citation2015a).
The instability of G-CSF at ambient temperature and the treatment plans for radiation-exposed victims in a radiological/nuclear incident scenario are issues still of concern. Another issue regarding G-CSF utility as a radiation countermeasure has been raised due to its potential effects on radiation-induced lung injury (Ding et al. Citation2013). Furthermore, pivotal G-CSF studies have used intensive supportive care. In a mass casualty scenario, such treatments will be definitely limited (Farese et al. Citation2013). Additional concerns are the negative effects of G-CSF which include fever, hypoxia, myalgia, splenomegaly, respiratory distress, sickle cell crisis, and Sweet’s syndrome (Reeves Citation2014). G-CSF administration has delayed platelet recovery in exposed victims (International Atomic Energy Agency Citation2000). A World Health Organization panel of experts strongly indorsed G-CSF or GM-CSF administration within 24 h of exposure, to victims exposed to doses >2 Gy, if the neutrophil count is below 0.5 × 109 cells/l and likely to persist for a week or more (Dainiak et al. Citation2011a).
PEGylated G-CSF/neulasta/PEGylated filgrastim
Similar to G-CSF, PEGylated G-CSF also has well-documented radioprotective, survival-promoting efficacies against potential lethal radiation exposures in both mice and NHP (Chua et al. Citation2014; Hankey et al. Citation2015). Studies clearly demonstrated that PEGylated G-CSF can be administered less often than G-CSF and still maintain the therapeutic efficacy of a more extensive dosing schedule; i.e. two weekly injections of PEGylated G-CSF are equivalent to or significantly better than 17–21 daily injections of G-CSF (Farese et al. Citation2012b; Hankey et al. Citation2015). An additional study demonstrated that PEGylated G-CSF limits the severity of the radiation-induced cytopenias in the rodent ARS model. However, this modified recombinant unfortunately appears to be less efficacious than G-CSF in treating irradiated animals with significant skin burns (15% total body surface area skin burns) (Kiang et al. Citation2014a). Similar to G-CSF, PEGylated G-CSF has also been used in several radiation accident victims with positive outcomes (Reeves Citation2014; Singh et al. Citation2015a). In November 2015, PEGylated G-CSF was approved by the FDA for adult and pediatric human use as a radiomitigator for H-ARS (National Institute of Allergic and Infectious Diseases Citation2015).
Countermeasures approved for internally deposited radionuclides and emesis
These countermeasures have been included in part two of this three article series.
Conclusions and future directions
The availability of suitable experimental animal models is one of the limiting factors for developing radiation countermeasures and the identification of appropriate biomarkers of radiation exposure (Augustine et al. Citation2005). For investigating the nature of radiation injury within large animals, only NHP and canine models have been suitably characterized (Williams et al. Citation2010). The NHP is considered by some investigators and regulatory authorities alike to be the gold standard for drug development and FDA approval under the Animal Rule. Additional suitable large animal models for H-ARS and GI-ARS need to be developed and validated in order to expedite not only the R&D of radiation countermeasures but also their regulatory approval. Swine have been suggested to be one such new and promising animal model for drug evaluation (Augustine et al. Citation2005; Singh et al. Citation2016c). Although the minipig is an intriguing model, it is in a primitive state as an ARS animal model compared to the mouse, canine, and NHP (Singh et al. Citation2016c). Further, it has been recently suggested that the minipig’s high radiosensitivity and early death patterns following acute irradiation are not necessarily due to a failure of function of either the hematopoietic or gastrointestinal systems per se, but perhaps mediated largely by DIC. Specifically, recent reports have indeed demonstrated that DIC induced by irradiation may be a promoting factor for the radiosensitivity of minipigs (Krigsfeld et al. Citation2014a, Citation2014b). It needs to be noted that the latter study was conducted using an X-ray source and not γ-ray source. However, it is expected that these reported findings will be comparable to future studies conducted using different qualities of ionizing radiation. Although such a study has been conducted with one strain of minipigs, additional strains still need to be studied relative to acute radiosensitivity and mode and mechanism(s) of early mortality. Recent studies have used relatively short observation period of 30 days following irradiation, but perhaps it would be more appropriate to use a longer observational period in order to better optimize the use of this animal model. An additional concern about this model is the limited availability of reagents for conducting in-depth investigations to understand the mechanism of action of radiation countermeasures under development (Singh et al. Citation2016c).
Finally, an additional concern about the use of this minipig model is the very limited experience the regulatory agencies have in reviewing swine-associated data presented to them for their analyses and deliberations relative to drug approval. This is specifically important since countermeasures for ARS are being developed using the Animal Rule where a human efficacy study is not practicable due to ethical reasons. Drug approval will be based on efficacy in animal models and safety in healthy human volunteers.
Only two radiation countermeasures have been fully approved by the FDA for the mitigation of acute radiation injuries. Both agents are radiomitigators for H-ARS and have been extensively studied for efficacy and safety using a variety of small and large animal models, gaining approval from regulatory authority for use in humans following the Animal Rule. To date, no radioprotector has been approved by the FDA specifically for either H-ARS or GI-ARS. Similarly, no agent has been approved for GI-ARS, neither protector nor mitigator.
Notes on contributors
Vijay K. Singh is a well-recognized radiation biologist and involved in advanced development of promising radiation countermeasures. The primary focus of his research is to understand the mechanism of action of radiation countermeasures and to elucidate the pathways involved in radiation injury. His research is well-funded with various government agencies.
Thomas M. Seed currently serves in a consulting capacity for both public and private organizations alike with vested interests in the nature of ionizing radiation injuries and agents and methods designed to counter those injuries.
Acknowledgements
The opinions or assertions contained herein are the private views of the authors and are not necessarily those of the Armed Forces Radiobiology Research Institute, the Uniformed Services University of the Health Sciences, or the Department of Defense. Mention of specific therapeutic agents does not constitute endorsement by the U.S. Department of Defense, and trade names are used only for the purpose of clarification. We apologize to those having contributed substantially to the topics discussed herein that we were unable to cite because of space constraints.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abdel-Mageed AS, Senagore AJ, Pietryga DW, Connors RH, Giambernardi TA, Hay RV, Deng W. 2009. Intravenous administration of mesenchymal stem cells genetically modified with extracellular superoxide dismutase improves survival in irradiated mice. Blood. 113:1201–1203.
- Aebersold P. 2012. FDA experience with medical countermeasures under the Animal Rule. Adv Prev Med. 2012:507571.
- Agay D, Scherthan H, Forcheron F, Grenier N, Herodin F, Meineke V, Drouet M. 2010. Multipotent mesenchymal stem cell grafting to treat cutaneous radiation syndrome: development of a new minipig model. Exp Hematol. 38:945–956.
- Aleksandrova EN, Kalendo GS, Serebriakov NG. 1980. Radiation block of mitoses and the action of stimulating factors. Tsitologiia. 22:1453–1457.
- Altas E, Ertekin MV, Kuduban O, Gundogdu C, Demirci E, Sutbeyaz Y. 2006. Effects of piracetam supplementation on cochlear damage occurring in guinea pigs exposed to irradiation. Biol Pharm Bull. 29:1460–1465.
- Armed Forces Radiobiology Research Institute. 2013. Medical management of radiological casualities. Bethesda, MD: Armed Forces Radiobiology Research Institute.
- Augustine AD, Gondre-Lewis T, McBride W, Miller L, Pellmar TC, Rockwell S. 2005. Animal models for radiation injury, protection and therapy. Radiat Res. 164:100–109.
- Basile LA, Ellefson D, Gluzman-Poltorak Z, Junes-Gill K, Mar V, Mendonca S, Miller JD, Tom J, Trinh A, Gallaher TK. 2012. HemaMax™, a recombinant human interleukin-12, is a potent mitigator of acute radiation injury in mice and non-human primates. PLoS One. 7:e30434.
- Benjamin GC, McGeary M, McCutchen SR. 2009. Assessing medical preparedness to respond to a terrorist nuclear event: workshop report. Washington, DC: The National Academies Press.
- Benjamin SA, Lee AC, Angleton GM, Jaenke BS, Saunders WJ, Miller GK, Brewster RD. 1986. Life-span radiation effects studies in prenatally and postnatally exposed beagle dogs at Colorado State University. In: Thompson RC, Mahafffey JA, editors. Life span radiation effects studies in animals: what can they tell us? Springfield, VA: National Technical Information Service.
- Benson KG, Paul-Murphy J, Hart AP, Keuler NS, Darien BJ. 2008. Coagulation values in normal ferrets (Mustela putorius furo) using selected methods and reagents. Vet Clin Pathol. 37:286–288.
- Bergonie J, Tribondeau L. 1959. Interpretation of some results of radiotherapy and an attempt at determining a logical technique of treatment. Radiat Res. 11:587–588.
- Bey E, Prat M, Duhamel P, Benderitter M, Brachet M, Trompier F, Battaglini P, Ernou I, Boutin L, Gourven M, et al. 2010. Emerging therapy for improving wound repair of severe radiation burns using local bone marrow-derived stem cell administrations. Wound Repair Regen. 18:50–58.
- Bianchi E. 1963. Radioresistance induced by total-body grid irradiation in small doses and the behavior of guinea pigs after a subsequent lethal dose of X-ray. Minerva Radiol Fisioter Radiobiol. 14:500–506.
- Bohuslavizki KH, Brenner W, Klutmann S, Hubner RH, Lassmann S, Feyerabend B, Luttges J, Tinnemeyer S, Clausen M, Henze E. 1998. Radioprotection of salivary glands by amifostine in high-dose radioiodine therapy. J Nucl Med. 39:1237–1242.
- Bohuslavizki KH, Klutmann S, Jenicke L, Kroger S, Buchert R, Mester J, Clausen M. 1999. Salivary gland protection by S-2-(3-aminopropylamino)-ethylphosphorothioic acid (amifostine) in high-dose radioiodine treatment: results obtained in a rabbit animal model and in a double-blind multi-arm trial. Cancer Biother Radiopharm. 14:337–347.
- Booth C, Tudor G, Tudor J, Katz BP, MacVittie TJ. 2012. Acute gastrointestinal syndrome in high-dose irradiated mice. Health Phys. 103:383–399.
- Brennan PC, Carr KE, Seed T, McCullough JS. 1998. Acute and protracted radiation effects on small intestinal morphological parameters. Int J Radiat Biol. 73:691–698.
- Brizel DM. 2007. Pharmacologic approaches to radiation protection. J Clin Oncol. 25:4084–4089.
- Carnes BA, Grdina DJ. 1992. In vivo protection by the aminothiol WR-2721 against neutron-induced carcinogenesis. Int J Radiat Biol. 61:567–576.
- Carpenter M, Epperly MW, Agarwal A, Nie S, Hricisak L, Niu Y, Greenberger JS. 2005. Inhalation delivery of manganese superoxide dismutase-plasmid/liposomes protects the murine lung from irradiation damage. Gene Ther. 12:685–693.
- Carr KE. 2001. Effects of radiation damage on intestinal morphology. Int Rev Cytol. 208:1–119.
- Carsten AL. 1984. Acute lethality: the hemopoietic syndrome in different species. In: Broerse JJ, MacVittie TJ, editors. Response of different species to total body irradiation. Dordrecht (The Netherlands): Martinus Niijhoff.
- Carter AB, May MM, Perry WJ. 2007. The day after: action following a nuclear blast in a US city. Washington Quart. 30:19–32.
- Cary LH, Ngudiankama BF, Salber RE, Ledney GD, Whitnall MH. 2012. Efficacy of radiation countermeasures depends on radiation quality. Radiat Res. 177:663–675.
- Casarett GW. 1980. Radiation histopathology. Boca Raton, FL: CRC Press.
- Centers for Disease Control and Prevention. 2016. Emergency Preparedness and Response [Online]. Available from: http://emergency.cdc.gov/radiation/countermeasures.asp
- Ch’ang HJ, Maj JG, Paris F, Xing HR, Zhang J, Truman JP, Cardon-Cardo C, Haimovitz-Friedman A, Kolesnick R, Fuks Z. 2005. ATM regulates target switching to escalating doses of radiation in the intestines. Nat Med. 11:484–490.
- Chen L, Brizel DM, Rabbani ZN, Samulski TV, Farrell CL, Larrier N, Anscher MS, Vujaskovic Z. 2004. The protective effect of recombinant human keratinocyte growth factor on radiation-induced pulmonary toxicity in rats. Int J Radiat Oncol Biol Phys. 60:1520–1529.
- Chua HL, Plett PA, Sampson CH, Katz BP, Carnathan GW, MacVittie TJ, Lenden K, Orschell CM. 2014. Survival efficacy of the PEGylated G-CSFs, Maxy-G34, and Neulasta in a mouse model of lethal H-ARS, and residual bone marrow damage in treated survivors. Health Phys. 106:21–38.
- Cohen EP, Bedi M, Irving AA, Jacobs E, Tomic R, Klein J, Lawton CA, Moulder JE. 2012. Mitigation of late renal and pulmonary injury after hematopoietic stem cell transplantation. Int J Radiat Oncol Biol Phys. 83:292–296.
- Dagle GE, Sanders CL. 1984. Radionuclide injury to the lung. Environ Health Perspect. 55:129–137.
- Dainiak N. 2002. Hematologic consequences of exposure to ionizing radiation. Exp Hematol. 30:513–528.
- Dainiak N, Gent RN, Carr Z, Schneider R, Bader J, Buglova E, Chao N, Coleman CN, Ganser A, Gorin C, et al. 2011a. First global consensus for evidence-based management of the hematopoietic syndrome resulting from exposure to ionizing radiation. Disaster Med Pub Health Prep. 5:202–212.
- Dainiak N, Gent RN, Carr Z, Schneider R, Bader J, Buglova E, Chao N, Coleman CN, Ganser A, Gorin C, et al. 2011b. Literature review and global consensus on management of acute radiation syndrome affecting nonhematopoietic organ systems. Disaster Med Pub Health Prep. 5:183–201.
- Daly MJ. 2012. Death by protein damage in irradiated cells. DNA Repair (Amst). 11:12–21.
- Danquechin Dorval E, Mueller GP, Eng RR, Durakovic A, Conklin JJ, Dubois A. 1985. Effect of ionizing radiation on gastric secretion and gastric motility in monkeys. Gastroenterology. 89:374–380.
- Denham JW, Hauer-Jensen M, Peters LJ. 2001. Is it time for a new formalism to categorize normal tissue radiation injury? Int J Radiat Oncol Biol Phys. 50:1105–1106.
- DiCarlo AL, Jackson IL, Shah JR, Czarniecki CW, Maidment BW, Williams JP. 2012. Development and licensure of medical countermeasures to treat lung damage resulting from a radiological or nuclear incident. Radiat Res. 177:717–721.
- DiCarlo AL, Ramakrishnan N, Hatchett RJ. 2010. Radiation combined injury: overview of NIAID research. Health Phys. 98:863–867.
- Ding NH, Li JJ, Sun LQ. 2013. Molecular mechanisms and treatment of radiation-induced lung fibrosis. Curr Drug Targets. 14:1347–1356.
- Doctrow SR, Lopez A, Schock AM, Duncan NE, Jourdan MM, Olasz EB, Moulder JE, Fish BL, Mader M, Lazar J, et al. 2013. A synthetic superoxide dismutase/catalase mimetic EUK-207 mitigates radiation dermatitis and promotes wound healing in irradiated rat skin. J Invest Dermatol. 133:1088–1096.
- Dorr H, Meineke V. 2011. Acute radiation syndrome caused by accidental radiation exposure – therapeutic principles. BMC Med. 9:126.
- Drouet M, Mourcin F, Grenier N, Leroux V, Denis J, Mayol JF, Thullier P, Lataillade JJ, Herodin F. 2004. Single administration of stem cell factor, FLT-3 ligand, megakaryocyte growth and development factor, and interleukin-3 in combination soon after irradiation prevents nonhuman primates from myelosuppression: long-term follow-up of hematopoiesis. Blood. 103:878–885.
- Dubois A, Jacobus JP, Grissom MP, Eng RR, Conklin JJ. 1984. Altered gastric emptying and prevention of radiation-induced vomiting in dogs. Gastroenterology. 86:444–448.
- Dumont F, Le Roux A, Bischoff P. 2010. Radiation countermeasure agents: an update. Expert Opin Ther Pat. 20:73–101.
- Elliott TB, Bolduc DL, Ledney GD, Kiang JG, Fatanmi OO, Wise SY, Romaine PL, Newman VL, Singh VK. 2015. Combined immunomodulator and antimicrobial therapy eliminates polymicrobial sepsis and modulates cytokine production in combined injured mice. Int J Radiat Biol. 91:690–702.
- Elliott TB, Deutz NE, Gulani J, Koch A, Olsen CH, Christensen C, Chappell M, Whitnall MH, Moroni M. 2014. Gastrointestinal acute radiation syndrome in Göttingen minipigs (Sus scrofa domestica). Comp Med. 64:456–463.
- Epperly MW, Carpenter M, Agarwal A, Mitra P, Nie S, Greenberger JS. 2004. Intraoral manganese superoxide dismutase-plasmid/liposome (MnSOD-PL) radioprotective gene therapy decreases ionizing irradiation-induced murine mucosal cell cycling and apoptosis. In Vivo. 18:401–410.
- Farese AM, Casey DB, Smith WG, Vigneulle RM, McKearn JP, MacVittie TJ. 2001. Leridistim, a chimeric dual G-CSF and IL-3 receptor agonist, enhances multilineage hematopoietic recovery in a nonhuman primate model of radiation-induced myelosuppression: effect of schedule, dose, and route of administration. Stem Cells. 19:522–533.
- Farese AM, Cohen MV, Katz BP, Smith CP, Gibbs A, Cohen DM, MacVittie TJ. 2013. Filgrastim improves survival in lethally irradiated nonhuman primates. Radiat Res. 179:89–100.
- Farese AM, Cohen MV, Katz BP, Smith CP, Jackson W, 3rd, Cohen DM, MacVittie TJ. 2012a. A nonhuman primate model of the hematopoietic acute radiation syndrome plus medical management. Health Phys. 103:367–382.
- Farese AM, Cohen MV, Stead RB, Jackson W 3rd, MacVittie TJ. 2012b. Pegfilgrastim administered in an abbreviated schedule, significantly improved neutrophil recovery after high-dose radiation-induced myelosuppression in rhesus macaques. Radiat Res. 178:403–413.
- Farese AM, MacVittie TJ. 2015. Filgrastim for the treatment of hematopoietic acute radiation syndrome. Drugs Today. 51:537–548.
- Fliedner TM, Dorr DH, Meineke V. 2005. Multi-organ involvement as a pathogenetic principle of the radiation syndromes: a study involving 110 case histories documented in SEARCH and classified as the bases of haematopoietic indicators of effect. BJR. (Suppl 27):1–8.
- Forcheron F, Agay D, Scherthan H, Riccobono D, Herodin F, Meineke V, Drouet M. 2012. Autologous adipocyte derived stem cells favour healing in a minipig model of cutaneous radiation syndrome. PLoS One. 7:e31694.
- Gao F, Fish BL, Szabo A, Doctrow SR, Kma L, Molthen RC, Moulder JE, Jacobs ER, Medhora M. 2012. Short-term treatment with a SOD/catalase mimetic, EUK-207, mitigates pneumonitis and fibrosis after single-dose total-body or whole-thoracic irradiation. Radiat Res. 178:468–480.
- Gao F, Narayanan J, Joneikis C, Fish BL, Szabo A, Moulder JE, Molthen RC, Jacobs ER, Rao RN, Medhora M. 2013. Enalapril mitigates focal alveolar lesions, a histological marker of late pulmonary injury by radiation to the lung. Radiat Res. 179:465–474.
- Garofalo M, Bennett A, Farese AM, Harper J, Ward A, Taylor-Howell C, Cui W, Gibbs A, Lasio G, Jackson W 3rd, et al. 2014a. The delayed pulmonary syndrome following acute high-dose irradiation: a rhesus macaque model. Health Phys. 106:56–72.
- Garofalo MC, Ward AA, Farese AM, Bennett A, Taylor-Howell C, Cui W, Gibbs A, Prado KL, Macvittie TJ. 2014b. A pilot study in rhesus macaques to assess the treatment efficacy of a small molecular weight catalytic metalloporphyrin antioxidant (AEOL 10150) in mitigating radiation-induced lung damage. Health Phys. 106:73–83.
- Geary RS, Swynnerton NF, Miller MA, Mangold DJ, Ludden T. 1989. Intraduodenal administration of ethiofos (WR-2721): dose proportionality study in the rhesus monkey. Res Commun Chem Pathol Pharmacol. 65:147–159.
- Georges GE, Kuver RP, Jordan R, Aragon A, Yang Y, Lesnikova M, Lesnikov V, Sale GE, McDonald GB. 2012. Post-exposure oral 17,21-beclomethasone dipropionate (BDP) improves survival in a canine gastrointestinal acute radiation syndrome (GI-ARS) model. Poster presented at 58th Annual Meeting of the Radiation Research Society; San Juan, Puerto Rico.
- Geraci JP, Jackson KL, Mariano MS. 1985. The intestinal radiation syndrome: sepsis and endotoxin. Radiat Res. 101:442–450.
- Geraci JP, Sun MC, Mariano MS. 1995. Amelioration of radiation nephropathy in rats by postirradiation treatment with dexamethasone and/or captopril. Radiat Res. 143:58–68.
- Ghosh SN, Zhang R, Fish BL, Semenenko VA, Li XA, Moulder JE, Jacobs ER, Medhora M. 2009. Renin-angiotensin system suppression mitigates experimental radiation pneumonitis. Int J Radiat Oncol Biol Phys. 75:1528–1536.
- Gianni AM, Bregni M, Siena S, Villa S, Sciorelli GA, Ravagnani F, Pellegris G, Bonadonna G. 1989. Rapid and complete hemopoietic reconstitution following combined transplantation of autologous blood and bone marrow cells. A changing role for high dose chemo-radiotherapy? Hematol Oncol. 7:139–148.
- Gluzman-Poltorak Z, Mendonca SR, Vainstein V, Kha H, Basile LA. 2014a. Randomized comparison of single dose of recombinant human IL-12 versus placebo for restoration of hematopoiesis and improved survival in rhesus monkeys exposed to lethal radiation. J Hematol Oncol. 7:31.
- Gluzman-Poltorak Z, Vainstein V, Basile LA. 2014b. Recombinant interleukin-12, but not granulocyte-colony stimulating factor, improves survival in lethally irradiated nonhuman primates in the absence of supportive care: evidence for the development of a frontline radiation medical countermeasure. Am J Hematol. 89:868–873.
- Goans RE, Holloway EC, Berger ME, Ricks RC. 1997. Early dose assessment following severe radiation accidents. Health Phys. 72:513–518.
- Gokare P, Navaraj A, Zhang S, Motoyama N, Sung SS, Finnberg NK. 2016. Targeting of Chk2 as a countermeasure to dose-limiting toxicity triggered by topoisomerase-II (TOP2) poisons. Oncotarget. 7:29520–29530.
- Gourmelon P, Benderitter M, Bertho JM, Huet C, Gorin NC, De Revel P. 2010. European consensus on the medical management of acute radiation syndrome and analysis of the radiation accidents in Belgium and Senegal. Health Phys. 98:825–832.
- Gratwohl A, John L, Baldomero H, Roth J, Tichelli A, Nissen C, Lyman SD, Wodnar-Filipowicz A. 1998. FLT-3 ligand provides hematopoietic protection from total body irradiation in rabbits. Blood. 92:765–769.
- Grdina DJ, Carnes BA, Grahn D, Sigdestad CP. 1991. Protection against late effects of radiation by S-2-(3-aminopropylamino)-ethylphosphorothioic acid. Cancer Res. 51:4125–4130.
- Gulbahar O, Aricioglu A, Akmansu M, Turkozer Z. 2009. Effects of radiation on protein oxidation and lipid peroxidation in the brain tissue. Transplant Proc. 41:4394–4396.
- Hahn FF, Muggenburg BA, Menache MG, Guilmette RA, Boecker BB. 1999. Comparative stochastic effects of inhaled alpha- and beta-particle-emitting radionuclides in beagle dogs. Radiat Res. 152:S19–S22.
- Hakim SG, Kosmehl H, Lauer I, Nadrowitz R, Wedel T, Sieg P. 2005. A comparative study on the protection profile of lidocaine, amifostine, and pilocarpin on the parotid gland during radiotherapy. Cancer Res. 65:10486–10493.
- Hall EJ, Giaccia AJ. 2012. Radiobiology for the radiobiologist. Philadelphia, PA: Lippincott Williams and Wilkins.
- Hankey KG, Farese AM, Blaauw EC, Gibbs AM, Smith CP, Katz BP, Tong Y, Prado KL, MacVittie TJ. 2015. Pegfilgrastim improves survival of lethally irradiated nonhuman primates. Radiat Res. 183:643–655.
- Haston CK, Zhou X, Gumbiner-Russo L, Irani R, Dejournett R, Gu X, Weil M, Amos CI, Travis EL. 2002. Universal and radiation-specific loci influence murine susceptibility to radiation-induced pulmonary fibrosis. Cancer Res. 62:3782–3788.
- Herodin F, Mestries JC, Janodet D, Martin S, Mathieu J, Gascon MP, Pernin MO, Ythier A. 1992. Recombinant glycosylated human interleukin-6 accelerates peripheral blood platelet count recovery in radiation-induced bone marrow depression in baboons. Blood. 80:688–695.
- Herodin F, Roy L, Grenier N, Delaunay C, Bauge S, Vaurijoux A, Gregoire E, Martin C, Alonso A, Mayol JF, et al. 2007. Antiapoptotic cytokines in combination with pegfilgrastim soon after irradiation mitigates myelosuppression in nonhuman primates exposed to high irradiation dose. Exp Hematol. 35:1172–1181.
- Herrera JL, Vigneulle RM, Gage T, MacVittie TJ, Nold JB, Dubois A. 1995. Effect of radiation and radioprotection on small intestinal function in canines. Dig Dis Sci. 40:211–218.
- Hilf R, Adachi R, Eckfeldt G. 1961. Effect of dietary supplementation with broccoli on X-irradiation-induced enzyme changes in the guinea pig. Radiat Res. 15:86–93.
- Hill RP. 2005. Radiation effects on the respiratory system. BJR. (Suppl. 27):75–81.
- Hoel DG, Carnes BA, Dedrick RL, Fry RJM, Grahn D, Griffith WC, Groer PG, Preston RJ, Clifton KH, Miller SC, Shuller HM, Seed TM. 2005. Extrapolation of radiation-induced cancer risks from nonhuman experimental systems to humans. Bethesda, MD: National Council of Radiation Protection.
- Holler V, Buard V, Gaugler MH, Guipaud O, Baudelin C, Sache A, Perez Mdel R, Squiban C, Tamarat R, Milliat F, et al. 2009. Pravastatin limits radiation-induced vascular dysfunction in the skin. J Invest Dermatol. 129:1280–1291.
- Hopewell JW, Rezvani M, Moustafa HF. 2000. The pig as a model for the study of radiation effects on the lung. Int J Radiat Biol. 76:447–452.
- Hosoi Y, Kurishita A, Ono T, Sakamoto K. 1992. Effect of recombinant human granulocyte colony-stimulating factor on survival in lethally irradiated mice. Acta Oncol. 31:59–63.
- Inoue T, Hirabayashi Y, Mitsui H, Sasaki H, Cronkite EP, Bullis JE Jr, Bond VP, Yoshida K. 1995. Survival of spleen colony-forming units (CFU-S) of irradiated bone marrow cells in mice: evidence for the existence of a radioresistant subfraction. Exp Hematol. 23:1296–1300.
- International Atomic Energy Agency. 2000. The radiological accident in Istanbul [Online]. Available from: http://www-pub.iaea.org/books/IAEABooks/6071/The-Radiological-Accident-in-Istanbul
- Jackson IL, Vujaskovic Z, Down JD. 2010. Revisiting strain-related differences in radiation sensitivity of the mouse lung: recognizing and avoiding the confounding effects of pleural effusions. Radiat Res. 173:10–20.
- Jackson IL, Vujaskovic Z, Down JD. 2011. A further comparison of pathologies after thoracic irradiation among different mouse strains: finding the best preclinical model for evaluating therapies directed against radiation-induced lung damage. Radiat Res. 175:510–518.
- Jackson IL, Xu P, Hadley C, Katz BP, McGurk R, Down JD, Vujaskovic Z. 2012. A preclinical rodent model of radiation-induced lung injury for medical countermeasure screening in accordance with the FDA animal rule. Health Phys. 103:463–473.
- Jacquet P, Vankerkom J, Lambiet-Collier M. 1994. The female guinea pig, a useful model for the genetic hazard of radiation in man; preliminary results on germ cell radiosensitivity in foetal, neonatal and adult animals. Int J Radiat Biol. 65:357–367.
- Jahraus CD, Schemera B, Rynders P, Ramos M, Powell C, Faircloth J, Brawner WR. 2010. Rifaximin diminishes neutropenia following potentially lethal whole-body radiation. Exp Biol Med (Maywood). 235:900–905.
- Jenrow KA, Brown SL, Liu J, Kolozsvary A, Lapanowski K, Kim JH. 2010. Ramipril mitigates radiation-induced impairment of neurogenesis in the rat dentate gyrus. Radiat Oncol. 5:6.
- Jiao W, Kiang JG, Cary L, Elliott TB, Pellmar TC, Ledney GD. 2009. COX-2 inhibitors are contraindicated for treatment of combined injury. Radiat Res. 172:686–697.
- Jourdan MM, Lopez A, Olasz EB, Duncan NE, Demara M, Kittipongdaja W, Fish BL, Mader M, Schock A, Morrow NV, et al. 2011. Laminin 332 deposition is diminished in irradiated skin in an animal model of combined radiation and wound skin injury. Radiat Res. 176:636–648.
- Jurkovic V, Vokrouhlicky L. 1963. Cardiovascular changes during radiation disease. Sb Ved Pr Lek Fak Karlovy Univerzity Hradci Kralove. 6:5–10.
- Kam WW, Banati RB. 2013. Effects of ionizing radiation on mitochondria. Free Radic Biol Med. 65:607–619.
- Kataoka Y, Basic I, Perrin J, Grdina DJ. 1992. Antimutagenic effects of radioprotector WR-2721 against fission-spectrum neurons and 60Co gamma-rays in mice. Int J Radiat Biol. 61:387–392.
- Kiang JG, Garrison BR, Burns TM, Zhai M, Dews IC, Ney PH, Cary LH, Fukumoto R, Elliott TB, Ledney GD. 2012. Wound trauma alters ionizing radiation dose assessment. Cell Biosci. 2:20.
- Kiang JG, Jiao W, Cary LH, Mog SR, Elliott TB, Pellmar TC, Ledney GD. 2010. Wound trauma increases radiation-induced mortality by activation of iNOS pathway and elevation of cytokine concentrations and bacterial infection. Radiat Res. 173:319–332.
- Kiang JG, Smith JT, Anderson MN, Swift JM, Christensen CL, Gupta P, Balakathiresan N, Maheshwari RK. 2015. Hemorrhage exacerbates radiation effects on survival, leukocytopenia, thrombopenia, erythropenia, bone marrow cell depletion and hematopoiesis, and inflammation-associated microRNAs expression in kidney. PLoS One. 10:e0139271.
- Kiang JG, Zhai M, Liao PJ, Bolduc DL, Elliott TB, Gorbunov NV. 2014a. Pegylated G-CSF inhibits blood cell depletion, increases platelets, blocks splenomegaly, and improves survival after whole-body ionizing irradiation but not after irradiation combined with burn. Oxid Med Cell Longev. 2014:481392.
- Kiang JG, Zhai M, Liao PJ, Elliott TB, Gorbunov NV. 2014b. Ghrelin therapy improves survival after whole-body ionizing irradiation or combined with burn or wound: amelioration of leukocytopenia, thrombocytopenia, splenomegaly, and bone marrow injury. Oxid Med Cell Longev. 2014:215858.
- Kim JH, Jenrow KA, Brown SL. 2014. Mechanisms of radiation-induced normal tissue toxicity and implications for future clinical trials. Radiat Oncol J. 32:103–115.
- Kim JH, Kolozsvary A, Jenrow KA, Brown SL. 2012. Plerixafor, a CXCR4 antagonist, mitigates skin radiation-induced injury in mice. Radiat Res. 178:202–206.
- King GL. 1988. Characterization of radiation-induced emesis in the ferret. Radiat Res. 114:599–612.
- King GL, Rabin BM, Weatherspoon JK. 1999. 5-HT3 receptor antagonists ameliorate emesis in the ferret evoked by neutron or proton radiation. Aviat Space Environ Med. 70:485–492.
- Kma L, Gao F, Fish BL, Moulder JE, Jacobs ER, Medhora M. 2012. Angiotensin converting enzyme inhibitors mitigate collagen synthesis induced by a single dose of radiation to the whole thorax. J Radiat Res. 53:10–17.
- Kobayashi T, Ohmori T, Yanai M, Kawanishi G, Mitsuyama M, Nomoto K. 1991. The analysis of the defense mechanism against indigenous bacterial translocation in X-irradiated mice. Microbiol Immunol. 35:315–324.
- Kouvaris JR, Kouloulias VE, Vlahos LJ. 2007. Amifostine: the first selective-target and broad-spectrum radioprotector. Oncologist. 12:738–747.
- Krigsfeld GS, Savage AR, Billings PC, Lin L, Kennedy AR. 2014a. Evidence for radiation-induced disseminated intravascular coagulation as a major cause of radiation-induced death in ferrets. Int J Radiat Oncol Biol Phys. 88:940–946.
- Krigsfeld GS, Shah JB, Sanzari JK, Lin L, Kennedy AR. 2014b. Evidence of disseminated intravascular coagulation in a porcine model following radiation exposure. Life Sci Space Res (Amst). 3:1–9.
- Krimsky M, Dagan A, Aptekar L, Ligumsky M, Yedgar S. 2000. Assessment of intestinal permeability in rats by permeation of inulin-fluorescein. J Basic Clin Physiol Pharmacol. 11:143–153.
- Krivokrysenko VI, Shakhov AN, Singh VK, Bone F, Kononov Y, Shyshynova I, Cheney A, Maitra RK, Purmal A, Whitnall MH, et al. 2012. Identification of granulocyte colony-stimulating factor and interleukin-6 as candidate biomarkers of CBLB502 efficacy as a medical radiation countermeasure. J Pharmacol Exp Ther. 343:497–508.
- Krivokrysenko VI, Toshkov IA, Gleiberman AS, Krasnov P, Shyshynova I, Bespalov I, Maitra RK, Narizhneva NV, Singh VK, Whitnall MH, et al. 2015. The Toll-like receptor 5 agonist Entolimod mitigates lethal acute radiation syndrome in non-human primates. PLoS One. 10:e0135388.
- Lang IM, Sarna SK, Condon RE. 1986. Gastrointestinal motor correlates of vomiting in the dog: quantification and characterization as an independent phenomenon. Gastroenterology. 90:40–47.
- Lataillade JJ, Doucet C, Bey E, Carsin H, Huet C, Clairand I, Bottollier-Depois JF, Chapel A, Ernou I, Gourven M, et al. 2007. New approach to radiation burn treatment by dosimetry-guided surgery combined with autologous mesenchymal stem cell therapy. Regen Med. 2:785–794.
- Laterveer L, Zijlmans JM, Liehl E, Willemze R, Fibbe WE. 1996. Accelerated platelet reconstitution following transplantation of bone marrow cells derived from IL-6-treated donor mice. Ann Hematol. 73:239–245.
- Lee JG, Park S, Bae CH, Jang WS, Lee SJ, Lee DN, Myung JK, Kim CH, Jin YW, Lee SS, et al. 2016. Development of a minipig model for lung injury induced by a single high-dose radiation exposure and evaluation with thoracic computed tomography. J Radiat Res. 57:201–209.
- Lemaire G, Maas J. 1984. Haematopoietic syndrome in pigs. In: Broerse JJ, Macvittie TJ, editors. Response of different species to total body irradiation. Dordrecht, The Netherlands: Martinus Niijhoff.
- Li M, Yu ZY, Xing S, Ou HL, Xiong GL, Xie L, Zhao YF, Han AR, Shan YJ, Liu XL, et al. 2011. High dose granulocyte colony-stimulating factor enhances survival and hematopoietic reconstruction in canines irradiated by 2.3 Gy mixed fission neutron and gamma ray. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 19:991–998.
- Lopez M, Martin M. 2011. Medical management of the acute radiation syndrome. Rep Pract Oncol Radiother. 16:138–146.
- Lutgens LC, Blijlevens NM, Deutz NE, Donnelly JP, Lambin P, de Pauw BE. 2005. Monitoring myeloablative therapy-induced small bowel toxicity by serum citrulline concentration: a comparison with sugar permeability tests. Cancer. 103:191–199.
- MacVittie TJ, Monroy RL, Farese AM, Seiler FR, Williams D. 1991. Cytokine therapy in canine and primate models of radiation-induced marrow aplasia. Behring Inst Mitt. 90:1–13.
- MacVittie TJ, Farese AM, Patchen ML, Myers LA. 1994. Therapeutic efficacy of recombinant interleukin-6 (IL-6) alone and combined with recombinant human IL-3 in a nonhuman primate model of high-dose, sublethal radiation-induced marrow aplasia. Blood. 84:2515–2522.
- MacVittie TJ, Monroy RL, Patchen ML, Souza LM. 1990. Therapeutic use of recombinant human G-CSF (rhG-CSF) in a canine model of sublethal and lethal whole-body irradiation. Int J Radiat Biol. 57:723–736.
- Mahmud N, Pang W, Cobbs C, Alur P, Borneman J, Dodds R, Archambault M, Devine S, Turian J, Bartholomew A, et al. 2004. Studies of the route of administration and role of conditioning with radiation on unrelated allogeneic mismatched mesenchymal stem cell engraftment in a nonhuman primate model. Exp Hematol. 32:494–501.
- Makrauer FL, Oates E, Becker J, Abrams R, O’Connor T, McCallum R, Shumaker J. 1999. Does local irradiation affect gastric emptying in humans? Am J Med Sci. 317:33–37.
- Mandel L, Travnicek J, Talafantova M, Zahradnickova M. 1980. The LD50/30 and the survival time in whole-body gamma-irradiated conventional and germfree Minnesota miniature piglets. Z Versuchstierkd. 22:96–100.
- Marks LB, Yu X, Vujaskovic Z, Small W Jr, Folz R, Anscher MS. 2003. Radiation-induced lung injury. Semin Radiat Oncol. 13:333–345.
- Martin C, Roman V, Agay D, Fatome M. 1998. Anti-emetic effect of ondansetron and granisetron after exposure to mixed neutron and gamma irradiation. Radiat Res. 149:631–636.
- Mason KA, Withers HR, McBride WH, Davis CA, Smathers JB. 1989. Comparison of the gastrointestinal syndrome after total-body or total-abdominal irradiation. Radiat Res. 117:480–488.
- Mavragani IV, Laskaratou DA, Frey B, Candeias SM, Gaipl US, Lumniczky K, Georgakilas AG. 2016. Key mechanisms involved in ionizing radiation-induced systemic effects. A current review. Toxicol Res. 5:12–33.
- Mays CW, Taylor GN, Lloyd RD. 1986. Toxicity ratios: Their use and abuse in predicting the risk from induced cancer. In: Thompson RC, Mahaffey JA, editors. Life span radiation effects studies in animals: what can they tell us? Springfield, VA: NTIS; p. 299–310.
- McCann DGC. 2006. Radiation poisoning: current concepts in the acute radiation syndrome. Am J Clin Med. 3:13–21.
- McLaughlin RF Jr, Tyler WS, Canada RO. 1966. Subgross pulmonary anatomy of the rabbit, rat, and guinea pig, with additional notes on the human lung. Am Rev Respir Dis. 94:380–387.
- Medhora M, Gao F, Fish BL, Jacobs ER, Moulder JE, Szabo A. 2012. Dose-modifying factor for captopril for mitigation of radiation injury to normal lung. J Radiat Res. 53:633–640.
- Medhora M, Gao F, Wu Q, Molthen RC, Jacobs ER, Moulder JE, Fish BL. 2014. Model development and use of ACE inhibitors for preclinical mitigation of radiation-induced injury to multiple organs. Radiat Res. 182:545–555.
- Moroni M, Coolbaugh TV, Lombardini E, Mitchell JM, Moccia KD, Shelton LJ, Nagy V, Whitnall MH. 2011a. Hematopoietic radiation syndrome in the Göttingen minipig. Radiat Res. 176:89–101.
- Moroni M, Lombardini E, Salber R, Kazemzedeh M, Nagy V, Olsen C, Whitnall MH. 2011b. Hematological changes as prognostic indicators of survival: similarities between Göttingen minipigs, humans, and other large animal models. PLoS One. 6:e25210.
- Moroni M, Ngudiankama BF, Christensen C, Olsen CH, Owens R, Lombardini ED, Holt RK, Whitnall MH. 2013. The Göttingen minipig is a model of the hematopoietic acute radiation syndrome: G-colony stimulating factor stimulates hematopoiesis and enhances survival from lethal total-body γ-irradiation. Int J Radiat Oncol Biol Phys. 86:986–992.
- Moulder JE. 2014. 2013 Dade W. Moeller lecture: medical countermeasures against radiological terrorism. Health Phys. 107:164–171.
- Moulder JE, Cohen EP. 2005. Radiation-induced multi-organ involvement and failure: the contribution of radiation effects on the renal system. BJR. (Suppl. 27):82–88.
- Moulder JE, Robbins ME, Cohen EP, Hopewell JW, Ward WF. 1998. Pharmacologic modification of radiation-induced late normal tissue injury. Cancer Treat Res. 93:129–151.
- Muggenburg BA, Hahn FF, Menache MG, Guilmette RA, Boecker BB. 1999. Comparative deterministic effects of inhaled, insoluble alpha- and beta-particle-emitting radionuclides in dogs. Radiat Res. 152:S23–S26.
- Mujica-Mota MA, Salehi P, Devic S, Daniel SJ. 2014. Safety and otoprotection of metformin in radiation-induced sensorineural hearing loss in the guinea pig. Otolaryngol Head Neck Surg. 150:859–865.
- Nash RA, Schuening FG, Seidel K, Appelbaum FR, Boone T, Deeg HJ, Graham TC, Hackman R, Sullivan-Pepe M, Storb R. 1994. Effect of recombinant canine granulocyte-macrophage colony-stimulating factor on hematopoietic recovery after otherwise lethal total body irradiation. Blood. 83:1963–1970.
- National Institute of Allergic and Infectious Diseases. 2015. Pegfilgrastim approved for treating acute radiation syndrome [Online]. Available from: https://www.niaid.nih.gov/topics/radnuc/Pages/pegfilgrastim.aspx
- Norol F, Drouet M, Mathieu J, Debili N, Jouault H, Grenier N, Laplanche A, Vainchenker W, Herodin F. 2000. Ex vivo expanded mobilized peripheral blood CD34+ cells accelerate haematological recovery in a baboon model of autologous transplantation. Br J Haematol. 109:162–172.
- Nothdurft W, Kreja L, Selig C. 1997. Acceleration of hemopoietic recovery in dogs after extended-field partial-body irradiation by treatment with colony-stimulating factors: rhG-CSF and rhGM-CSF. Int J Radiat Oncol Biol Phys. 37:1145–1154.
- Nothdurft W, Selig C, Fliedner TM, Hintz-Obertreis P, Kreja L, Krumwieh D, Kurrle R, Seiler FR, Weinsheimer W. 1992. Haematological effects of rhGM-CSF in dogs exposed to total-body irradiation with a dose of 2.4 Gy. Int J Radiat Biol. 61:519–531.
- O’Connor MM, Mayberg MR. 2000. Effects of radiation on cerebral vasculature: a review. Neurosurgery. 46:138–149; discussion 150-131.
- Okayasu R, Suetomi K, Yu Y, Silver A, Bedford JS, Cox R, Ullrich RL. 2000. A deficiency in DNA repair and DNA-PKcs expression in the radiosensitive BALB/c mouse. Cancer Res. 60:4342–4345.
- Okunieff P, Xu J, Hu D, Liu W, Zhang L, Morrow G, Pentland A, Ryan JL, Ding I. 2006. Curcumin protects against radiation-induced acute and chronic cutaneous toxicity in mice and decreases mRNA expression of inflammatory and fibrogenic cytokines. Int J Radiat Oncol Biol Phys. 65:890–898.
- Osmond DG, Roylance PJ, Lee WR, Ramsell TG, Yoffey JM. 1966. The effect of unilateral limb shielding on the haemopoietic response of the guinea-pig to gamma irradiation (150 r.). Br J Haematol. 12:365–375.
- Otterson MF, Sarna SK, Leming SC, Moulder JE, Fink JG. 1992. Effects of fractionated doses of ionizing radiation on colonic motor activity. Am J Physiol. 263:G518–G526.
- Otterson MF, Sarna SK, Moulder JE. 1988. Effects of fractionated doses of ionizing radiation on small intestinal motor activity. Gastroenterology. 95:1249–1257.
- Patchen ML, MacVittie TJ. 1994. Granulocyte colony-stimulating factor and amifostine (Ethyol) synergize to enhance hemopoietic reconstitution and increase survival in irradiated animals. Semin Oncol. 21:26–32.
- Peebles DD, Soref CM, Copp RR, Thunberg AL, Fahl WE. 2012. ROS-scavenger and radioprotective efficacy of the new PrC-210 aminothiol. Radiat Res. 178:57–68.
- Pellmar TC, Rockwell S. 2005. Priority list of research areas for radiological nuclear threat countermeasures. Radiat Res. 163:115–123.
- Peter RU, Braun-Falco O, Birioukov A, Hacker N, Kerscher M, Peterseim U, Ruzicka T, Konz B, Plewig G. 1994. Chronic cutaneous damage after accidental exposure to ionizing radiation: the Chernobyl experience. J Am Acad Dermatol. 30:719–723.
- Peter RU, Gottlober P. 2002. Management of cutaneous radiation injuries: diagnostic and therapeutic principles of the cutaneous radiation syndrome. Mil Med. 167:110–112.
- Peter RU, Gottlober P, Nadeshina N, Krahn G, Plewig G, Kind P. 1997. Radiation lentigo. A distinct cutaneous lesion after accidental radiation exposure. Arch Dermatol. 133:209–211.
- Prise KM, Schettino G, Folkard M, Held KD. 2005. New insights on cell death from radiation exposure. Lancet Oncol. 6:520–528.
- Reeves G. 2014. Overview of use of G-CSF and GM-CSF in the treatment of acute radiation injury. Health Phys. 106:699–703.
- Rivina L, Davoren M, Schiestl RH. 2014. Radiation-induced myeloid leukemia in murine models. Hum Genomics. 8:13.
- Robbins ME, Zhao W, Garcia-Espinosa MA, Diz DI. 2010. Renin-angiotensin system blockers and modulation of radiation-induced brain injury. Curr Drug Targets. 11:1413–1422.
- Rosen EM, Day R, Singh VK. 2015. New approaches to radiation protection. Front Oncol. 4:381.
- Rosenthal SA, Harber LC, Baer RL, Friedman M. 1960. Survival of guinea pigs after lethal irradiation and homologus bone marrow injections. Radiat Res. 13:496–501.
- Ryan JL, Heckler CE, Ling M, Katz A, Williams JP, Pentland AP, Morrow GR. 2013. Curcumin for radiation dermatitis: a randomized, double-blind, placebo-controlled clinical trial of thirty breast cancer patients. Radiat Res. 180:34–43.
- Saha S, Bhanja P, Kabarriti R, Liu L, Alfieri AA, Guha C. 2011. Bone marrow stromal cell transplantation mitigates radiation-induced gastrointestinal syndrome in mice. PLoS One. 6:e24072.
- Sanzari JK, Krigsfeld GS, Shuman AL, Diener AK, Lin L, Mai W, Kennedy AR. 2015. Effects of a granulocyte colony stimulating factor, Neulasta, in mini pigs exposed to total body proton irradiation. Life Sci Space Res (Amst). 5:13–20.
- Sanzari JK, Wan XS, Krigsfeld GS, King GL, Miller A, Mick R, Gridley DS, Wroe AJ, Rightnar S, Dolney D, et al. 2013. Effects of solar particle event proton radiation on parameters related to ferret emesis. Radiat Res. 180:166–176.
- Satyamitra MM, DiCarlo AL, Taliaferro L. 2016. Understanding the pathophysiology and challenges of development of medical countermeasures for radiation-induced vascular/endothelial cell injuries: report of a NIAID workshop, August 20, 2015. Radiat Res. 186:99–111.
- Schuening FG, Appelbaum FR, Deeg HJ, Sullivan-Pepe M, Graham TC, Hackman R, Zsebo KM, Storb R. 1993. Effects of recombinant canine stem cell factor, a c-kit ligand, and recombinant granulocyte colony-stimulating factor on hematopoietic recovery after otherwise lethal total body irradiation. Blood. 81:20–26.
- Schuening FG, Storb R, Goehle S, Graham TC, Appelbaum FR, Hackman R, Souza LM. 1989. Effect of recombinant human granulocyte colony-stimulating factor on hematopoiesis of normal dogs and on hematopoietic recovery after otherwise lethal total body irradiation. Blood. 74:1308–1313.
- Schultheiss TE, Kun LE, Ang KK, Stephens LC. 1995. Radiation response of the central nervous system. Int J Radiat Oncol Biol Phys. 31:1093–1112.
- Seed TM. 2005. Radiation protectants: current status and future prospects. Health Phys. 89:531–545.
- Seed TM, Fritz TE, Tolle DV, Poole CM, Lomard LS, Doyle DE, Kaspar LV. 1984. Survival patterns and hemopathological responses of dogs under continuous gamma irradiation. In: Broerse JJ, Macvittie TJ, editors. Response of different species to total body irradiation. Dordrecht, The Netherlands: Martinus Niijhoff.
- Seed TM, Fry SA, Neta R, Weiss JF, Jarrett DG, Thomassen D. 2002a. Prevention and treatments: summary statement. Mil Med. 167:87–93.
- Seed TM, Inal C, Dobson ME, Ghose S, Hilyard E, Tolle D, Fritz TE. 2002b. Accommodative responses to chronic irradiation: effects of dose, dose rate, and pharmacological response modifiers. Mil Med. 167:82–86.
- Seed TM, Tolle DV, Fritz TE, 2002c. Haematological responses to chronic irradiation: The past Argonne experience and future AFRRI initiatives. In: Fliedner TM, Feinendegen LE, Hopewell JW, editors. Chronic irradiation: tolerance and failure in complex biological systems. Advanced research workshop on protracted, intermittent or chronic irradiation: biological effects and mechanisms of tolerance (2001: Ulm, Germany). London, UK: British Institute of Radiology.
- Semont A, Francois S, Mouiseddine M, Francois A, Sache A, Frick J, Thierry D, Chapel A. 2006. Mesenchymal stem cells increase self-renewal of small intestinal epithelium and accelerate structural recovery after radiation injury. Adv Exp Med Biol. 585:19–30.
- Sevan’kaev AV, Kozlov VM. 1974. Mitotic activity of human lymphocytes irradiated at different stages of the cell cycle. Radiobiologiia. 14:117–119.
- Sharplin J, Franko AJ. 1989a. A quantitative histological study of strain-dependent differences in the effects of irradiation on mouse lung during the early phase. Radiat Res. 119:1–14.
- Sharplin J, Franko AJ. 1989b. A quantitative histological study of strain-dependent differences in the effects of irradiation on mouse lung during the intermediate and late phases. Radiat Res. 119:15–31.
- Shim S, Jang WS, Lee SJ, Jin S, Kim J, Lee SS, Bang HY, Jeon BS, Park S. 2014. Development of a new minipig model to study radiation-induced gastrointestinal syndrome and its application in clinical research. Radiat Res. 181:387–395.
- Shin SC, Kang YM, Kim HS. 2010. Life span and thymic lymphoma incidence in high- and low-dose-rate irradiated AKR/J mice and commonly expressed genes. Radiat Res. 174:341–346.
- Singh VK, Beattie LA, Seed TM. 2013. Vitamin E: tocopherols and tocotrienols as potential radiation countermeasures. J Radiat Res. 54:973–988.
- Singh VK, Ducey EJ, Brown DS, Whitnall MH. 2012. A review of radiation countermeasure work ongoing at the Armed Forces Radiobiology Research Institute. Int J Radiat Biol. 88:296–310.
- Singh VK, Kulkarni S, Fatanmi OO, Wise SY, Newman VL, Romaine PL, Hendrickson H, Gulani J, Ghosh SP, Kumar KS, et al. 2016a. Radioprotective efficacy of gamma-tocotrienol in nonhuman primates. Radiat Res. 185:285–298.
- Singh VK, Newman VL, Romaine PL, Wise SY, Seed TM. 2014a. Radiation countermeasure agents: an update (2011–2014). Expert Opin Ther Pat. 24:1229–1255.
- Singh VK, Newman VL, Seed TM. 2015a. Colony-stimulating factors for the treatment of the hematopoietic component of the acute radiation syndrome (H-ARS): a review. Cytokine. 71:22–37.
- Singh VK, Romaine PL, Newman VL, Seed TM. 2016b. Medical countermeasures for unwanted CBRN exposures: part II radiological and nuclear threats with review of recent countermeasure patents. Expert Opin Ther Pat. 26:1399–1408.
- Singh VK, Romaine PL, Seed TM. 2015b. Medical countermeasures for radiation exposure and related injuries: characterization of medicines, FDA-approval status and inclusion into the Strategic National Stockpile. Health Phys. 108:607–630.
- Singh VK, Thrall KD, Hauer-Jensen M. 2016c. Minipigs as models in drug discovery. Expert Opin Drug Discov. 11:1131–1134.
- Singh VK, Wise SY, Fatanmi OO, Beattie LA, Seed TM. 2014b. Preclinical development of a bridging therapy for radiation casualties: appropriate for high risk personnel. Health Phys. 106:689–698.
- Singh VK, Wise SY, Fatanmi OO, Scott J, Romaine PL, Newman VL, Verma A, Elliott TB, Seed TM. 2014c. Progenitors mobilized by gamma-tocotrienol as an effective radiation countermeasure. PLoS One. 9:e114078.
- Slauson DO, Hahn FF, Benjamin SA, Chiffelle TL, Jones RK. 1976. Inflammatory sequences in acute pulmonary radiation injury. Am J Pathol. 82:549–572.
- Soligenix Inc. 2016. OrbeShield™ for gastrointestinal acute radiation syndrome (GI ARS) [Online]. Princeton, NJ: Soligenix Inc. Available from: http://www.soligenix.com/pipeline/vaccinesbiodefense/orbeshield-for-gastrointestinal-acute-radiation-syndrome-gi-ars/
- Stickney DR, Dowding C, Authier S, Garsd A, Onizuka-Handa N, Reading C, Frincke JM. 2007. 5-androstenediol improves survival in clinically unsupported rhesus monkeys with radiation-induced myelosuppression. Int Immunopharmacol. 7:500–505.
- Stickney DR, Dowding C, Garsd A, Ahlem C, Whitnall M, McKeon M, Reading C, Frincke J. 2006. 5-androstenediol stimulates multilineage hematopoiesis in rhesus monkeys with radiation-induced myelosuppression. Int Immunopharmacol. 6:1706–1713.
- Stone HB, Moulder JE, Coleman CN, Ang KK, Anscher MS, Barcellos-Hoff MH, Dynan WS, Fike JR, Grdina DJ, Greenberger JS, et al. 2004. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries. Report of an NCI Workshop, December 3–4, 2003. Radiat Res. 162:711–728.
- Sureda A, Kadar E, Valls A, Garcia-Lopez J. 1998. Granulocyte colony-stimulating factor administered as a single intraperitoneal injection modifies the lethal dose95/30 in irradiated B6D2F1 mice. Haematologica. 83:863–864.
- Swift JM, Smith JT, Kiang JG. 2015. Ciprofloxacin therapy results in mitigation of ATP loss after irradiation combined with wound trauma: preservation of pyruvate dehydrogenase and inhibition of pyruvate dehydrogenase kinase 1. Radiat Res. 183:684–692.
- Tanikawa S, Nose M, Aoki Y, Tsuneoka K, Shikita M, Nara N. 1990. Effects of recombinant human granulocyte colony-stimulating factor on the hematologic recovery and survival of irradiated mice. Blood. 76:445–449.
- Thompson RC. 1989. Life-span effects of ionizing radiation in the beagle dog [Online]. Pacific Northwest Laboratory, Battelle Memorial Institute. Available from: http://www.iaea.org/inis/collection/NCLCollectionStore/_Public/20/071/20071717.pdf
- Travis EL. 2001. Organizational response of normal tissues to irradiation. Semin Radiat Oncol. 11:184–196.
- Travis EL, Tucker SL. 1986. The relationship between functional assays of radiation response in the lung and target cell depletion. Br J Cancer Suppl. 7:304–319.
- U.S. Food and Drug Administration. 2015a. FDA approves Neupogen for treatment of patients with radiation-induced myelosuppression following a radiological/nuclear incident [Online]. Silver Spring, MD: U.S. Food and Drug Administration. Available from: http://www.fda.gov/EmergencyPreparedness/Counterterrorism/MedicalCountermeasures/AboutMCMi/ucm443245.htm
- U.S. Food and Drug Administration. 2015b. Guidance for industry: product development under the Animal Rule [Online]. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM399217.pdf
- Vasin MV, Semenov LF, Suvorov NN, Antipov VV, Ushakov IB, Ilyin LA, Lapin BA. 2014. Protective effect and the therapeutic index of indralin in juvenile rhesus monkeys. J Radiat Res. 55:1048–1055.
- Vriesendorp HM, van Bekkum DW, 1984. Susceptibility to total body irradiation. In: Broerse JJ, Macvittie TJ, editors. Response of different species to total body irradiation. Dordrecht, The Netherlands: Martinus Niijhoff.
- Vujaskovic Z, Batinic-Haberle I, Rabbani ZN, Feng QF, Kang SK, Spasojevic I, Samulski TV, Fridovich I, Dewhirst MW, Anscher MS. 2002. A small molecular weight catalytic metalloporphyrin antioxidant with superoxide dismutase (SOD) mimetic properties protects lungs from radiation-induced injury. Free Radic Biol Med. 33:857–863.
- Vujaskovic Z, Marks LB, Anscher MS. 2000. The physical parameters and molecular events associated with radiation-induced lung toxicity. Semin Radiat Oncol. 10:296–307.
- Wagemaker G, Neelis KJ, Hartong SCC, Wognum AW, Thomas GR, Fielder PJ, Eaton DL. 1998. The efficacy of recombinant TPO in murine and nonhuman primate models for myelosuppression and stem cell transplantation. Stem Cells. 16(Suppl. 2):127–141.
- Wang Z, Yang WL, Jacob A, Aziz M, Wang P. 2015. Human ghrelin mitigates intestinal injury and mortality after whole body irradiation in rats. PLoS One. 10:e0118213.
- Ward WF, Molteni A, Ts’ao CH. 1989. Radiation-induced endothelial dysfunction and fibrosis in rat lung: modification by the angiotensin converting enzyme inhibitor CL242817. Radiat Res. 117:342–350.
- Warrington JP, Ashpole N, Csiszar A, Lee YW, Ungvari Z, Sonntag WE. 2013. Whole brain radiation-induced vascular cognitive impairment: mechanisms and implications. J Vasc Res. 50:445–457.
- Waselenko JK, MacVittie TJ, Blakely WF, Pesik N, Wiley AL, Dickerson WE, Tsu H, Confer DL, Coleman CN, Seed T, et al. 2004. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med. 140:1037–1051.
- Weinstock DM, Case C Jr, Bader JL, Chao NJ, Coleman CN, Hatchett RJ, Weisdorf DJ, Confer DL. 2008. Radiologic and nuclear events: contingency planning for hematologists/oncologists. Blood. 111:5440–5445.
- Weiss JF, Landauer MR. 2009. History and development of radiation-protective agents. Int J Radiat Biol. 85:539–573.
- Williams JP, Brown SL, Georges GE, Hauer-Jensen M, Hill RP, Huser AK, Kirsch DG, Macvittie TJ, Mason KA, Medhora MM, et al. 2010. Animal models for medical countermeasures to radiation exposure. Radiat Res. 173:557–578.
- Williams JP, Jackson IL, Shah JR, Czarniecki CW, Maidment BW, DiCarlo AL. 2012. Animal models and medical countermeasures development for radiation-induced lung damage: report from an NIAID Workshop. Radiat Res. 177:e0025–0039.
- Wilson DA, Brigantic A, Morgan WF. 2011. The association of inbreeding with lung fibrosis incidence in Beagle dogs that inhaled 238PuO2 or 239PuO2. Radiat Res. 176:781–786.
- Wisniewski K, Piekarska T, Danysz A. 1972. Reaction of the small intestine of guinea pigs to barium chloride in irradiation sickness. Acta Physiol Pol. 23:159–166.
- Withers HR, Elkind MM. 1968. Dose-survival characteristics of epithelial cells of mouse intestinal mucosa. Radiology. 91:998–1000.
- Yu ZY, Li M, Han AR, Xing S, Ou HL, Xiong GL, Xie L, Zhao YF, Xiao H, Shan YJ, et al. 2011. RhG-CSF improves radiation-induced myelosuppression and survival in the canine exposed to fission neutron irradiation. J Radiat Res. 52:472–480.
- Zawaski JA, Yates CR, Miller DD, Kaffes CC, Sabek OM, Afshar SF, Young DA, Yang Y, Gaber MW. 2014. Radiation combined injury models to study the effects of interventions and wound biomechanics. Radiat Res. 182:640–652.
- Zhang K, Tian Y, Yin L, Zhang M, Beck LA, Zhang B, Okunieff P, Zhang L, Vidyasagar S. 2011. Fibroblast growth factor-peptide improves barrier function and proliferation in human keratinocytes after radiation. Int J Radiat Oncol Biol Phys. 81:248–254.
- Zhu X, Liu Y. 2009. Effects of intranasal corticosteroids on radiated nasal mucosa of guinea pig. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 23:364–368.
- Zoetelief J, Hennen LA, Broerse JJ. 1984. Some practical aspects of dosimetry and dose specification for whole body irradiation. In: Broerse JJ, Macvittie TJ, editors. Response of different species to total body irradiation. Netherlands: Springer.
